Abstract
The presence of mural calcification has, for decades, been recognized as a marker for atheromatous plaque in the coronary arteries and the aorta, but only in the past decade has the application of noncontrast computed tomography (CT) been shown to be a reproducible, safe, and convenient test, which now is available worldwide. However, awareness of coronary artery calcium scanning is insufficient and the practitioner must be aware of the available literature as well as understanding clinical recommendations for applications and interpretation. It is best applied in the medium/intermediate risk, asymptomatic adult regardless of ethnicity across broad age ranges for both men and women; additional prognostic information is also afforded from the calcium distribution in the coronary artery system. Additionally, information can also be derived from the same CT scan regarding heart and aorta size and assessment of the epicardial fat pad (an anatomic marker for the metabolic syndrome). Details of how this test can aid in cardiovascular risk assessment and management in adults are provided.
Keywords: coronary artery calcium, coronary artery disease, electron beam computed tomography, multidetector computed tomography, National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III), epicardial fat
Introduction
In 2004, 17.1 million individuals died of cardiovascular diseases, representing 29% of all global deaths; in the United State alone 650,000 individuals each year present with an acute coronary event as the initial symptom of developed coronary artery disease (CAD).1 In up to 20% of such individuals, this is their first and last (ie, fatal) symptom. Furthermore, it has been shown that 68% of acute coronary events2 occur in arteries that prior to the event were without a hemodynamically significant obstructive lesion as might have been defined by stress testing or direct, invasive angiography. It has been estimated that up to 75% of asymptomatic individuals destined to suffer from coronary heart disease were not aware of their sub-clinical atherosclerosis as they had no symptoms and may well have passed a standard stress test. These observations and others decry the need for a diagnostic tool to estimate focal severity of coronary sub-clinical atherosclerosis in asymptomatic individuals that is more sensitive than stress testing and a more accurate predictor of medium and long term events than office based conventional Framingham risk factor analysis.
Coronary artery calcification (CAC) and coronary atherosclerosis was described by Virchow3 in 1858 as ‘plates of bone’. In 1961 Blankenhorn4 found, in 3,500 coronary segments from autopsies, that all CAC was associated with intimal atherosclerosis. The first description of CAC using X-ray computed tomography (CT [electron beam CT or EBT]) was in 1987.5 In 1990, Agatston and colleagues6 used high-resolution, thin collimation, ‘step and shoot’, ECG-gated ‘heartscans’ and introduced the ‘Agatston’ total calcium scoring method, now almost universally applied in published research studies using conventional noncontrast CT (EBT and/or 64+-slice multidetector CT [MDCT]).
During the past 20 years, literally thousands of papers on CAC scoring have been published defining the role of the coronary calcium score in qualifying coronary atherosclerosis. In particular, data have shown its clinical value, incremental and complementary to conventional risk factors, as an aid to diagnostics and prognostication in patients at medium/intermediate risk for coronary disease. Additionally, more recent investigations have indicated that there remains further information that can be derived from the low radiation dose, noncontrast ‘heartscan’. These include data regarding the applicability of CAC across ethnic sub-groups, use of CAC for prognostication in the elderly, use of CAC in defining ‘heart age’, defining CAC distribution in the coronary system as an additional factor above CAC score alone, and looking beyond the coronary arteries – regarding left ventricular size, aortic root/thoracic aorta diameter, and epicardial fat.
In this context, CAC quantitation has been the subject of extensive investigations (Table 1) that have confirmed its incremental value above conventional measures of predicting cardiovascular outcomes in asymptomatic individuals.7–15
Table 1.
Published prognostic studies using CT and CAC in asymptomatic individuals
| Author | No of subjects | Mean age (years) | Follow up duration (years) | CAC score cutpoint | Comparison group | Risk ratio |
|---|---|---|---|---|---|---|
| Raggi7 | 632 | 52 | 2.7 | Highest quartile | Lowest quartile | 13 |
| Wong8 | 926 | 54 | 3.3 | Highest quartile | Lowest quartile | 8.8 |
| Arad9 | 1,173 | 53 | 3.6 | CAC Score ≥160 | CAC Score <160 | 20.2 |
| Kondos10 | 5,635 | 51 | 3.1 | CAC Score >0 | CAC Score = 0 | 10.5 |
| Shaw11 | 10,377 | 53 | 5 | CAC >400 | CAC ≤10 | 8.4 |
| Greenland12 | 66 | 66 | 7 | CAC >300 | CAC = 0* | 3.9* |
| Arad13 | 5,585 | 49 | 4.3 | CAC ≥ 100 | CAC <100 | 10.7 |
| Budoff14 | 25,253 | 56 | 6.8 | CAC >400 | CAC = 0 | 9.2 |
Note:
see caveat on interpretation on this study in text.
Abbreviations: CT, computed tomography; CAC, coronary artery calcification.
CAC scanning was incorporated into the European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (2003)16 stating that “Coronary calcium scanning is thus especially suited for patients at medium risk”; thus supporting use of CAC to supplement conventional risk analysis. The American Heart Association Guidelines for Cardiovascular Disease Prevention in Women (2004) listed the finding of CAC as an example of subclinical cardiovascular disease placing certain women in the higher 10-year risk category stating that “some patients with subclinical CVD will have >20% 10-year CHD risk and should be elevated to the high-risk category”.17
It is essential that clinicians understand what the CAC score provides in terms of diagnosis and the implications especially for medium to long term prognosis in individual patients The purpose of this review is to define a framework based upon published literature and the author’s experience for the incorporation of CAC scanning into the current practice paradigm of the FRS18 and the National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines,19 by providing recommendations for patient selection and cardiovascular risk assessment and management of medium/intermediate risk asymptomatic adults. Furthermore, new data ‘beyond the coronary arteries’ that can be derived from the noncontrast CT regarding cardiovascular health are reviewed.
Overview of CAC
EBT utilizes a rotating electron beam to acquire triggered, tomographic 100 millisecond X-ray images at 3 mm intervals in the space of a short breath-hold, and quantifies the calcified plaque in the epicardial coronary arteries. Current state of the art MDCT employs a rotating gantry with a special X-ray tube and 64 (or more) rows of detectors, with 165 millisecond or faster imaging at 3.0 mm intervals. Cardiac scans using <64 slice MDCT remain suspect as to their accuracy to quantify CAC due to motion and scan timing issues.
CAC is virtually always associated with mural atheromatous plaque.20,21 A strong direct relationship has been established between CAC as measured by CT and both histological22 and in-vivo intravascular ultrasound23,24 measures of combined calcified and noncalcified plaque. Thus, CAC provides a viable estimate of total coronary plaque burden for a given individual and has been found to be a powerful predictor of future cardiac events, providing independent and incremental information over risk factor based assessment in the asymptomatic patient.
The original coronary calcium score as published by Agatston and Janowitz6 is determined by site-by-site calcified plaque area and calcium lesion peak intensity (density). Proper application of the ‘Agatston’ calcium score requires ‘rules’ for scanner settings (see Table 2) and any deviation from these rules invalidates the measurement. It is important to note that the original application was defined using EBT and it is essential that MDCT scanners be standardized to these parameters for any confident comparison to established scoring guidelines and for application of scoring based upon prior published works. Scanning requires a 3 mm CT slice thickness and a threshold for CAC of ≥130 Hounsfield units (CT density) involving ≥ 1 mm2 area/lesion. MDCT scanners set to <3 mm slice thickness result in ‘oversampling’ and calculated scores higher than that from EBT and scanners set to >3 mm slice thickness result in ‘undersampling’ and calculated scores less than that of the EBT published standards.
Table 2.
Cardiac CT scanning and scoring parameters for application of Agatston coronary calcium scoring (see text for details)
| CT Scanner FOV | 26 cm |
| Minimal CT density for calcium | ≥130 HU |
| CT scanner slice collimation | 3.0 mm |
| Minimal calcium area | 1 mm2 (3 pixels) |
| Scoring by calcified lesion | calcium area (mm2) × 1 for maximum HU 130–199 |
| Total Agatston Score = sum of all | calcium area (mm2) × 2 for maximum HU 200–299 |
| scores for all calcified lesions in all | calcium area (mm2) × 3 for maximum HU 300–399 |
| coronary arteries | calcium area (mm2) × 4 for maximum HU ≥400 |
Abbreviations: CT, computed tomography; FOV, field of view; HU, hounsfield units.
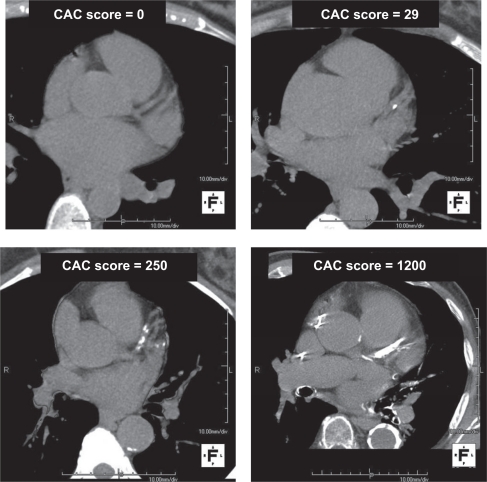
Conventional categories for CAC scoring was originally put forward by Rumberger et al25 and the plaque burden quantitatively characterized as follows: the zero score (no measurable calcified plaque), a score of 1–10 as minimal, a score of 11–100 as mild, a score of 101–400 as moderate and a score >400 as extensive. Example images representing these categories are shown in Figure 1. The calcium volume score26 is a more reproducible parameter independent of maximum calcium density per lesion and considered to be better suited for serial studies to track progression or regression of atherosclerosis; most available computer workstations that allow convenient measurements of the calcium score report data for both the Agatston score and the volume score but most published investigations report data from the Agatston calcium score alone. By comparing a subject’s Agatston calcium score to others of the same age and gender through the use of large databases of asymptomatic subjects, a calcium score percentile rank for any given individual patient can be determined.27,28 This is an index of the severity but also prematurity or, alternatively, latency of atherosclerosis development at a given chronological age and gender. Although these widely utilized nomograms are useful, it should be understood that variations according to ethnicity have been described29–32 but this subject is discussed in a later section of this manuscript.
Figure 1.
Examples of noncontrast coronary calcium CT scans at the base of the heart: top left, CAC score = 0; top right, CAC score = 29; bottom left, CAC score = 250; bottom right, CAC score = 1200.
Abbreviations: CT, computed tomography; CAC, coronary artery calcification.
Risk stratification
Key studies
The report of the NCEP ATP III guidelines33 made the following recommendation on the basis of existing data at the time publication (2002): “Therefore, measurement of coronary calcium is an option for advanced risk assessment in appropriately selected persons. In persons with multiple risk factors, high coronary calcium scores (eg, >75th percentile for age and sex) denotes advanced coronary atherosclerosis and provides a rationale for intensified LDL-lowering therapy”.
Subsequent to the NCEP guidelines, several major reports have highlighted the incremental value of CAC to conventional risk factor assessment. In a retrospective analysis Kondos et al10 (5,635 asymptomatic, predominantly low to medium conventional (Framingham) risk, middle-aged patients followed for 37 ± 12 months) found that the presence of any CAC by CT was associated with a relative risk (RR) for future cardiac events of 10.5, compared to 1.98 and 1.4 for the presence of the conventional risk factors diabetes and smoking, respectively. In women, only CAC was linked to future events, with a RR of 2.6; conventional risk factors were not related. The presence of CAC also provided prognostic information incremental to the subject’s chronological age.
Shaw et al11 retrospectively analyzed 10,377 asymptomatic patients with a 5-year follow up after an initial noncontrast CT evaluation. All-cause mortality (United States National Death Index listing at follow up) increased proportional to baseline CAC score, which was an independent predictor of risk after adjusting for all Framingham risk factors (P < 0.001). Superiority of CAC to conventional Framingham risk factor assessment was also demonstrated by a significantly greater area under the receiver operating characteristic (ROC) curves (0.73 vs 0.67; P < 0.001). Incremental value of CAC to Framingham risk was also established by a significant increase of the area under the ROC curves, from 0.72 for Framingham risk to 0.78 with the addition of CAC (P < 0.001). Stratification of all-cause mortality risk by CAC score was as effective in women as in men. A recent study published by Budoff et al using also the National Death Index looked at all-cause mortality in >25,000 initially asymptomatic subjects and found similar data for prognostication using the baseline or initial CAC score.14
Greenland et al12 analyzed a population based study of 1,461 prospectively followed, older asymptomatic subjects, who were predominantly medium to high risk, and found that CAC scores >300 significantly added prognostic information to Framingham risk analysis in the intermediate, 10%–20% Framingham risk category.
In the Saint Francis Heart Study,13 a prospective, population based study of 5,585 asymptomatic patients, CAC scores >100 were associated with RR from 10.4 to 32, and transformed conversion of Framingham intermediate risk individuals to high or very high risk status. In a subset of 1,817 patients with clinical risk factor data, incremental information over Framingham scores was documented, with areas under the ROC curves of 0.79 for CAC and 0.69 for Framingham (P = 0.0006). The greatest separation of patients who developed nonfatal myocardial infarction (MI) or cardiac death in follow-up from those who did not was at the Agatston CAC score of 100; which has for nearly the past decade been considered the cutpoint between mild and moderate coronary atherosclerosis.25 Importantly, the 15% of patients in the Saint Frances study with initial CAC scores in the 100–400 range had a 10-fold increase in RR for any cardiovascular event compared to the 33% of individuals entered into the study protocol with a zero CAC score.
Applications of total CAC score in ethnic sub-groups
In non-Hispanic Caucasians, CT ‘heartscan’ derived measures of the total CAC score predict incident coronary heart disease independent of traditional coronary risk factors, as discussed above. However, it is has been controversial as to whether the CAC score predicts coronary heart disease and prognosis across racial or ethnic subgroups. The Multi-Ethnic Study of Atherosclerosis (MESA) collected data on risk factors and performed noncontrast CT for coronary calcium in a population-based, multiethnic sample of 6,722 men and women, of whom 38.6% were Caucasian-American, 27.6% were African-American, 21.9% were Hispanic-American, and 11.9% were Chinese-American. The subjects had no known clinical cardiovascular disease at study entry and were followed for a median of 3.8 years. In comparison with subjects with no coronary calcium (ie, a CAC score of zero), the adjusted risk of a coronary event was increased by a factor of 7.73 among participants with coronary calcium scores between 101 and 300 and by a factor of 9.67 among participants with scores above 300 (P < 0.001 for both comparisons).34 Among the four ethnic sub-groups, a doubling of the calcium score increased the RR of a major coronary event by 15% to 35% and the risk of any coronary event by 18% to 39%. Furthermore, the areas under the ROC curves for the prediction of both major coronary events and any coronary event were higher when the calcium score was added to standard risk factors. The investigators concluded that the total Agatston CAC score was a strong predictor of incident coronary heart disease and provided similar predictive information beyond that provided by standard risk factors in each of the four major racial/ethnic sub-groups investigated.
Application of total CAC scores in the elderly
The majority of data using the predictive power of the total Agatston CAC score have been derived from studies done in individuals between 40 and 65 years of age. It is well known that the CAC score increases as a function of age in both men and women and many have suggested that the predictive power of the CAC score may be diminished in the elderly in whom the prevalence of any CAC is common.
Raggi et al35 reported on the predictive power of the total CAC score in a total of 35,383 individuals referred by their primary care physician for a ‘heartscan’. Of this group, 3,570 were above the age of 70 years. The investigators evaluated the predictive power of CAC scoring in 6 age deciles from individuals <40 to >80 years of age. The study reaffirmed that overall mortality rate increased with each age decile (hazard ratio [HR], 1.09) with mortality rates greater for men than women (HR, 1.53; P < 0.0001). The authors concluded that CAC risk was different in men and women but that even in elderly patients, CAC score was an independent prognostic indicator.
Defining heart age vs chronological age
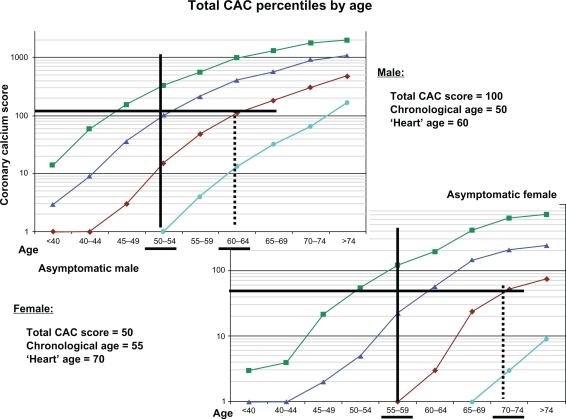
The Framingham risk score (FRS) assesses future ‘risk’ from standard risk variables including chronological age of the patient, gender, total cholesterol value, high-density lipoprotein (HDL) cholesterol value, smoking history, and blood pressure (noting if the patient is or is not currently taking hypertensive medications). Of these values, the variables with the greatest influence within the FRS model are age and gender. Overall, the FRS defines the ‘median’ risk for a group of individuals of similar age and risk factor status, however conceptually this can be further individualized through refinement of patient age as ‘heart age’ for the FRS calculation. Grundy36 was the first to suggest this approach. Nassir and Rumberger37 expanded on this concept by redefining the traditional FRS scoring tables substituting ‘heart’ age for chronological age using percentile ranking of total CAC scores.
Estimation of an individual’s ‘heart age’ can be done in a straightforward manner using previously published database information. Figure 2 shows a graphical representation of CAC score data from asymptomatic men and women originally presented by Hoff et al.27 Upon this figure is indicated information from a male aged 50 years with a total CAC score of 100 and a female, aged 55 years, with a total CAC score of 50. The ‘median’ CAC score for a 50-year-old man is 15 and the corresponding ‘median’ score for a 50-year-old woman is 1. From the data as shown in Figure 2, these individual total CAC scores are more representative of median scores from a man age 60 years (ie, 10 years older) and a woman age 70 years (ie, 15 years older). To illustrate further, assume that both are nonsmokers, have a total cholesterol value of 5.83 mmol/L (225 mg/dL), an HDL of 1.09 mmol/L (42 mg/dL), and both have mild, untreated hypertension with resting systolic blood pressures of 135 mmHg. The subsequent calculated Framingham risk18 for the man (based on chronological age) would be 0.7%/year and for the woman 0.2%/year. Using the ‘heart age’ (but not changing the point scores based on chronological age points accumulated for the other risk factors) the cardiovascular ‘risk’ for developing symptomatic disease would be 1.2%/year and 0.6%/year for the man and women, respectively. These revised risk estimates can then be used clinically to guide the level of aggressiveness for medical interventions. Heart age and a FRS integrating conventional risk factors and a given individual’s CAC score can be conveniently calculated at the MESA website at http://www.mesa-nhlbi.org/CACReference.aspx.
Figure 2.
Graphical representation of data presented by Hoff27 showing distribution of total CAC scores in 35,246 men and women. The red line represents the median score as a function of age. Superimposed are information regarding determination of ‘heart age’ (see text for details) in a 50 year old man with a CAC score of 100 and a 55 year old woman with a CAC score of 50.
Abbreviation: CAC, coronary artery calcification.
Coronary calcium pattern and distribution
The majority of data regarding the prognostic power of CAC has been reported using the total Agatston coronary calcium score whereas little has been published regarding calcification patterns and prognosis. A common question is: “does a total calcium score of X, attributed to a single site in a single coronary artery invoke the same prognostic power as the same total calcium score in an individual where the distribution is diffuse and found in three separate coronary arteries or the left main artery?”
Ehara et al38 studied preintervention intracoronary ultrasound in patients with acute coronary syndromes and in patients with stable coronary artery disease (CAD) and noted that ‘spotty’ coronary calcium (ie, a focus <3 mm in size) was more likely to be associated with an acute presentation. Schmermund et al had noted previously that a pattern of ‘spotty’ (mild to moderate diffuse versus moderate to severe focal) coronary calcium by CT was more commonly associated with diffuse coronary plaque and focal outward (ie, positive) coronary artery remodeling at angiography.39 Most recently, Williams et al40 reported on 14,759 individuals for all-cause mortality at an average of 6.8 years of follow up. They noted that the mortality rate exceeded 2% per year (considered ‘coronary artery disease’ risk equivalent by the NCEP) in the setting of more than 20 calcified lesions. Additionally, the finding of CAC in the left main coronary artery as opposed to any other anatomic site was associated with increased all-cause mortality.
Quantification of the distribution of coronary artery calcium was recently proposed with a ‘calcium coverage score’ (CCS) calculated as the proportion of 5 mm coronary arterial segments affected by calcific plaque.41 In the MESA, after 41 months of follow-up, a two-fold increase in CCS was associated with a 34% increased risk of a hard coronary disease event, defined as definite adjudicated myocardial infarction (MI), coronary death, and/or angina, compared with just a 14% increased risk indicated by the total CAC Agatston score. Additional validation work is needed on the CCS, but in the future, application of the CCS may result in further refinement in the assessment of individual risk over and above the total CAC score.
The zero score
Asymptomatic individuals with zero CAC score have not yet developed detectable, calcified coronary plaque but may have fatty streaking and early stages of a plaque and have a 1%–2% chance of focal obstructive coronary artery disease. Atherosclerotic plaques are present in many young adults,42 but the event rate in patients with CAC score of zero is very low.7,10,12,13 Raggi et al7 have demonstrated an annual event rate of 0.11% in asymptomatic subjects with 0 scores, and in the St Francis Heart Study,13 scores of 0 were associated with a 0.12% annual event rate over the ensuing 4.3 years. Greenland et al12 in a higher risk asymptomatic cohort, noted a higher annual event rate (0.62%) with 0 CAC scores; however a less sensitive CAC detection technique (significant ‘under sampling’ and thus ‘under-scoring’ by using higher thresholds for positive scans, ie, nonstandard Agatston scoring) and marked ethnic heterogeneity may have contributed to their different findings.43
A more definitive study (again using the National Death Index) which addressed the issue of a zero CAC score and all-cause mortality was reported by Blaha and colleagues.44 Annualized all-cause mortality was assessed in 44,052 consecutive asymptomatic adults (age 54 ± 10 years) referred for risk assessment using noncontrast CT and CAC scoring. Follow up was done at 5.6 ± 2.6 years. A total of 19,898 subjects had no detectable CAC on initial cardiac CT examination. Annualized all-cause mortality rates for CAC = 0 were 0.87 deaths/1,000 person years (0.087%/year). Mortality rates for a CAC score of 1–10 and >10 were higher at 1.92 and 7.48/1,000 person years, respectively. This confirms that a zero CAC score in an asymptomatic individual puts them at a <0.1%/year mortality risk, but scores above zero represent incrementally and substantially increased cardiac risk.
Distribution of CAC
There is often confusion as to what would be the typical distribution of calcium scores as applied to an asymptomatic group of intermediate risk individuals. Misconceptions abound as many symptomatic individuals undergoing CT coronary angiography are often found to have very high calcium scores and many physicians that I have consulted with seem to think that ‘everybody who is middle aged has some CAC’. This is simply not true.
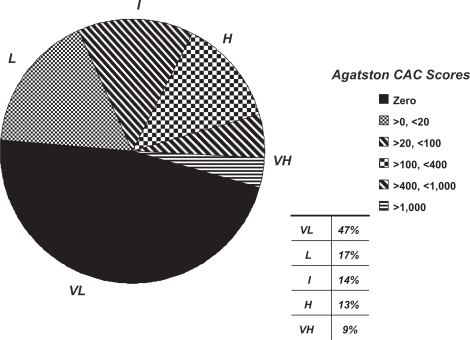
As an example, I offer information on 5,192 randomly assessed, asymptomatic individuals referred to me personally for cardiovascular risk assessment by their primary care doctors. The average age was 53 ± 10 years (60% men); 31% had total cholesterol above 5.18 mmol/L (200 mg/dl), 22% had a systolic blood pressure >140 mmHg, 7% were current smokers, and 23% had a family history of premature heart disease in a first degree relative. Calcium scores ranged from 0 to 5808 (mean 128 ± 378). A zero CAC score was found in 47% while a score above 1000 was found in only 3.5%. A pie chart of the distributions of calcium scores in this asymptomatic group is found in Figure 3.
Figure 3.
Pie chart representing the distribution of coronary calcium scores in >5,000 asymptomatic, middle aged adults referred to one scanning center for assistance in cardiovascular risk stratification. Subsequent stratification of risk is altered based upon the total calcium scores (see text for detail) as very low (VL = <0.1%/year), low (L = <1%/year), true intermediate (I = 1%–2%/year), high (H = >2%/year), and very high (VH = >3%/year).
Clinical applications
Patient selection
Recommendations for CAC scanning are not based on age and gender alone. Rather, the FRS, which incorporates both age and gender, is recommended as the initial step in selecting the appropriate test populations. Asymptomatic patients in the 10%–20% Framingham 10 year risk category (medium/intermediate risk) comprise of the group that presents the greatest challenge to the treating physician, and are those in whom the application of CAC scoring is considered most appropriate. This group represents up to 40% of the population that might be seen sitting in the waiting room of the average internist.45 While this application was proposed as being reasonable in the early American College of Cardiology/American Heart Association (ACC/AHA) consensus statement,46 the recent additional evidence of risk-stratification by CAC in this group has resulted in a greater acceptance of its benefits, and was included in the 2005 AHA Scientific Statement on noninvasive testing in women for use in the intermediate risk population.17
Selected patients with less than intermediate Framingham risk may also benefit. For instance, most young patients with a family history for premature CAD (1st degree relatives with documented heart disease below the age of 55) will not have sufficient risk factors to even warrant Framingham scoring (lower NCEP risk), or will be in the low (1%–10%) 10 year Framingham risk group.18,47 Data from Schmermund et al24 and Pohle et al48 indicate that 95% of acute MI patients would have been identified by CAC plaque imaging, even in those with a mean age of 41 years. On the basis of these observations, the use of CAC scoring should be considered in patients with a family history of premature CAD, especially if their Framingham risk is intermediate (although many would advocate this use even if the initial Framingham risk was calculated as low; since family history remains one of the most positive risk factors and is NOT considered part of conventional Framingham Risk scoring).
Selective application of CAC scanning to patients with Framingham high risk (>20%/decade or >2%/year) may also be warranted. For instance, some Framingham high risk patients may be intolerant of statins or may strongly prefer alternative-medicine approaches. In these patients, CAC confirmation of high risk may be used to reinforce the necessity for finding a statin that can be tolerated and for persuading the refractory patient of the need for aggressive treatment. Conversely, the absence of any CAC for age or gender may permit relaxation of the treatment goals, an approach that appears justified by the low event rate in the 0 score CAC group.7,10,12–14
Initiation and goals of drug therapy
The presence or absence and amount of CAC can be useful for clinical decision making, as previously recommended in the AHA Prevention V Update.49 As an extension of this report, based on recent data, Table 3 provides simple, easily implemented treatment paradigms for combining risks of varying CAC scores with the most recent NCEP recommendations. Patients in the 10%–20% 10 year risk category that are identified to be at higher risk by CAC become candidates for secondary prevention lipid goals regardless of their baseline lipid level. This would apply even for patients with low-density lipoprotein (LDL) cholesterol <2.59 mmol/L (<100 mg/dL), as implied by the Heart Protection Study50 and stated in the prior NCEP report.
Table 3.
Guidelines for treatment of LDL cholesterol in asymptomatic individuals classified as intermediate risk patients by NCEP (Framingham 10%–20% 10 year risk)
| CAC score and percentile ranking | Framingham risk group equivalent | Target LDL goal mmol/L (mg/dl) | Pharmacologic therapy indicated @ mmol/L (mg/dl) |
|---|---|---|---|
| Zero | Very low risk (10 year risk <1%) | <4.14 (160) | ≥4.92 (190) |
| >0, <10 AND <75th percentile | Low risk (10 year risk >1% but <10%) | <3.37 (130) | ≥4.14 (160) |
| 11–100 AND <75th percentile | Intermediate risk (10 year risk >10% but <20%) | <3.37 (130) | ≥3.37 (130) |
| 101–400 OR ≥ 75th but <90th percentile | High risk (coronary disease risk equivalent; 10 year risk ≥20%) | <2.59 (100) | ≥2.59 (100) |
| >400 OR ≥90th percentile | Very high risk (10 year risk >30%) | <1.29 1.81 (50 70) | any LDL level |
Abbreviations: LDL, low-density lipoprotein; NCEP, National Cholesterol Education Program; CAC, coronary artery calcification.
Based on prognostic data, CAC scores >100 and/or >75th percentile for age/gender serves to define a CAD risk equivalent (ie, >20% over the next decade). In the St Francis Heart Study,13 the CAC cutpoint for secondary prevention risk equivalency in the Framingham intermediate 10%–20% 10 year risk group was a score >100; or, additionally at a score >75th percentile for age as suggested by the NCEP guidelines. In this regard, CAC scores >400 or >90th percentile for age/gender are associated with a very high annual risk (4.8% and 6.5% respectively),7,51 and these individuals are candidates for an even more aggressive approach (ie, LDL < 1.81 mmol/L (<70 mg/dL) as suggested by the latest update to the NCEP19) and possibly further stratification with stress testing (see Discussion below).
In the Framingham 10%–20% 10 year risk population, patients with CAC scores ≤100 and/or ≤75th percentile remain in the same risk group or are transformed to lower risk categories depending on the actual score or percentile rank. A reasonable approach is to leave the patients with CAC scores from 10–100 and <75th percentile in the intermediate risk (10%–20% 10 year risk), and reclassify patients with CAC scores from 1–10 and <75th percentile as low risk (<10% 10 year risk) and treat accordingly. CAC scores of 0 would reclassify the patient to the very low risk category.
As noted above, some patients in the lower risk groups based on Framingham scores, such as younger patients (35–45 years of age) with a strong family history of premature coronary heart disease, may be appropriately tested with CAC scanning. In such patients, the recommendations in Table 3 would also apply. While it is widely accepted that high CAC scores reinforce the intensity of medical therapy, how low CAC scores should affect therapy is not yet clear. It would appear reasonable that in high risk asymptomatic patients who have undergone imaging, CAC scores ≤100, and in particular, ≤10, imply a lower than expected risk and may reduce the intensity of therapy. The rationale for this lowest category is as follows. If the use of cholesterol lowering medications (such as statins) can across the board, lower the risk of an MI by 1/3, this is significantly of value when the risk is found to be high, but is less practical when the risk is very low; for instance, if the ‘risk’ of a cardiovascular event is 0.1%/year for a zero CAC score (as supported by the published literature), then using a cholesterol medication may be of limited incremental value if the risk reduces to 0.07%.
It is important to note, however, that a CAC score of 0 does not imply that no treatment is necessary. Rather, it is used to shift the patient to a lowest risk group. For example, early information has shown that the event rate in diabetics with 0 CAC scores is as low as in nondiabetics with 0 scores,52 potentially creating a group of diabetics who would not have to be considered to have ‘coronary heart disease’ equivalence. More data is needed in these groups for determining the therapeutic implications of the absence of CAC.
Evaluation of treatment
The use of changes in CAC score rather than changes in lipid values to track treatment effects has been under investigation. Serial CT scanning has shown that aggressive lipid lowering therapy may slow progression of calcified plaque53–58 although it is far from infallible.59 Clearly, a noninvasive tool with which sequential testing could be performed safely and reliably would be highly desirable provided the results are associated with significant prognostic value. Raggi et al have demonstrated that CAC progression was greater in patients with future MI58 whereas LDL levels on treatment were similar in patients with and without events. Progression was associated with a worse prognosis compared to stabilization, irrespective of baseline CAC score. However, more studies are required to justify the broad based use of serial CAC scanning to monitor treatment efficacy.
Triage for stress testing
The asymptomatic middle aged population has a low pretest likelihood for significant obstructive disease (>50% stenosis); nonetheless, stress testing in this group is common even though there are no guideline sanctioned recommendations. Bayes Theorem60 dictates that stress testing of any kind will yield a large proportion of false positives in this low pretest likelihood group. CAC scoring is better suited than stress testing as the initial assessment; stress testing may be of value in selected patients after the CAC test to determine whether ischemia is present in order to further guide management and in particular to determine whether coronary angiography should be considered. Stress testing performed to evaluate cardio-respiratory fitness and blood pressure response to exercise should not be influenced by CAC.
Based on studies in relatively large groups of patients, it is clear that stress testing is not needed in the vast majority of asymptomatic patients with CAC scores >0 (see score distributions in a sample of such patients in Figure 3). In 411 patients without established coronary disease undergoing CAC scanning and myocardial perfusion imaging (MPI), He et al61 found 0% positive nuclear tests with CAC scores of 0 and 1–10, 2.6% with CAC scores 11–100, 11.6% with CAC scores 101–399, and 46% with CAC scores ≥400. However, these high percentages of abnormal MPI in various CAC score categories are at odds with the observations of Berman et al.62 In 1195 patients without known disease, Berman noted 1.6% abnormal MPI with CAC scores of 0, 0% with scores of 1–9, 2.4% with scores of 10–99, 5.2% with scores of 100–399, 8.9% from 400–999, and 19.9% with scores ≥1000. However, both studies concluded that sufficient pretest likelihood of an abnormal MPI is present in the CAC score >400 group and in only selected patients in the 100–400 CAC score group. Many clinicians now suggest stress testing when the baseline CAC score exceeds 400, even if they are asymptomatic. This was the same conclusion reached by Rumberger and colleagues in the first guidelines paper on CAC scoring, published more than a decade ago.25
The study of Berman et al also illustrated that the normal MPI study alone, performed without the CAC score, was insufficient to place patients into a group not requiring aggressive medical therapy, even if the short term prognosis is benign. They found that 56% of 1195 patients with normal MPI had CAC scores >100, and 31% had CAC scores >400. Thus, if the likelihood of CAD was sufficiently high to warrant stress testing with any modality, CAC scanning should be considered after a normal stress evaluation to guide the aggressiveness of medical therapy.62
Regardless of the CAC score, stress testing should precede coronary arteriography in the asymptomatic patient, and coronary angiography should be reserved for those patients deemed to be at high risk on the basis of the stress test results. However, this group may alternatively be considered for direct CT angiography (CTA) using 64+−slice MDCT.
Limitations
As with any diagnostic method, CAC testing has limitations. It does not evaluate the degree of coronary stenosis; the specificity of a positive CAC score for the presence of obstructive disease is in the 40% range.63 In addition, the specificity of CAC for cardiac events is quite low, since only a minority of patients with CAC experience events. However, the specificity of CAC for the diagnostic goal, the identification of sub-clinical atherosclerosis, is nearly 100%. CAC does not visualize noncalcified (incorrectly deemed ‘soft’) plaque, and patients with exclusively noncalcified plaque will escape detection; however, although discussed by clinicians, such a situation of significant noncalcified plaque in the absence of concomitant calcified plaque somewhere within the coronary system is rare in the pathology literature.
CAC scanning is associated with a radiation dose which ranges from 0.9 mSv (milli-Severt) for EBT and up to 2.5 mSv for prospectively gated MDCT. However, awareness of the need to reduce radiation doses for routine CT scanning has resulted in major improvements in scanning algorithms from the MDCT manufacturers. Currently, a retrospective CAC scan can be done in almost all situations with radiation doses <1.5 mSv and commonly at <1 mSv with the latest scanners (the radiation dose for a standard two-view mammogram is about 0.75 mSv, to put this in perspective). However, the fact that a cardiac CT examination of any type does necessitate radiation exposure to the patient emphasizes the importance of appropriate patient selection to ensure that the benefits outweigh the risks.
The major limitation of this report is the absence of evidence based confirmation of the CAC based proposed modifications of the NCEP-III guidelines, and the reliance on the clinical experience of the authors and extrapolation of risk from key clinical studies, some of which are retrospective.
Beyond the coronary arteries
A noncontrast CT of the heart can provide additional anatomic information beyond just defining the amount or pattern of CAC. There has been renewed interest in using all potential scan information as it might relate to an individual’s development of or risk for developing cardiac disease. In particular three new areas have been most recently examined.
Heart size
Nasir et al64 used data from the MESA study to compare left ventricular size from noncontrast CT scans of the heart with magnetic resonance imaging (MRI) determinations of left ventricular muscle mass and volumes in the same subjects. A total of 5,004 participants were included in the investigation. From the ‘heartscan’, a single midventricular CT slice was defined at the level containing the coronary sinus or the first level below the left atrium. Then a straight line connecting the anterior-posterior junction of both ventricles was drawn to divide the left and right ventricles and the subsequent area of the left ventricle was then measured with planimetry and left ventricular (LV, middiastolic) volume was estimated using a previously validated algorithm developed by Mao.65 Left ventricular measurements with CT correlated well with MRI regarding both LV volume (r = 0.73; P < 0.01) and LV muscle mass (r = 0.74; P = 0.01). Further studies will be required, but these data clearly indicate that an additional important parameter for cardiovascular diagnosis and prognosis, namely estimates of LV volume/mass, are possible from a standardized ‘heartscan’.
Thoracic aorta size
Dilation of the aortic root and the thoracic aorta is common in aortic valve disease, primary diseases of the aorta, and systemic hypertension. Two-dimensional echocardiography is most commonly used clinically for these measurements; however, similar anatomic areas are also imaged as part of a standard ‘heartscan’. Lin et al66 evaluated noncontrast, ungated, scans of the chest/heart in 103 normotensive, nonobese individuals who were free of known cardiovascular disease. They reported that normal aortic root size (2.5–3.7 cm), ascending aorta diameter (2.1–3.5 cm), and thoracic aorta (1.7–2.6 cm) diameters were very similar to what had been previously reported using standard transthoracic echocardiography. These data imply that prospective application of these normative values to individuals undergoing ‘heartscans’ for assessment of coronary calcium may provide additional diagnostic information.
Pericardial/epicardial fat
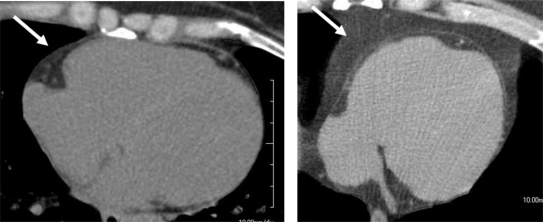
Visceral abdominal/adipose fat has a known association with the metabolic syndrome67 and its measurement from abdominal CT scans has been standardized for some time.68 Recently, an association of visceral adipose fat with epicardial adipose fat on MDCT scans has been noted69 where both were found to correlate with body mass index, waist circumference, and other known cardiovascular risk factors such as coronary and vascular calcification. Recently Nichols and colleagues70 have evaluated a three-dimensional volumetric measure of epicardial fat using contrast-enhanced MDCT and have found it be highly reproducible. However, further data are needed to determine the algorithms application to the more commonly obtained noncontrast scans done as part of standard CAC assessment. An example of a single slice, mid LV ‘heartscan’ from a patient without and a patient with metabolic syndrome, demonstrating the epicardial fat distribution, is shown on Figure 4. A reliable and reproducible measure of epicardial fat would provide yet another potential for the ‘heartscan’ to be used in cardiovascular risk stratification, especially in individuals with the metabolic syndrome.
Figure 4.
A single, mid LV slice from a noncontrast CT of the heart (ie, a ‘heartscan’) demonstrating the epicardial fat distribution in a patient without metabolic syndrome (Left arrow) and a patient with known metabolic syndrome (right arrow). See text for details.
Abbreviations: LV, left ventricle; CT, computed tomography.
Conclusions
The increasing use of CAC scanning for risk assessment is now supported by extensive evidence in appropriately selected patients. Critical to its implementation is the ability of practitioners to understand the CAC scoring data and limitations of the test, as outlined in this review. Only then can they appropriately utilize this knowledge, including data ‘beyond the coronary arteries’, to improve cardiovascular risk assessment and management in their individual patients.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Heart and Stroke Statistical Update. Dallas, TX: American Heart Association; 2001. [Google Scholar]
- 2.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 3.Virchow R. Die Cellulärpathologie in Ihrer Begrundung auf physiologische und pathologische Gewebesslehre. Berlin: August Hirschwald; 1858. [Google Scholar]
- 4.Blankenhorn DH. Coronary arterial calcification: a review. Am J Med Sci. 1961;242:1–9. [Google Scholar]
- 5.Tannenbaum SR, Kondos GT, Veselik KE, Prendergast MR, Brundage BH, Chomka EV. Detection of calcific deposits in coronary arteries by ultrafast computed tomography and correlation with angiography. Am J Cardiol. 1989;63:870–872. doi: 10.1016/0002-9149(89)90060-x. [DOI] [PubMed] [Google Scholar]
- 6.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Callister TQ, Cooil B, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 8.Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–498. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 9.Arad Y, Spadaro L, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253–1260. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 10.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 13.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein and atherosclerotic cardiovascular disease events: the St Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 14.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcium: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 15.Schmermund A, Denktas AE, Rumberger JA, et al. Independent and incremental value of coronary artery calcium for predicting the extent of angiographic coronary artery disease: comparison with cardiac risk factors and radionuclide perfusion imaging. J Am Coll Cardiol. 1999;34:777–786. doi: 10.1016/s0735-1097(99)00265-x. [DOI] [PubMed] [Google Scholar]
- 16.DeBacker G, Ambrosioni E, Borch-Johnson K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third joint task force of European and other countries in cardiovascular disease in clinical practice. Eur Heart J. 2003;24:1601–1610. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 17.Mieres JH, Shaw LJ, Andrew Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PWF, D’Agostino B, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 20.Rifkin RD, Parisi AF, Folland E. Coronary calcification in the diagnosis of coronary artery disease. Am J Cardiol. 1979;44:141–147. doi: 10.1016/0002-9149(79)90263-7. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy JH, Palmer FJ. Incidence and significance of coronary artery calcification. Br Heart J. 1974;36:499–506. doi: 10.1136/hrt.36.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium areas by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 23.Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol. 1997;30:57–64. doi: 10.1016/s0735-1097(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 24.Schmermund A, Baumgart D, Gorge G, et al. Coronary artery calcium in acute coronary syndromes: a comparative study of electron beam ct, coronary angiography, and intracoronary ultrasound in survivors of acute myocardial infarction and unstable angina. Circulation. 1997;96:1461–1469. doi: 10.1161/01.cir.96.5.1461. [DOI] [PubMed] [Google Scholar]
- 25.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic individuals. Mayo Clin Proc. 1999;74:243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 26.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 27.Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87:1335–1339. doi: 10.1016/s0002-9149(01)01548-x. [DOI] [PubMed] [Google Scholar]
- 28.Schmermund A, Erbel R, Silber S, MUNICH Registry Study Group Age and gender distribution of coronary artery calcium measured by four-slice computed tomography in 2,030 persons with no symptoms of coronary artery disease. Am J Cardiol. 2002;90:169–173. doi: 10.1016/s0002-9149(02)02445-1. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Yang TP, Shavelle RM, Lamont DH, Brundage BH. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol. 2002;39:408–412. doi: 10.1016/s0735-1097(01)01748-x. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22:424–430. doi: 10.1161/hq0302.105357. [DOI] [PubMed] [Google Scholar]
- 31.Khuran C, Rosenbaum CG, Howard BV, et al. Coronary artery calcification in black women and white women. Am Heart J. 2003;145:724–729. doi: 10.1067/mhj.2003.99. [DOI] [PubMed] [Google Scholar]
- 32.Jain T, Peshock R, Darren K, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 33.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. NIH Publication No. 02-5215. 2002 Sep. [PubMed]
- 34.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 35.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM. Coronary plaque as a replacement for age as a risk factor in global risk assessment. Am J Cardiol. 2001;88(2A):8E–11E. doi: 10.1016/s0002-9149(01)01712-x. [DOI] [PubMed] [Google Scholar]
- 37.Nasir K, Vasameddy C, Blumenthal RS, Rumberger JA. Comprehensive coronary risk determination in primary prevention: an imaging and clinical based definition combining computed tomographic coronary artery calcium score and national education program risk score. Int J Cardiol. 2006;110:129–136. doi: 10.1016/j.ijcard.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 39.Schmermund A, Rumberger JA, Colter JF, Sheedy PF, Schwartz RS. Angiographic correlates of “spotty” coronary artery calcium detected by electron beam computed tomography in patients with normal or near-normal coronary angiograms. Am J Cardiol. 1998;82:508–511. doi: 10.1016/s0002-9149(98)00372-5. [DOI] [PubMed] [Google Scholar]
- 40.Williams M, Shaw LJ, Raggi P, et al. Prognostic value of number and site of calcified coronary lesions compared with the total score. JACC Cardiovasc Imaging. 2008;1:61–69. doi: 10.1016/j.jcmg.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Brown ER, Kronmal RA, Bluemke DA, et al. Coronary calcium coverage score: determination, correlates, and predictive accuracy in the multi-ethnic study of atherosclerosis. Radiology. 2008;247:669–675. doi: 10.1148/radiol.2473071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 43.Budoff MJ, Ehrlich J, Hecht HS, Rumberger JR. Letter to the Editor. JAMA. 2004;291:1822. doi: 10.1001/jama.291.15.1832-a. [DOI] [PubMed] [Google Scholar]
- 44.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Img. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Greenland P, Smith SC, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104:1863–1867. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 46.O’Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000;102:126–140. doi: 10.1161/01.cir.102.1.126. [DOI] [PubMed] [Google Scholar]
- 47.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 48.Pohle K, Ropers D, Mäffert R, et al. Coronary calcifications in young patients with first, unheralded myocardial infarction: a risk factor matched analysis by electron beam tomography. Heart. 2003;89:625–628. doi: 10.1136/heart.89.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SC, Greenland P, Grundy SM. AHA Conference Proceedings. Prevention Conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention. executive summary. Circulation. 2000;101:111–116. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 50.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial Lancet 20023607–22.12114036 [Google Scholar]
- 51.Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J. 2001;141:375–382. doi: 10.1067/mhj.2001.113220. [DOI] [PubMed] [Google Scholar]
- 52.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 53.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 54.Budoff MJ, Lane KL, Bakhsheshi H, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86:8–11. doi: 10.1016/s0002-9149(00)00820-1. [DOI] [PubMed] [Google Scholar]
- 55.Achenbach S, Ropers D, Pohle K, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002;106:1077–1082. doi: 10.1161/01.cir.0000027567.49283.ff. [DOI] [PubMed] [Google Scholar]
- 56.Shavelle DM, Takasu J, Budoff MJ, et al. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002;359(9312):1125–1126. doi: 10.1016/S0140-6736(02)08161-8. [DOI] [PubMed] [Google Scholar]
- 57.Raggi P, Cooil B, Shaw L, et al. Progression of coronary calcification on serial electron beam tomography scanning is greater in patients with future myocardial infarction. Am J Cardiol. 2003;92:827–829. doi: 10.1016/s0002-9149(03)00892-0. [DOI] [PubMed] [Google Scholar]
- 58.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 59.Hecht HS, Harman SM. Evaluation by electron beam tomography in treated and untreated asymptomatic patients. Am J Cardiol. 2003;91:1131–1134. doi: 10.1016/s0002-9149(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 60.Diamond GA, Forester JS. Analysis of probability as an aid in the clinical diagnosis of coronary artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 61.He Z, Hedrick TD, Pratt CM, et al. Severity of coronary calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–251. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 62.Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–930. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 63.Haberl R, Becker A, Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–457. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- 64.Nasir K, Kata R, Mao S, et al. Comparison of left ventricular size by computed tomography with magnetic resonance imaging measures of left ventricular mass and volumes: the multi-ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. 2008;2:141–148. doi: 10.1016/j.jcct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Mao S, Budoff MJ, Oudiz RJ, Bakhsheshi H, Wang S, Brundage BH. A simple single slice method for measurement of left and right ventricular enlargement by electron beam tomography. Int J Card Imaging. 2000;16:383–390. doi: 10.1023/a:1026523924838. [DOI] [PubMed] [Google Scholar]
- 66.Lin FY, Devereux RB, Roman MJ, et al. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovas Comput Tomogr. 2008;2:298–308. doi: 10.1016/j.jcct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 68.Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437–445. [PubMed] [Google Scholar]
- 69.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 70.Nichols JH, Samy B, Nasir K, et al. Volumetric measurement of pericardial adipose tissue from contrast-enhanced coronary computed tomography angiography: A reproducibility study. J Cardiovasc Comput Tomogr. 2008;2:288–295. doi: 10.1016/j.jcct.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]