Abstract
Lipoperoxidative damage to the respiratory chain proteins may account for disruption in mitochondrial electron transport chain (ETC) function and could lead to an augment in the production of reactive oxygen species (ROS). To test this hypothesis, we investigated the effects of lipoperoxidation on ETC function and cytochromes spectra of Saccharomyces cerevisiae mitochondria. We compared the effects of Fe2+ treatment on mitochondria isolated from yeast with native (lipoperoxidation-resistant) and modified (lipoperoxidation-sensitive) fatty acid composition. Augmented sensitivity to oxidative stress was observed in the complex III-complex IV segment of the ETC. Lipoperoxidation did not alter the cytochromes content. Under lipoperoxidative conditions, cytochrome c reduction by succinate was almost totally eliminated by superoxide dismutase and stigmatellin. Our results suggest that lipoperoxidation impairs electron transfer mainly at cytochrome b in complex III, which leads to increased resistance to antimycin A and ROS generation due to an electron leak at the level of the QO site of complex III.
Keywords: Lipoperoxidation, Cytochromes, Yeast mitochondria, Iron, Electron transport chain
Introduction
The study of mechanisms underlying oxidative stressrelated mitochondrial dysfunction has become a relevant research field due to participation of mitochondrial oxidative stress in the development of a wide variety of disorders such as liver damage by hepatitis B virus (Lee et al, 2004), heart ischemia-reperfusion (Lucas and Szweda 1998), aging (Wei et al. 1998, 2001) cancer (Lemire et al. 2003), diabetes (Green et al. 2004), and neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis (Tanusree et al. 2007). The mitochondrial electron transport chain (ETC) is the primary source of reactive oxygen species (ROS) in the cell. Generally, ROS are short-lived species that react readily with molecules surrounding its generation site. Among cellular biomolecules, polyunsaturated fatty acids display the highest propensity to ROS attack (Pamplona et al. 1998), making unsaturated fatty acids from mitochondrial membranes the main target of ROS during mitochondrial oxidative stress.
Lipoperoxides inactivate enzymes bound to biological membranes and decrease membrane fluidity (Rice-Evans 1994). Consequently, ETC function becomes impaired during oxidative stress and several reports have recognized lipoperoxidation as the major cause of mitochondrial dysfunction (Petrosillo et al. 2003; Paradies et al. 1999, 2002, 2004; Cardoso et al. 1999; Forsmark-Andreé et al. 1997). In these studies, cardiolipin and quinone loss have been identified as the fundamental cause of ETC dysfunction. However, the damaging effects of lipoperoxidation in other redox components of the ETC (i.e. cytochromes) have not been further explored. In one of these studies (Forsmark-Andreé et al. 1997), it was observed that the content of cytochrome c and the reduction of cytochrome b by respiratory substrates decrease by the treatment with ascorbate and ADP/Fe3+. Nevertheless, from these results it seems difficult to establish a direct correlation between anomalies in cytochromes reduction and lipoperoxidation because of the direct effect of ROS on cytochromes was not properly discarded.
Impairment of cytochromes by lipoperoxidation is an important issue to address because in the ubiquinol-cytochrome c oxidoreductase complex (complex III), cytochromes participate in electron transfer at quinone redox sites, which produce ROS from mitochondria. According to the Q-cycle (Hunte et al. 2003), during bifurcated quinol oxidation at the quinol oxidase (QO) site, one electron is transferred to the cytochrome b subunit and another electron is transferred to cytochrome c1 via the Rieske iron-sulfur protein (ISP) rotation between the QO site and the cytochrome c1 vicinity. Inhibition of electron transfer during complex III turnover by treatment with inhibitors of quinone redox sites induces the apparition of reactions that bypass the Q-cycle which are responsible for ROS production (Muller et al. 2003). For example, treatment of complex III with antimycin A blocks reoxidation of cytochrome b at the Qi site, leading to electrons accumulation in b hemes and reverse transfer of electrons to quinone at the QO site to produce semiquinone, which can reduce oxygen to superoxide (Muller et al. 2002).
There are functional, specific binding of phospholipid molecules with protein moieties of complex III. For example, a molecule of phosphatidylinositol was found to be bound to a position near the flexible linker region of ISP in the yeast complex III. This interaction was proposed to facilitate the rotation of the ISP catalytic, head domain (Lange et al. 2001). As well, delipidation of complex III impairs antimycin A binding in a reversible way through cardiolipin addition, reflecting a requirement of cytochrome b for lipids to achieve an adequate stabilization of its structure (Tsai and Palmer 1986). Taking into account these facts, it is feasible to expect that an augment on lipoperoxidation sensitivity could impair electron transfer at the cytochromes level, which may lead to an increased susceptibility of ETC to inhibition by oxidative stress and the apparition of Q-cycle bypass reactions responsible for undesirable ROS production. To test this hypothesis, mitochondria from the yeast Saccharomyces cerevisiae represent an ideal research model because yeast membranes are resistant to lipoperoxidation (which allow an investigation of the effects of ROS on the ETC that are independent of lipoperoxidation) (Cortés-Rojo et al. 2007) and the sensitivity of yeast to this process can be increased by manipulation of the membranes fatty acid content.
In the present work, we studied the effects of Fe2+ on levels of lipoperoxidation, ETC function and cytochromes status in yeast mitochondria with native (lipoperoxidation-resistant) and modified (lipoperoxidation-sensitive) fatty acid composition. Lipoperoxidation increased the sensitivity of ETC to inhibition by oxidative stress except at the level of complex II. Furthermore, lipoperoxidation induced a resistance to the inhibitory effects of antimycin A on cytochrome c reduction, which was associated with an increment in superoxide (O2•−) formation and a defect in cytochrome b reduction that was prevented by an anti-lipoperoxidative agent.
Materials and methods
Materials
α-Linolenic acid (cis,cis,cis-9,12,15-octadecatrienoic acid, purity ≥99%), Igepal CA-630, succinic acid, 2,6-dichlorophenolindophenol (DCIP), potassium cyanide, cytochrome c, antimycin A, were obtained from Sigma Chemical. Co. (St. Louis, MO, USA). Stigmatellin and dithionite were obtained from Fluka Chemie GmbH, (Buchs, Switzerland). Zymolyase 20T was obtained from ICN Biomedicals, Inc. (Aurora, OH USA). All other reagents were of the highest purity commercially available.
Cell growth and mitochondria isolation
For mitochondria isolation, an industrial wild type diploid strain of Saccharomyces cerevisiae, Yeast Foam was used (Kind gift from Professor Michel Rigoulet, IBGC, U of Bordeaux-2, France). Growth medium (YLac: 0.12% (NH4)2SO4, 0.1% KH2PO4, 1% yeast extract, 2% DL-lactate, pH 5.0 with NaOH) was inoculated to an optical density of 0.03 by using cells from 24-h starter cultures grown in YLac. Cells were grown aerobically at 28°C with orbital shaking at 180 RPM and harvested in mid-exponential growth phase (OD550=3.5–4.0). For yeast fatty acid content manipulation, 1 mM of linolenic acid (C18:3) solubilised with 5% (v/v) Igepal CA-630 was added to growth medium immediately previous to inoculation. Mitochondria were isolated from spheroplasts as described previously (Guérin et al. 1979; Avéret et al. 1998) except that zymoliase 20T was used instead of cytohelicase. Mitochondrial protein concentration was measured by the Biuret assay.
Lipid extraction and fatty acid analysis
Lipids were extracted from mitochondrial homogenates by the method of Bligh and Dyer (1959). For fatty acid analysis, methyl esters were generated by the BF3-methanol assay of Morrison and Smith (1964). After extraction with n-hexane, samples of methyl esters were separated by gas chromatography (Perkin Elmer Clarus 500) on a 30 m×0.25 mm Omegawax capillary column using high-purity N2 as carrier gas. Fatty acids were identified and quantified by comparison of their retention time with those of authentic standards.
Induction of oxidative stress
Oxidative stress was established by incubating mitochondria (at proper concentrations for each determination as indicated below) at 4°C during 30 min in 50 mM KH2PO4 buffer (pH 7.6 with NaOH) with Fe2+ at the concentrations indicated in the legend to each figure. It has been showed that Fe2+ can initiate the lipoperoxidative process in phosphate buffer through the generation of a strong oxidant that has hydroxyl radical (OH•) characteristics (North et al. 1992). Iron was added from a stock solution of 12.5 mM FeSO4•7H2O which was acidified with four drops of concentrated H2SO4 to maintain iron in the Fe2+ form. For experiments of protection with an antilipoperoxidative agent, mitochondria were incubated with 5 µM butylated hydroxytoluene (BHT) during 15 min previous to treatment with Fe2+. Controls were treated in the same way except by iron addition.
Lipid peroxidation measurements
Changes in lipid peroxide levels were determined with the thiobarbituric acid (TBA) assay (Buege and Aust 1978). Mitochondria were treated as indicated above and mixed with 2 ml of an acid mixture containing 0.25 N HCl, 15% w/v trichloroacetic acid and 0.375% w/v thiobarbituric acid. The mixtures were boiled for 15 min, cooled on ice and centrifuged at 1,000 g for 5 min. The absorbance of the supernatant was measured at 535 nm (Perkin Elmer Lambda 18 UV/vis spectrophotometer) against a blank containing all the reagents minus mitochondrial protein. The results were calculated using the molar extinction coefficient for malondialdehyde and expressed in terms of nanomoles of TBA reactive species (TBARS) per milligram of protein.
Succinate-DCIP oxidoreductase activity
This enzymatic activity was measured at room temperature following the succinate-stimulated secondary reduction of DCIP (Uribe et al. 1985). For optimal determination of ETC reactions, mitochondria were permeabilized by mild detergent treatment with Triton X-100 (Hallberg et al. 1993) and treated with Fe2+ and BHT as indicated above. The reaction mixture contained 50 mM KH2PO4 phosphate buffer (pH 7.6), 0.3 mg/ml permeabilized mitochondria, 80 µM DCIP, 1 mg antimycin A and 0.75 mM KCN in a final 1 ml volume. 0.2 mM EDTA was also added to eliminate unspecific reduction of DCIP by Fe2+. After incubating for 5 min with inhibitors and EDTA, the reaction was initiated with 10 mM sodium succinate (pH 7.6). Absorbance changes were recorded in a Perkin Elmer Lambda 18 UV/vis spectrophotometer at 600 nm. The rate of DCIP reduction was calculated from the slope of the absorbance plot using the molar extinction coefficient of 21 mM−1 cm−1 for DCIP. The activity was calculated by subtracting the background rate reduction of DCIP in the absence of succinate to the reduction rate of DCIP stimulated with succinate.
Succinate cytochrome c oxidoreductase activity
The antimycin A-sensitive succinate-mediated reduction of cytochrome c was followed by measuring at room temperature the reduction of cytochrome c in permeabilized mitochondria treated with Fe2+and BHT as describe above. The reaction mixture contained 50 mM KH2PO4 (pH 7.6), 0.1 mg/ml permeabilized mitochondria, 1.5 mg cytochrome c, and 0.75 mM KCN in a final 1 ml volume. After incubating for 5 min with inhibitors, the reaction was initiated by adding 10 mM sodium succinate (pH 7.6). The reaction was stopped by the addition of 1 µg antimycin A. Alternatively, where indicated, reaction was stopped by 2 µg stigmatellin. Absorbance changes were recorded in a Perkin Elmer Lambda 18 UV/vis spectrophotometer at 550 nm. The rate of cytochrome c reduction was calculated from the slopes of the absorbance plots using a molar extinction coefficient of 19.1 mM−1 cm−1 for cytochrome c. The antimycin A-sensitive reduction of cytochrome c was calculated by subtracting the activity in the presence of antimycin A plus succinate to the activity stimulated only with succinate.
Cytochrome c oxidase activity
This activity was evaluated by measuring the cyanide-sensitive oxidation of reduced cytochrome c in permeabilized mitochondria treated with Fe2+ and BHT as described above. The reaction mixture contained 50 mM KH2PO4 (pH 7.6), 0.1 mg/ml permeabilized mitochondria and 1 µg antimycin A in a final 1 ml volume. After incubating for 5 min with antimycin A, the reaction was initiated by adding 250 mg dithionite-reduced cytochrome c. The reaction was stopped by the addition of 0.75 mM KCN. Absorbance changes were recorded in a Perkin Elmer Lambda 18 UV/vis spectrophotometer at 550 nm. The rate of cytochrome c reduction was calculated from the slopes of the absorbance plots using a molar extinction coefficient of 19.1 mM−1 cm−1 for cytochrome c. The cyanide-sensitive reduction of cytochrome c was calculated by subtracting the activity in the presence of cyanide plus cytochrome c to the activity stimulated only with cytochrome c.
Assessment of superoxide production
O2•− production was estimated by evaluating the superoxide dismutase (SOD)-sensitive succinate-stimulated reduction of cytochrome c (Boveris and Cadenas 1975; Muller et al. 2002; Cape et al. 2005). The determinations were carried out under the same conditions used for succinate cytochrome c oxidoreductase activity except that 100 U/ml MnSOD, which is resistant to KCN (Muller et al. 2002), was added 5 min. before activity assays. Under our experimental conditions, 100 U/ml MnSOD inhibit the antimycin A-resistant reduction of cytochrome c (attributable to O2•− formation at the QO site of complex III) at levels comparable with the inhibition of cytochrome c reduction achieved with stigmatellin. Results were depicted as the rate of remaining cytochrome c reduction stimulated by succinate after MnSOD treatment.
Cytochromes spectra
Cytochromes spectra were obtained by measuring at room temperature the reduced minus oxidized spectra with a SLM Aminco DW2000 spectrophotometer fitted in the split mode. Mitochondria (1.5 mg/ml) were treated with Fe2+ (and BHT where indicated) as described above and placed in reference and sample cuvettes for recording of the baseline. 10 min before scanning of reduced spectra, ETC inhibitors (0.75 mM KCN and, where indicated, 1 µg antimycin A) and 10 mM succinate were added to sample cuvette. Spectra were scanned between 500 and 580 nm. For measurement of cytochromes concentration, the content of the reference cuvette was reduced by adding a few grains of solid sodium dithionite. Cytochromes contents were calculated using the following wavelengths and molar extinction coefficients: cytochrome c+cI, ΔA=552 nm minus 540 nm and ε=19.1 mM−1 cm−1; cytochrome b, ΔA=562 minus 575 nm and ε=20 mM−1 cm−1.
Results
Analysis of mitochondrial fatty acid composition
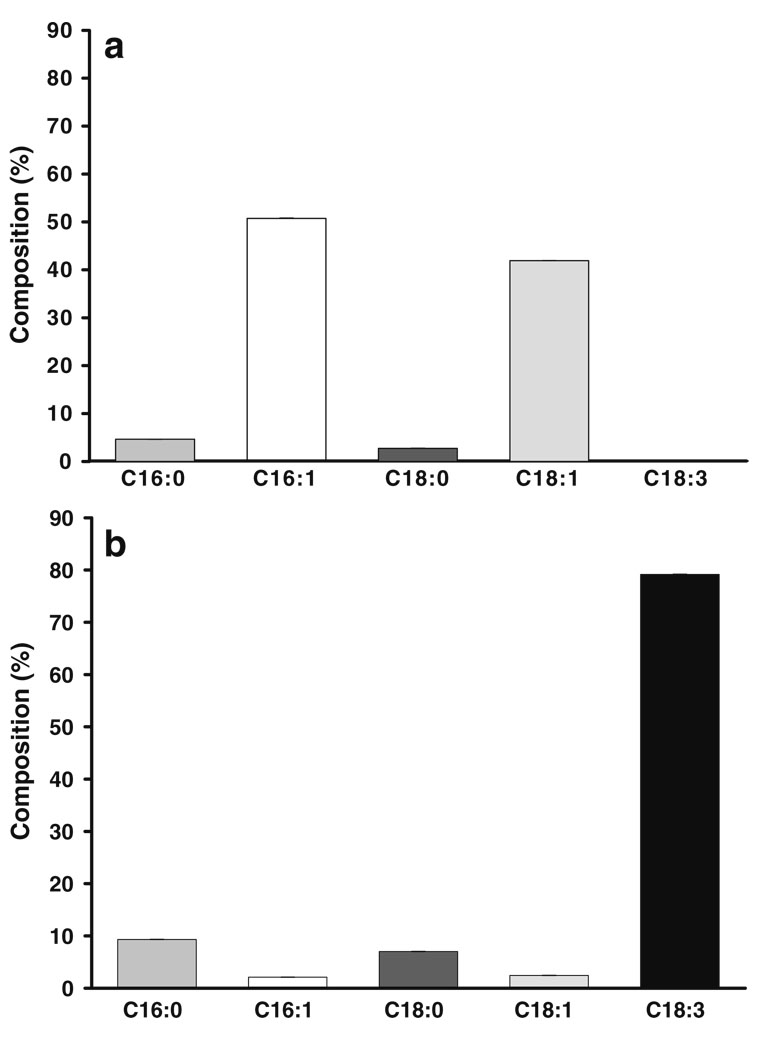
To know mitochondrial fatty acid composition of the Yeast Foam strain of S. cerevisiae and verify the incorporation of C18.3 into mitochondria, the composition and amount of fatty acid of isolated mitochondria was analyzed by gas chromatography. The percentage fatty acid composition is presented in Fig. 1. In mitochondria isolated from cells grown in the absence of C18:3 (−C18:3, panel a), the percentage composition consist mainly of the monounsaturated fatty acids palmitoleic acid (C16:1, 50.7%) and oleic acid (C18:1, 41.9%). Much lower amounts of the saturated fatty acids palmitic acid (C16:0, 4.6%) and stearic acid (C18:0 2.7%) were detected. When cells where grown in the presence of 10 mM C18.3 (+C18.3, panel b), the mitochondrial fraction becomes highly enriched in this fatty acid (79.2%). In comparison with −C18:3 mitochondria, the amount of saturated fatty acids in +C18:3 mitochondria increased to 9.3% and 7% for C16:0 and C18:0, respectively. In contrast, the quantity of monounsaturated fatty acids decreased to 2.1% and 2.4% for C16:0 and C18:0, respectively.
Fig. 1.
Effect of C18:3 supplementation on fatty acid profile of yeast mitochondria. Mitochondria were isolated from cells grown in the absence of C18:3 (a) or supplemented with 10 mM C18:3 (b) and lipids were extracted and analyzed from mitochondrial homogenates as described in “Materials and methods”. Data are presented as the mean ± SE of four determinations
Effect of C18:3 incorporation on mitochondrial sensitivity to lipoperoxidation
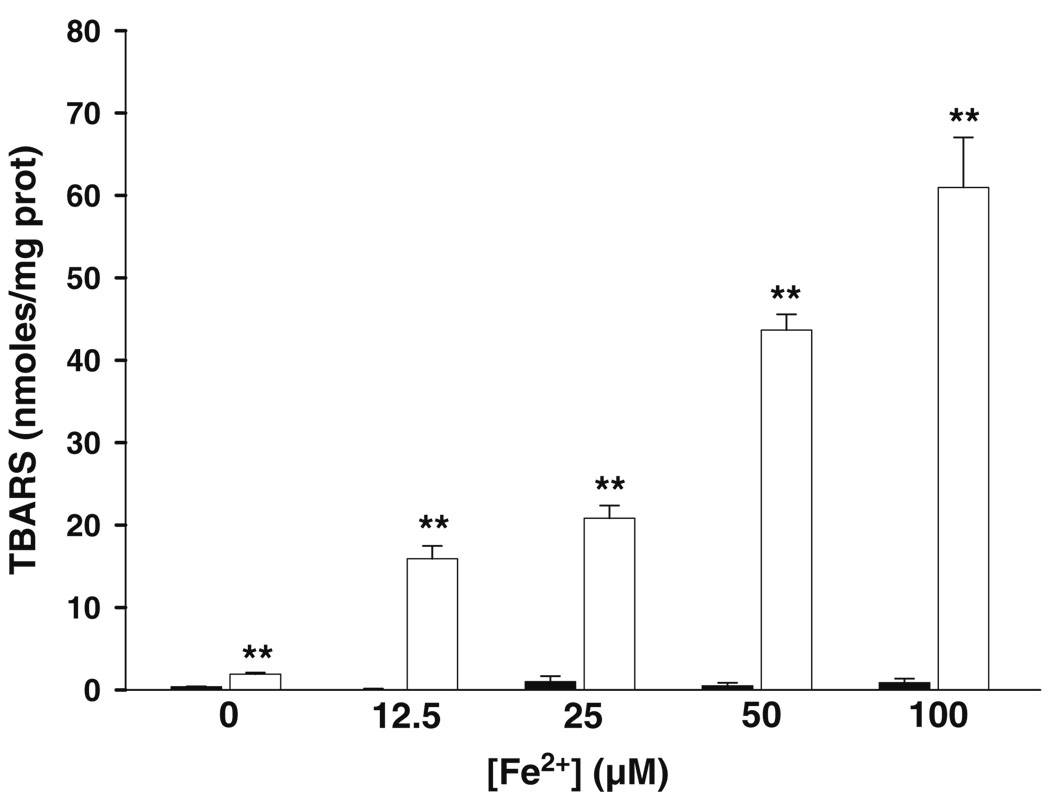
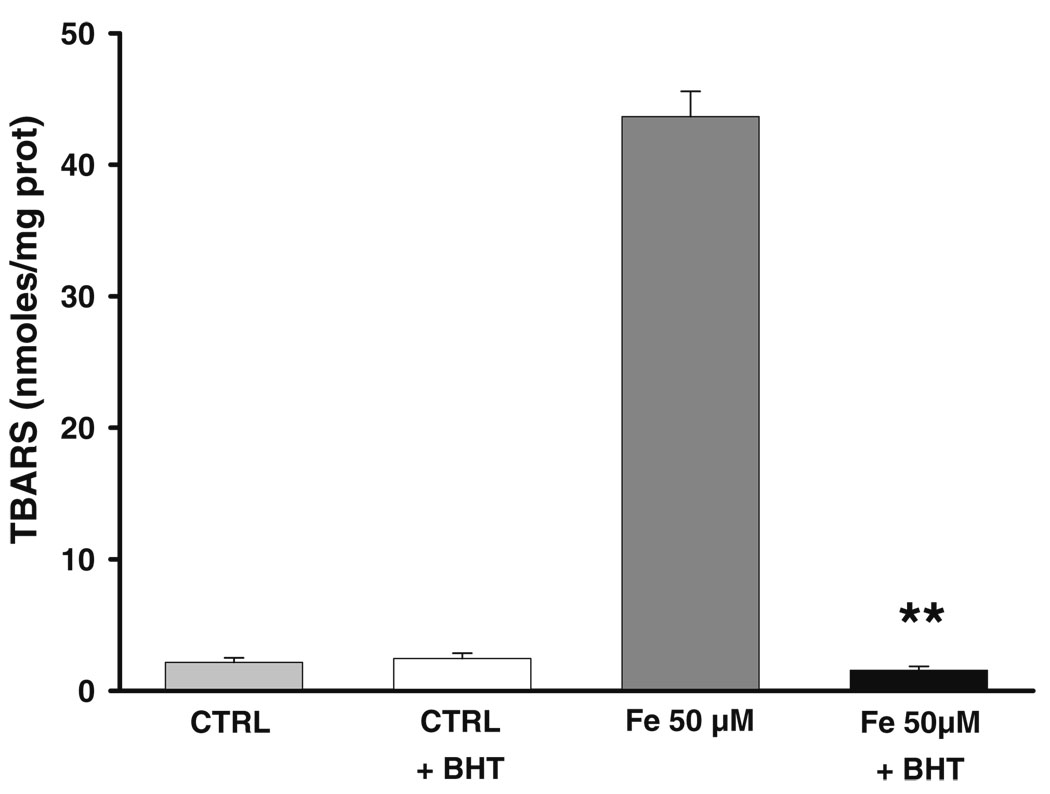
Mitochondria were challenged during 30 min with increasing amounts of Fe2+ to establish oxidative stress conditions and test whether enrichment with C18:3 can augment the sensitivity of mitochondria towards lipoperoxidation. A null effect on lipoperoxidation levels was observed in −C18:3 mitochondria even at the higher concentration of Fe2+ (Fig. 2a). The same treatment stimulated a notable rise in lipoperoxidation levels of +C18.3 mitochondria with all Fe2+ concentrations tested. These results indicate that incorporation of C18.3 sensitized mitochondria to the deleterious effects of oxidative stress. To further support this results and discard the possibility that the latter result could be the product of an unspecific reaction of thiobarbituric acid, +C18:3 mitochondria were pre-incubated with 5 µM of the anti-lipoperoxidative agent BHT during 15 min before Fe2+ treatment. Phenolic compounds like BHT inhibit lipoperoxidation by trapping intermediate peroxyl radicals that promote the propagative phase of this process (Porter 1986). Figure 3 shows that BHT fully inhibits the increment in lipoperoxidation obtained with 50 µM Fe2+ and does not exert any effect on the basal levels of this parameter.
Fig. 2.
Effect of Fe2+ on lipoperoxidation levels in −C18:3 (black bars) and +C18:3 (white bars) mitochondria. Mitochondria were challenged at 4°C during 30 min. with indicated concentrations of Fe2+. Measurements were made as described in “Materials and methods”. Data are presented as mean ± S.E. of at least three independent experiments. Significantly different when compared to −C18:3 mitochondria at the same Fe2+ concentration (**P<0.01, Student’s test)
Fig. 3.
Protective effect of BHT against lipoperoxidative damage induced in +C18:3 mitochondria by 50 µM Fe2+. To test protective effect of BHT, +C18:3 mitochondria were pre-incubated with 5 µM BHT 15 min. prior to treatment with Fe2+, which was carried out as described in Fig. 2. Controls were treated in the same way except by Fe2+ or BHT addition. Data are presented as mean ± S.E. of at least three independent experiments. Significantly different when compared to Fe2+ 50 µM (**P<0.01, Student’s test)
Influence of C18:3 incorporation on ETC sensitivity to Fe2+
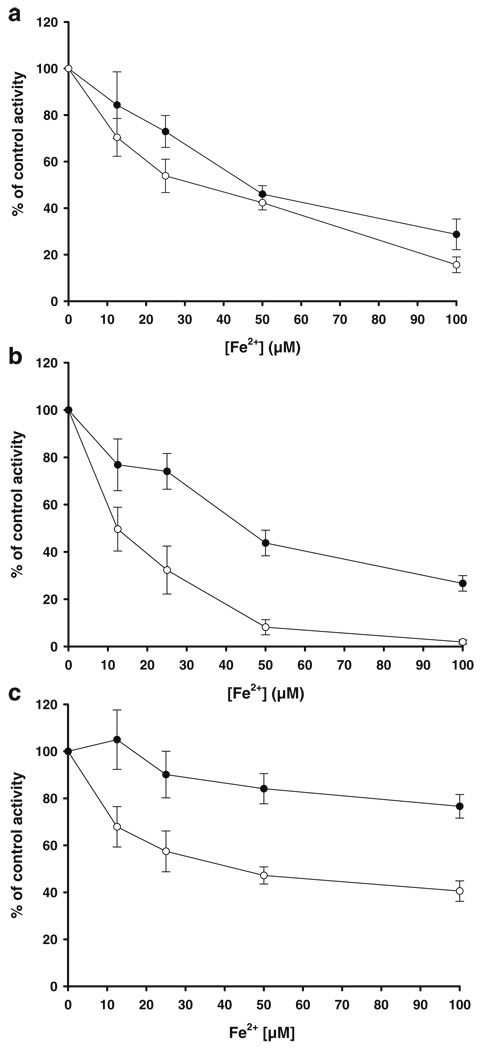
The sensitivities of ETC partial reactions to Fe2+ in −C18:3 and +C18:3 mitochondria were compared to investigate whether incorporation of C18:3 into mitochondria and the consequent augment in lipoperoxidation may enhance susceptibility of ETC function to oxidative stress. Mitochondria were treated during 30 min with Fe2+ and ETC activities were measured spectrophotometrically with appropriate substrates and inhibitors for each complex. Figure 4a shows a concentration-dependent progressive inhibition on succinate-DCIP oxidoreductase (complex II) activity. However no significative differences in sensitivity to Fe2+ were observed between −C18:3 and +C18:3 mitochondria, suggesting that lipoperoxidation may not participate in the mechanism of complex II inhibition.
Fig. 4.
Effect of Fe2+ on partial reactions of ETC in −C18:3 (black circles) and +C18:3 (white circles) mitochondria. Mitochondria were challenged at 4°C during 30 min. with the indicated concentrations of Fe2+. Succinate-DCIP oxidoreductase (a), antimycin A-sensitive succinate-cytochrome c oxidoreductase (b) or KCN-sensitive cytochrome c oxidase activities were measured as described in “Materials and methods”. Data are expressed as the percentage of the remaining activities with respect to control activities (0 µM Fe2+). The values of control activities (100%) were: 72.62±6.7 and 51.01±9.01 mmoles DCIP/min×mg protein for succinate-DCIP oxidoreductase activities in −C18:3 and +C18:3 mitochondria, respectively; 192.63±28.14 and 56.7±3.22 mmoles cytochrome c /min×mg protein for antimycine A-sensitive succinate-cytochrome c oxidoreductase activities in −C18:3 and +C18:3 mitochondria, respectively; 162.81±11.16 and 160.01± 26.7 mmoles cytochrome c /min×mg protein for KCN-sensitive cytochrome c oxidase activities in −C18:3 and +C18:3 mitochondria, respectively. Data are presented as mean ± S.E. of at least four independent experiments
Deeper differences in sensitivity to Fe2+ were observed when antimycin A-sensitive succinate-cytochrome c oxidoreductase activity (representative of the complex III activity using succinate and endogenous ubiquinone-6 as electron donor [Matsuno-Yagi and Hatefi 1996]), was assayed (Fig. 4b). In −C18:3 mitochondria, this activity was inhibited about 24% with low (12.5 µM) and intermediate (25 µM) concentrations of Fe2+. At high (50–100 µM) concentrations, a remaining activity of 43% and 26%, respectively, was observed. Significative differences in sensitivity with respect −C18:3 mitochondria where observed in +C18:3 mitochondria at concentrations above 12.5 µM Fe2+. Activity was inhibited by 67% and 92% with 25 µM and 50 µM Fe2+, respectively, while 100 µM Fe2+ almost fully inhibited this activity.
Cytochrome c oxidase (complex IV) activity (Fig. 4c) also becomes more sensitive to Fe2+ inhibition in +C18:3 mitochondria, although in a lower proportion than that observed in complex III activity, At high (50–100 µM) Fe2+ concentrations, oxidase activity was repressed in +C18:3 mitochondria by 53% and 60%, respectively, and 16% and 24% in −C18:3 mitochondria. It must be pointed out that overall resistance of cytochrome c oxidase to Fe2+ in −C18:3 mitochondria were higher in this complex than that observed for the other ETC complexes. Taken together, these results suggest that lipoperoxidation is involved in the augmented sensitivity of the complex III-IV segment of the ETC to oxidative stress.
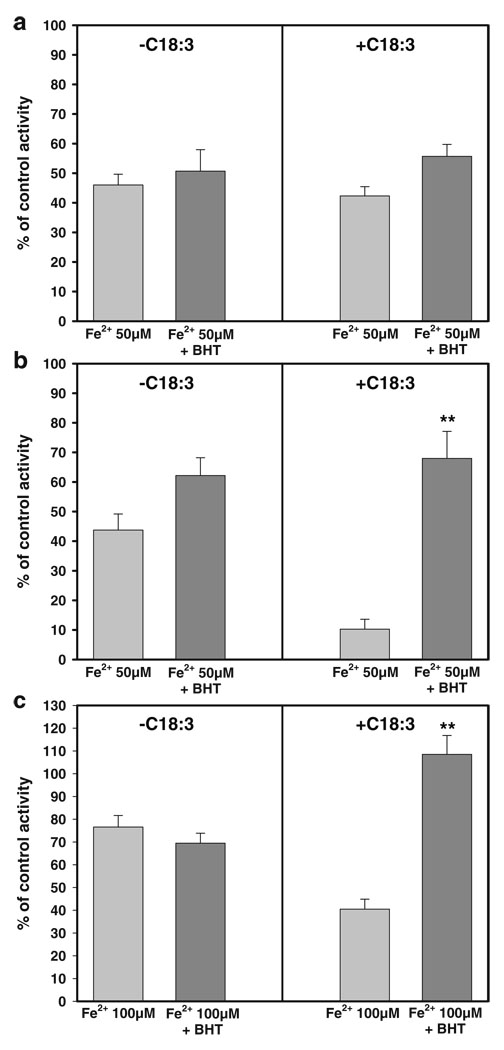
Protective effect of BHT on ETC inhibition by Fe2+
The protective effect of 5 µM BHT on the inhibition of ETC activities by 50 µM Fe2+ was assayed to further asses the participation of lipoperoxidation in the augmented sensitivity to Fe2+ observed in +C18:3 mitochondria. As observed in Fig. 5a, BHT did not exert a protective effect from the inhibition achieved in complex II with 50 µM Fe2+ neither in −C18:3 nor +C18:3 mitochondria, which confirm that complex II activity is not affected by lipoperoxidation. On the other hand, a recovery of up to 68% in the complex III activity was observed with BHT exclusively in +C18:3 mitochondria (Fig. 5b) indicating an important participation of lipoperoxidation in the inactivation of this complex. As expected, BHT had no effect on the inhibition observed in −C18:3 mitochondria.
Fig. 5.
Protective effect of BHT against the inhibition induced in ETC partial reactions by Fe2+. To test the protective effect of BHT, −C18:3 or +C18:3 mitochondria were pre-incubated with 5 µM BHT 15 min. prior to the treatment with the indicated concentrations of Fe2+, which was carried out as described in Fig. 2. Succinate-DCIP oxidoreductase (a), antimycin A-sensitive succinate-cytochrome c oxidoreductase (b) or KCN-sensitive cytochrome c oxidase (c) activities were measured as described in “Materials and methods”. Data are expressed as the percentage of the remaining activities with respect to control activities (0 µM Fe2+ in the absence of BHT or 0 µM Fe2+ plus 5 µM BHT when this agent was tested). The control values for succinate-DCIP oxidoreductase activities were: 72.62±6.7 and 76.73±2.23 mmoles DCIP/min×mg protein in −C18:3 mitochondria in the absence and the presence of BHT, respectively; 51.01±9.01 and 49.14±0.28 mmoles DCIP/min×mg protein in +C18:3 mitochondria in the absence and the presence of BHT, respectively. The control values for antimycine A-sensitive succinate-cytochrome c oxidoreductase activities were: 192.63±28.14 and 230.98±46.76 mmoles cytochrome c /min×mg protein in −C18:3 mitochondria in the absence and the presence of BHT, respectively; 56.7±3.22 and 56.78±6.74 mmoles cytochrome c / min×mg protein in +C18:3 mitochondria in the absence and the presence of BHT, respectively. The control values for KCN-sensitive cytochrome c oxidase activities were: 162.81±11.16 and 145.56±38.82 mmoles cytochrome c /min×mg protein in −C18:3 mitochondria in the absence and the presence of BHT, respectively; 160.01±26.7 and 157.59±5.93 mmoles cytochrome c /min×mg protein in +C18:3 mitochondria in the absence and the presence of BHT, respectively. Data are presented as mean ± S.E. of at least four independent experiments. Significantly different when compared to +C18:3 mitochondria with Fe2+ 50 µM (b) or +C18:3 mitochondria with Fe2+ 100 µM (c) (**P<0.01, Student’s test)
Considering the higher resistance of complex IV to inhibitory effects of Fe2+, we decided to test protective effects of BHT in the presence of 100 µM Fe2+ for a better discernment of the results. BHT fully protected complex IV activity exclusively in +C18:3 (Fig. 5c), confirming the notion that the increase in susceptibility to oxidative stress in this complex can be attributed to lipoperoxidation. A similar result was observed in the presence of 50 µM Fe2+ (data not shown). Taken together, these results confirm that an augment on the sensitivity of complex III–IV segment of ETC towards oxidative damage is attributable to lipoperoxidation.
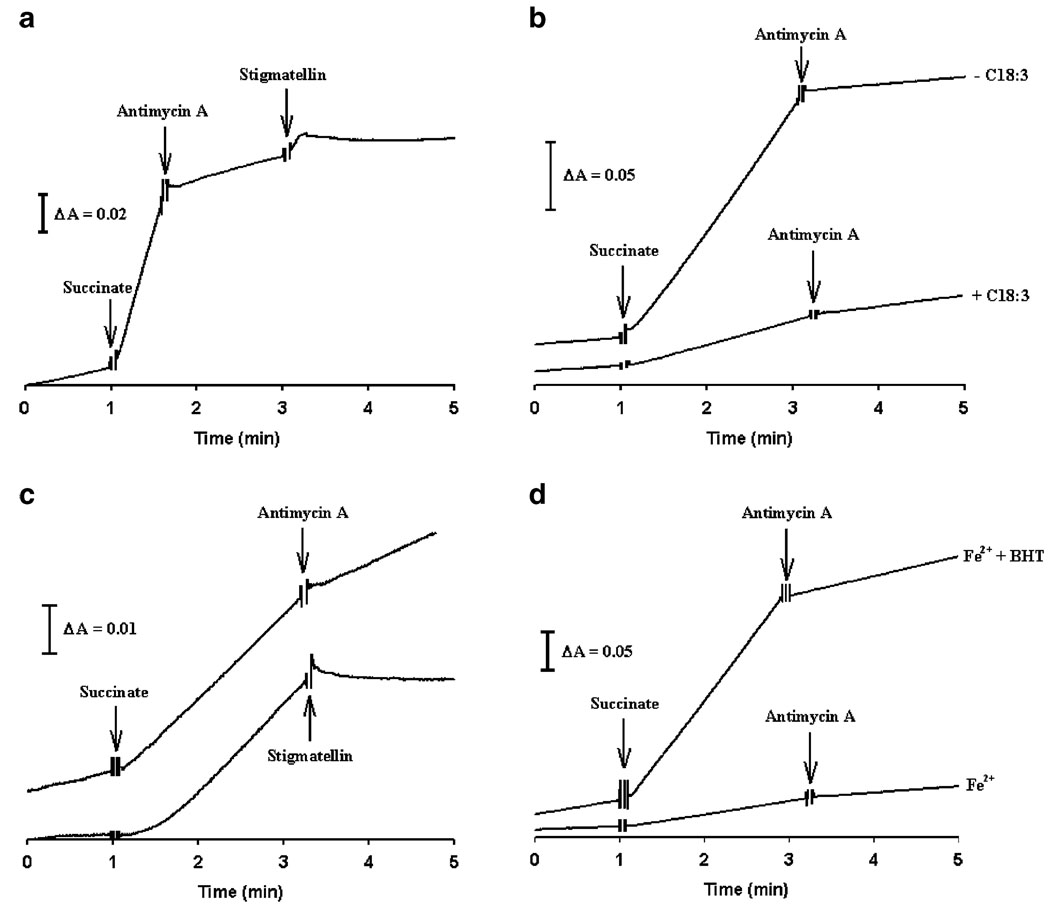
Influence of C18:3 incorporation and oxidative stress over the effects of complex III inhibitors and SOD on cytochrome c reduction
The effects of complex III inhibitors and MnSOD on cytochrome c reductions were evaluated as an approach to assess O2•− formation, identify its site of generation and investigate whether lipoperoxidative damage may enhance ROS production. Figure 6a illustrates the effect of complex III inhibitors on the traces of cytochrome c reduction by control mitochondria. Succinate addition stimulates a rapid reduction of cytochrome c. Antimycin A partially inhibits this reduction (97.6% in −C18:3 mitochondria and 94.5% in +C18:3 mitochondria) and is fully abolished by stigmatellin. Antimycin A-resistant cytochrome c reduction at complex III has been closely associated with O2•− production (Chance et al. 1979). Moreover, stigmatellin inhibit O2•− production by binding in the ubiquinol oxidation pocket at QO site (Muller et al. 2003; Kessl et al. 2003). Considering these facts, we assumed that the effects observed in Fig. 6a reflect O2•− generation at the QO site of complex III. Therefore, the effect of complex III inhibitors on cytochrome c reduction was analyzed in mitochondria treated with Fe2+ (50 µM). In −C18:3 mitochondria, the rate of cytochrome c reduction was inhibited with antimycin A by 93.5% (Fig. 6b), which was comparable with that observed in control treatment. Although the rate of cytochrome c reduction by succinate was lower in +C18:3 mitochondria, an increased resistance to antimycin A was detected in this case, since this inhibitor decreased the reduction rate by just 42.1% (Fig. 6b). Furthermore, stigmatellin abolished cytochrome c reduction in +C18:3 mitochondria (Fig. 6c). The loss of sensitivity to antimycin A observed in Fe2+-treated +C18:3 mitochondria was recovered by 82.8% through pre-treatment with BHT (Fig. 6d)
Fig. 6.
Time traces of cytochrome c reduction by succinate. Cytochrome c reduction was evaluated as described in “Materials and methods” for succinate cytochrome c oxidoreductase activity measurements. Complex III inhibitors were added at the times indicated by arrows. Where indicated, mitochondria were incubated with Fe2+ or BHT plus Fe2+ prior to determinations as described in Fig. 3. a Effect of consecutive addition of antimycin A and stigmatellin on cytochrome c reduction in control mitochondria. b Effect of antimycin A on cytochrome c reduction in −C18:3 and +C18:3 mitochondria treated with Fe2+ 50 µM. c Effect of antimycin A or stigmatellin on cytochrome c reduction in +C18:3 mitochondria treated with Fe2+ 50 µM. d Effect of antimycin A on cytochrome c reduction in +C18:3 mitochondria treated with Fe2+ 50 µM or BHT plus Fe2+ 50 µM. Data are from a representative experiment
Increased resistance to antimycin A induced by Fe2+ in +C18:3 mitochondria and the full inhibition of cytochrome c reduction by stigmatellin may implicate that under these conditions, a large proportion of the residual reduction of cytochrome c can be attributable to O2•− generation at QO site. To test this hypothesis, the effect of 100 U/ml MnSOD on cytochrome c reduction stimulated by succinate and in the absence of inhibitors was analyzed. Table 1 shows that this activity was unaffected by MnSOD in −C18:3 mitochondria. In contrast, MnSOD inhibited cytochrome c reduction by 95% in +C18:3 mitochondria. Together, these results indicate that lipoperoxidative damage in complex III increases its susceptibility to oxidative stress and stimulates O2•− generation at the QO site.
Table 1.
Effect of MnSOD on the rate of cytochrome c reduction stimulated by succinate in mitochondria exposed to Fe2+ 50 µM
| Treatments | −C18:3 | +C18:3 |
|---|---|---|
| Fe2+ 50 µM | 50.8±10.2 | 4.0±1.4 |
| Fe2+ 50 µM+MnSOD (100 U/ml) |
57.7±4.1 | 0.2±0.06* |
Rates are expressed in mmoles of reduced cytochrome c/min/mg protein. Data are presented as the mean ± SE of at least 3 independent experiments. Significantly different when compared to +C18:3 mitochondria treated with Fe2+ 50 µM
(P<0.05, Student’s test).
Influence of C18:3 incorporation and oxidative stress on cytochromes spectra
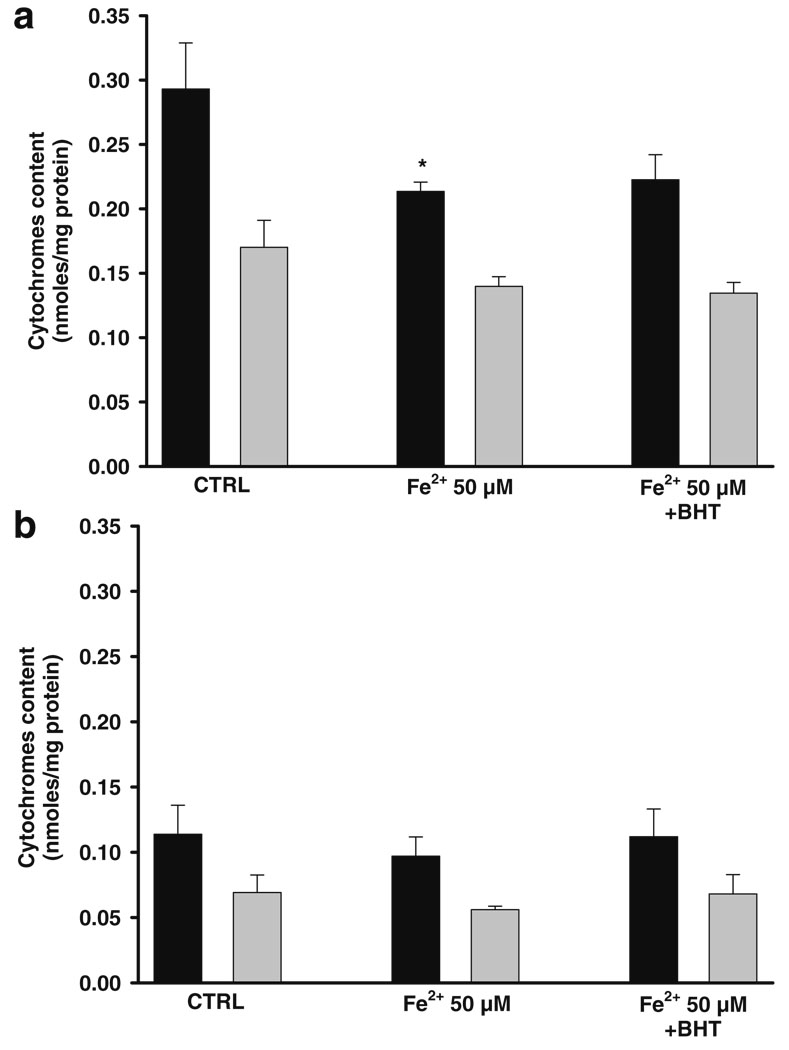
The effect of Fe2+ on absorption spectra of cytochromes was evaluated to reveal if the increased sensitivity of ETC to oxidative stress observed in +C18:3 mitochondria is related with impaired cytochrome(s) function and/or altered cytochrome(s) content. Figure 7 depicts the total amount of cytochromes reduced in the presence of dithionite. The amount of cytochrome b and c+c1 per milligram of protein was higher in −C18:3 (Fig. 7a) than in +C18:3 (Fig. 7b). Although their lower cytochromes content, no appreciable effect was exerted by Fe2+ on +C18:3 mitochondria. Conversely, the treatment with Fe2+ 50 µM decreased the content of cytochromes c+c1 in −C18:3 mitochondria. As expected, pre-treatment with BHT did not protect from cytochromes loss.
Fig. 7.
Effect of Fe2+ on cytochromes content of −C18:3 (a) and +C18:3 (b) mitochondria using dithionite as electron donor. Mitochondria were incubated with 50 µM Fe2+ or BHT plus 50 µM Fe2+ prior to determinations as described in Fig. 3. Cytochromes spectra were recorded as described in “Materials and methods”. Cytochromes content was calculated from wavelengths and molar extinction coefficients described in “Materials and methods”. Black bars: cytochromes c+c1. Gray bars: cytochrome b. Data are presented as mean ± S.E. of at least three independent experiments. Significantly different when compared to control (*P<0.05, Student’s test)
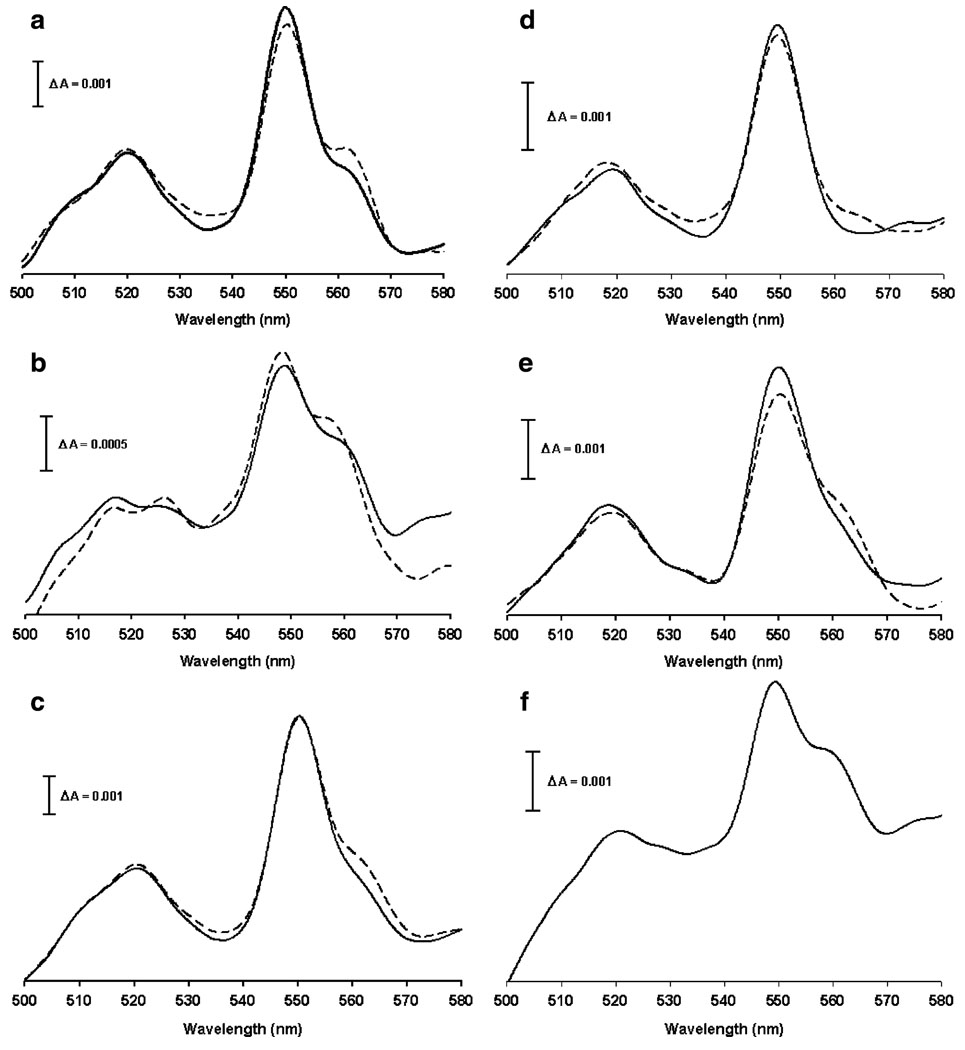
Figures 8a and b show cytochromes spectra of both −C18:3 and +C18:3 control mitochondria (solid line) using succinate as electron donor instead dithionite. The characteristic absorption peak of cytochromes c+c1 can be observed at 550 nm and the shoulder corresponding to cytochrome b appears at 562 nm. Absorption peak of cytochromes c+c1 and shoulder corresponding to cytochrome b were also observed when -C18:3 mitochondria were treated with 50 µM Fe2+ (Fig. 8c). However, in +C18.3 mitochondria, Fe2+ treatment caused the total disappearance of the shoulder corresponding to cytochrome b (Fig. 8d), suggesting an impairment on the reduction of this cytochrome. To further investigate this result, the effect of antimycin A in cytochrome b reduction was tested. Antimycin A blocks cytochrome b re-oxidation at Qi site which is reflected as an augment in the intensity of cytochrome b absorption in both −C18:3 and +C18:3 control mitochondria (Fig. 8a and b, dotted line). When oxidative stress was induced by Fe2+, the effect of antimycin A was also observed in −C18:3 mitochondria (Fig. 8c, dotted line), while in +C18:3 mitochondria, antimycin A induced just a very weak increment in absorption in the range of wavelengths between approx. 560 to 565 nm (Fig. 8d, dotted line). The impairment in cytochrome b reduction by succinate observed in +C18:3 mitochondria was not attributed to cytochromes loss as the cytochrome b shoulder was observed in Fe2+-treated +C18:3 mitochondria in the presence of dithionite (Fig. 8f) and cytochrome b content in +C18:3 mitochondria was not altered by Fe2+ treatment (Fig. 7b). To test whether the failure in cytochrome b reduction by succinate can be attributed to lipoperoxidative damage, the effect of BHT was tested on cytochromes spectra of Fe2+-treated +C18:3 mitochondria using succinate as electron donor. Figure 8e shows that the shoulder corresponding to cytochrome b is present when lipoperoxidation was prevented with BHT (solid line). Moreover, antimycin A produced an increment in cytochrome b absorption (dotted line), indicating that sensitivity to this inhibitor was recovered. Taken together, these results indicate lipoperoxidation does not affect the cytochromes content but impairs electron transfer at the level of cytochrome b, while under non-lipoperoxidative conditions, oxidative stress affects mainly the content of cytochromes c+c1.
Fig. 8.
Effect of Fe2+ on difference absorption spectra of cytochromes from −C18:3 and +C18:3 mitochondria. Mitochondria were incubated with 50 µM Fe2+ or BHT plus 50 µM Fe2+ prior to determinations as described in Fig. 3. Cytochromes spectra were recorded as described in “Materials and methods” using succinate as electron donor except by (f), where dithionite was used instead succinate. Solid lines: spectra in the absence of complex III inhibitors. Dotted lines: spectra in the presence of antimycin A. a Spectra of −C18:3 control mitochondria. b Spectra of +C18:3 control mitochondria. c Spectra of -C18:3 mitochondria treated with 50 µM Fe2+d Spectra of +C18:3 mitochondria treated with 50 µM Fe2+e Spectra of +C18:3 mitochondria treated with BHT plus 50 µM Fe2+f Spectra of +C18:3 mitochondria treated with 50 µM Fe2+ using dithionite as electron donor. Data are from a representative experiment
Discussion
The present study shows that an increase in mitochondrial sensitivity to lipoperoxidation brings important changes in the response of ETC to oxidative stress. This was related with incorporation into mitochondria of a fatty acid with a higher number of double bounds than that observed in the native fatty acids from yeast membranes. As demonstrated by this and other reports (Watson et al. 1975; Tuller et al. 1999; Priault et al. 2002), the fatty acid composition of S. cerevisiae mitochondria consist of saturated and monounsaturated fatty acids, the latter being the main membrane components when yeast grows in a non fermentable carbon source. In yeast, monounsaturated fatty acids are formed from saturated fatty acyl CoA precursors by the Ole1p Δ9-fatty acid desaturase (Martin et al. 2007). When supplemented in growth medium, unsaturated fatty acids are imported into the cell and subsequently activated to the corresponding CoA thioesthers (Johnson et al. 1994). The latter strongly repress desaturase activity of Ole1p due to the decrease of OLE1 mRNA levels (Bossie and Martin 1989). Thus, the final result of fatty acid supplementation is the induction of radical changes in the acyl composition of membranes lipids by replacement of the native monounsaturated fatty acids with the supplemented fatty acids (Martin et al. 2007). Our results show that mitochondria were successfully enriched with C18:3 through addition of this fatty acid to the culture medium. Also, C18:3 supplementation produced a drastic diminution in the amount of monounsaturated fatty acids (Fig. 1), which may be interpreted as the result of the repression exerted by C18:3 on Ole1p desaturase activity.
Lipoperoxidation is initiated by the abstraction of an H atom from a methylene group of a polyunsaturated fatty acid to produce a carbon-centered radical (North et al. 1992). This radical tends to be stabilized by a molecular rearrangement to give a stabilized conjugated dienyl radical that undergo rapid autoxidation. Methylene-interrupted diene fatty acids (i.e. C18:3) undergo autoxidation readily at room temperature via the stabilized dienyl radicals, while monounsaturated fatty acids only undergo autoxidation at elevated temperatures due to the impossibility to form a stabilized radical (Porter 1986). These data explain the insensitivity to peroxidation observed in saturated and monounsaturated fatty acids (Holman 1954) and are in concordance with the null sensitivity to lipoperoxidation observed in −C18:3 mitochondria, whose membranes are constituted of that type of fatty acids. Incorporation of C18:3 increased the sensitivity of mitochondria to lipoperoxidation, which is explained in view that sensitivity of fatty acids to peroxidation depends on the number of double bonds contained in its hydrophobic tail; the higher number of double bonds the higher susceptibility to peroxidation.
No differences in the sensitivity of complex II to Fe2+ were observed between −C18:3 and +C18:3 mitochondria. As well, inhibition of complex II activity was not prevented by BHT, suggesting that the contribution of lipoperoxidation is not determining the mechanism of inhibition of this complex. In concordance with this suggestion, in a study carried out in rat synaptosomes, it was observed that the inhibition in complex II activity with ascorbate/iron was not prevented by pre-incubation with the lipophilic anitoxidant vitamin E (Cardoso et al. 1999). A wide array of mitochondrial proteins depends on the phospholipid cardiolipin for its adequate functioning; however, there are no reports describing a cardiolipin requirement for adequate function of complex II (For a review see Chicco and Sparagna 2007). From this perspective, it can be suggested that peroxidation of cardiolipin and other phospholipids may not have a harmful effect on complex II function. Hence, other targets would be proposed to explain complex II inactivation by oxidative stress. One of these candidates could be oxidation of thiol groups that are essential for complex II activity (Lê-Quôc et al. 1981). In agreement with this idea, in a study carried out in rat synaptosomes, the inhibition of the complex II activity with ascorbate/iron was prevented by treatment with reduced glutathione (Cardoso et al. 1999), while in yeast mitochondria, the thiol reductant β-mercaptoethanol reversed the inhibition in complex II activity induced by H2O2 (Cortés-Rojo et al. 2007). Other possible candidates are the three iron-sulfur clusters that direct electron flow in complex II (Cheng et al. 2006), given that these kind of prosthetic groups can be damaged through overproduction of ROS caused by iron overload (Rustin et al. 1999).
Augmented susceptibility to oxidative stress was observed in complex III and complex IV activities from +C18:3 mitochondria. This effect was attributed to lipoperoxidative damage since BHT protected to a different degree the enzymatic activities of these complexes. In this regard, BHT fully protected complex IV activity in +C18:3 and no protection was observed in −C18:3 mitochondria, which discard the possibility of artifactual protection by BHT. There are reports pointing to cardiolipin as an essential phospholipid for complex IV functioning (Fry and Green 1980; Robinson 1993). As well, it has been demonstrated that peroxidative damage to membrane lipids affects complex IV activity in a reversible way through cardiolipin restitution (Paradies et al. 2000). From these data, it can be inferred that complex IV inactivation can be attributed totally to lipoperoxidation.
Complex III activity from +C18:3 mitochondria was the most sensitive segment of ETC to the damaging effects of oxidative stress since a concentration of 100 µM Fe2+ almost fully abolished this activity. Likewise, in a study carried out in rat liver mitochondria, it was observed that complex III behaved as the most sensitive enzyme against the attack of free radicals generated by the iron/ascorbate system (Trümper et al. 1988). In a subsequent study (Reinheckel et al. 1995), it was observed that high susceptibility of the complex III activity in comparison to the other complexes was not attributable to structural alterations in complex subunits given that changes in electrophoretic mobilities or specific losses of protein subunits were not found. Hence, it was suggested that the loss of activity has to be explained by specific impairment of the redox-active centers in the respiratory chain proteins, most likely by changes in phospholipid-protein interactions. In this work, we searched for alterations in cytochromes status caused by Fe2+ in an attempt to elucidate how a higher susceptibility to lipoperoxidation sensitize complex III activity against the damaging effects of oxidative stress. First, it was found that basal complex III activity was lower in +C18:3 mitochondria than in −C18:3 mitochondria. This may be attributed to an inferior capability of +C18:3 mitochondria for electron transport due to a lower concentration of cytochromes c+c1 and b. We do not have experimental evidence to explain the diminished cytochromes content when cells are supplemented with C18:3. In this regard, it can be speculated that lower content of cytochromes may be related with repression of desaturase activity by supplemented fatty acids. Removal of electrons and protons during fatty acid desaturation is a process energetically demanding (Fox et al. 2004) and requires oxygen (Martin et al. 2007). Therefore, energy expenditure and oxygen demand could considerably decrease when unsaturation is repressed by supplementation with C18:3 and the cell could respond to this change by diminishing energy production and oxygen consumption through down-regulation in cytochromes synthesis at a threshold level that allow aerobic growth in a nonfermentable carbon source. In any case, a lower amount of cytochromes was not related with a higher inhibition of complex III activity in +C18:3 mitochondria as the content of cytochromes was not altered by Fe2+ treatment. Indeed, a decrease in cytochromes c+c1 was observed only in −C18:3 mitochondria. Studies carried out in liposomes containing lipids with a variable unsaturation degree have demonstrated that the presence of unsaturated fatty acids protects structure and function of cytochrome c from oxidative damage caused by exposure to oxygen singlet (Rodrigues et al. 2007) or t-butylhydroperoxide (Nantes et al. 2000). In a similar way, it is reasonable to assume that tri-unsaturated lipids from +C18:3 mitochondria react preferentially with ROS produced by Fe2+ exposure, which may help to preserve structural characteristics responsible for light absorption of cytochromes. Conversely, it is possible that in −C18:3 mitochondria, cytochromes c+c1 becomes the main target of the ROS generated by Fe2+ given the low unsaturation of fatty acids from −C18:3 mitochondria and its null susceptibility to lipoperoxidation. As expected, BHT did not protect from inhibition complex III activity and cytochromes loss in −C18:3 mitochondria. This suggests that under non-lipoperoxidative conditions, the damage in cytochromes c+c1 is the main factor that limits electron transfer at complex III.
Although the cytochromes content was not affected by oxidative stress in +C18:3 mitochondria, a failure in cytochrome b reduction by succinate was observed. During the Q-cycle, reduction of cytochrome b is achieved by bifurcated sequential oxidation of quinol at the QO site of complex III. The first electron from quinol is transferred to the ISP, leaving a reactive radical semiquinone. The semiquinone then transfers an electron to hemes b contained in the cytochrome b subunit (Muller et al. 2003). Alternatively, a concerted mechanism for quinol oxidation has been proposed (Zhu et al. 2007). On the other hand, delipidation of complex III impairs antimycin A binding in a reversible way by cardiolipin restitution. Taking into account these data and the lipophilic nature of cytochrome b, it seems plausible to hypothesize that, without altering heme b concentration, lipoperoxidation may alter the cytochrome b conformation or redox potential of b hemes to an unfavourable value in such a way that electron transfer to hemes becomes impaired. As well, this argument is supported by the lack of antimycin A effects observed in the cytochromes spectra and the augmented resistance to this inhibitor observed in cytochrome c reduction. The role of lipoperoxidation in these effects is confirmed because when +C18:3 mitochondria were pre-incubated with BHT, Fe2+ treatment did not impair the cytochrome b reduction by succinate and the effects of antimycin A were recovered in both the cytochromes spectra and in cytochrome c reduction. Furthermore, disappearance of the cytochrome b shoulder and increased resistance to antimycin A were not observed in −C18:3 mitochondria.
Although cytochrome b reduction was impaired by lipoperoxidation, our results suggest that quinol binding and oxidation by the ISP protein at the QO site was not totally affected by lipoperoxidation since residual cytochrome c reduction was abolished by stigmatellin, a competitive inhibitor of quinol oxidation (Kessl et al. 2003). Also, contrary to the quinone destroyed by lipoperoxidation reported in another study (Forsmark-Andreé et al. 1997), residual cytochrome c reduction by succinate implicate that a fraction of endogenous ubiquinone was still available for electron transfer from complex II to complex III. Otherwise, cytochrome c reduction would be completely inhibited.
Specific interactions between membrane phospholipids and domains of ISP have been proposed to facilitate the rotation of the ISP catalytic, head domain (Lange et al. 2001). These interactions could be altered in +C18:3 mitochondria when lipoperoxidation was stimulated by Fe2+ treatment. In addition, it has been proposed that disruption in the electron transfer between b hemes impair the movement of the head domain of reduced ISP (Yang et al. 2008). Thus, these two factors may conjugate to impair ISP movement in +C18:3 mitochondria. As stated above, the stigmatellin-sensitive cytochrome c reduction suggest that quinol oxidation by ISP is still operative in +C18:3 mitochondria subjected to oxidative stress. Thus, quinol oxidation and the presumed impairment in ISP movement caused by lipoperoxidation could lead to increased O2•− production and non-catalytic reduction of cytochrome c. Disruption in cytochrome b reduction in mutants lacking b hemes leads to increased O2•− formation because the second electron from quinol is retained either in bL heme or semiquinone and reacts with oxygen to produce O2•− (Shang 2008). Similarly, the disruption in cytochrome b reduction observed in +C18:3 mitochondria may contribute to an electron leak at the QO site and O2•−production. In accordance with this argument, residual cytochrome c reduction was almost fully inhibited by MnSOD (or stigmatellin) only in +C18:3 mitochondria, indicating that under lipoperoxidative conditions, a large part of the cytochrome c reduction proceeds through O2•−generation at QO site.
Taken together with the data observed by others and the results obtained in this study, we suggest a mechanism by which lipoperoxidation could lead to decreased complex III activity and augmented O2•− production. Lipoperoxidation affects cytochrome b conformation, which would be reflected as a disruption in the reduction of b hemes and an increased resistance to antimycin A. At the same time, lipoperoxidation and/or the defect in electron transfer at cytochrome b may impair ISP movement between heme bL and cytochrome c1, which would lead to a decrease in catalytic cytochrome c reduction. Damage in ISP movement would not impair quinol oxidation (as reflected by inhibition of cytochrome c reduction by stigmatellin). The failure in electron transfer to cytochrome b and the impaired ISP movement would lead to the leak of electrons arising from quinol oxidation by ISP. These electrons may react with oxygen to produce O2•−, which in turn could reduce cytochrome c in a non-catalytic way (as reflected by inhibition of cytochrome c reduction MnSOD).
The total concentration of Fe2+ in yeast mitochondria has been estimated to be in the range between approximately 500–800 µM and it has been suspected that about 20% is in a free chelatable form (Hudder et al. 2007). The higher concentration of Fe2+ used in this study (100 µM) is out of the range of the free iron concentration mentioned above. Considering that even this concentration was not able to fully inhibit any of the activities measured in −C18:3 mitochondria, this indicates that yeast mitochondria are highly resistant to the damaging effects of oxidative stress, as was suggested in another report (Machida et al. 1998). A great part of this resistance must be attributed to the low unsaturation degree of yeast membranes. Lipid unsaturation of mitochondrial membranes and resistance to lipoperoxidation has generated a great interest in the scientific community that studies ageing mechanisms in superior organisms (For a review see Hulbert 2005). Peroxidisability index of mitochondrial membranes has been strongly correlated with the life span of superior organisms: the higher life span, the lower sensitivity of mitochondrial membranes to lipoperoxidation (Pamplona et al. 1998). The higher susceptibility to lipoperoxidation observed in organisms with shorter life spans would lead to an accelerated decay in mitochondrial function (maybe involving some aspects of mitochondrial dysfunction described in the present investigation), compromising the adequate function of the cell and predisposing it to early ageing. Taking into account the results obtained in the present study and the fact that yeast has been used as a model in ageing research (Breitenbach et al. 2003), we consider that yeast with manipulated fatty acid content may be useful to understand the underlying molecular mechanisms that connect mitochondrial lipoperoxidation with ageing.
Acknowledgements
This work was supported by CIC-UMSNH (2.16 to S.M.A and A.S.M.), COECYT 2008 (to S.M.A and A.S.M.), Fondos Mixtos CONACYT-Estado de Michoacán (to S.M.A and A.S. M.), NIAID (AI062885 to I.B), NIH/NIA (AG 021830 to I.B) and NIEHS Center Grant, EOS 006677. The authors appreciated the technical assistance from Dr. Emma Bertha Gutiérrez-Cirlos, UBIMED Iztacala. UNAM.
Contributor Information
Christian Cortés-Rojo, Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Edificio B-3. C.U., Morelia, Mich 58030, México.
Elizabeth Calderón-Cortés, Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Edificio B-3. C.U., Morelia, Mich 58030, México.
Mónica Clemente-Guerrero, Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Edificio B-3. C.U., Morelia, Mich 58030, México.
Mirella Estrada-Villagómez, Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Edificio B-3. C.U., Morelia, Mich 58030, México.
Salvador Manzo-Avalos, Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Edificio B-3. C.U., Morelia, Mich 58030, México.
Ricardo Mejía-Zepeda, Unidad de Biomedicina, Facultad de Estudios Superiores Iztacala, UNAM. D.F., Mexico, Mexico.
Istvan Boldogh, School of Medicine University of Texas, Medical Branch at Galveston, Galveston, TX, USA.
Alfredo Saavedra-Molina, Email: saavedra@umich.mx, Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Edificio B-3. C.U., Morelia, Mich 58030, México.
References
- Avéret N, Fitton V, Bunoust O, Rigoulet M, Guérin B. Mol Cell Biochem. 1998;184:67–79. [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. Can J Biochem Physiol. 1959;37:911–3917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bossie MA, Martin CE. J Bact. 1989;171:6409–6413. doi: 10.1128/jb.171.12.6409-6413.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Cadenas E. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- Breitenbach M, Madeo F, Laun P, Heeren G, Jarolim S, Fröhlich K-U, Wissing S, Pichova A. Topics in current genetics. Berlin and Heidelberg: Springer-Verlag; 2003. Yeast as a model for ageing and apoptosis research; pp. 61–97. [Google Scholar]
- Buege JA, Aust D. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Pereira C, Oliveira CR. Free Radic Biol Med. 1999;26:3–13. doi: 10.1016/s0891-5849(98)00205-6. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Cheng VWT, Zhao EMZ, Rothery RA, Weiner JH. J Biol Chem. 2006;281:27662–27668. doi: 10.1074/jbc.M604900200. [DOI] [PubMed] [Google Scholar]
- Chicco AJ, Sparagna GC. Am J Physiol Cell Physiol. 2007;292:33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- Cortés-Rojo C, Calderón-Cortes E, Clemente-Guerrero M, Manzo-Avalos S, Uribe S, Boldogh I, Saavedra-Molina A. Free Radic Res. 2007;41:1212–1223. doi: 10.1080/10715760701635082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsmark-Andreé P, Lee CP, Dallner G, Ernster L. Free Radic Biol Med. 1997;22:391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- Fry M, Green DE. Biochem Biophys Res Commun. 1980;93:1238–1246. doi: 10.1016/0006-291x(80)90622-1. [DOI] [PubMed] [Google Scholar]
- Green K, Brand MD, Murphy MP. Diabetes. 2004;53:110–118. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- Guérin B, Labbe P, Somlo M. Methods Enzymol. 1979;55:149–159. doi: 10.1016/0076-6879(79)55021-6. [DOI] [PubMed] [Google Scholar]
- Hallberg EM, Shu Y, Hallberg RL. Mol Cell Biol. 1993;13:3050–3057. doi: 10.1128/mcb.13.5.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RT. Autooxidation of fats and related substances. New York: Academic; 1954. pp. 51–98. [Google Scholar]
- Hudder BN, Morales JG, Stubna A, Münck E, Hendrich MP, Lindahl PA. J Biol Inorg Chem. 2007;12:1029–1053. doi: 10.1007/s00775-007-0275-1. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. J Theor Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hunte C, Palsdottir H, Trumpower BL. FEBS Lett. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Knoll LJ, Levin DE, Gordon JI. J Cell Biol. 1994;127:751–762. doi: 10.1083/jcb.127.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Nett JH, Trumpower BL, Hunte C. EMBO J. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YI, Hwang JM, Im JH, Lee YI, Kim DG, Yu DY, Moon HB, Park SK. J Biol Chem. 2004;279:15460–15471. doi: 10.1074/jbc.M309280200. [DOI] [PubMed] [Google Scholar]
- Lê-Quôc K, Lê-Quôc D, Gaudemer Y. Biochemistry. 1981;20:1705–1710. doi: 10.1021/bi00510a001. [DOI] [PubMed] [Google Scholar]
- Lucas DT, Szweda LI. Proc Natl Acad Sci USA. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K, Tanaka T, Fujita K-I, Taniguchi M. J Bact. 1998;180:4460–4465. doi: 10.1128/jb.180.17.4460-4465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Oh C-S, Jiang Y. Biochim Biophys Acta. 2007;1771:271–285. doi: 10.1016/j.bbalip.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A, Hatefi Y. J Biol Chem. 1996;271:6164–6171. doi: 10.1074/jbc.271.11.6164. [DOI] [PubMed] [Google Scholar]
- Morrison WR, Smith LM. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- Muller FL, Crofts AR, Kramer DM. Biochemistry. 2002;41:7866–7874. doi: 10.1021/bi025581e. [DOI] [PubMed] [Google Scholar]
- Muller FL, Roberts AG, Bowman MK, Kramer DM. Biochemistry. 2003;42:6493–6499. doi: 10.1021/bi0342160. [DOI] [PubMed] [Google Scholar]
- Nantes IL, Faljoni-Alario A, Nascimento OR, Bandy B, Gatti R, Bechara EJH. Free Radic Biol Med. 2000;28:786–796. doi: 10.1016/s0891-5849(00)00170-2. [DOI] [PubMed] [Google Scholar]
- North JA, Spector AA, Buettner GR. J Biol Chem. 1992;267:5743–5746. [PubMed] [Google Scholar]
- Pamplona R, Portero-Otín M, Riba D, Ruiz C, Prat J, Bellmunt JP, Barja G. J Lipid Res. 1998;39:1989–1994. [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, Ruggiero FM. Free Radic Biol Med. 1999;27:42–50. doi: 10.1016/s0891-5849(99)00032-5. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. FEBS Lett. 2000;466:323–326. doi: 10.1016/s0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federico A, Ruggiero FM. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- Porter NA. Acc Chem Res. 1986;19:262–268. [Google Scholar]
- Priault M, Bessoule J-J, Grelaud-Coq A, Camougrand N, Manon S. Eur J Biochem. 2002;269:5440–5450. doi: 10.1046/j.1432-1033.2002.03234.x. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA. Free radical damage and its control. Amsterdam: Elsevier Science; 1994. pp. 131–153. [Google Scholar]
- Robinson NC. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- Rodrigues T, de Franca LP, Kawai C, de Faria PA, Mugnol KCU, Braga FM, Tersariol ILS, Smaili SS, Nantes IL. J Biol Chem. 2007;282:25577–25587. doi: 10.1074/jbc.M700009200. [DOI] [PubMed] [Google Scholar]
- Rustin P, von Kleist-Retzow JC, Chantrel-Groussard K, Sidi D, Munnich A, Rötig A. Lancet. 1999;354:477–479. doi: 10.1016/S0140-6736(99)01341-0. [DOI] [PubMed] [Google Scholar]
- Trümper L, Hoffmann B, Wiswedel I, Augustin W. Biochim Biophys Acta. 1988;47:933–939. [PubMed] [Google Scholar]
- Tsai A, Palmer G. Biochim Biophys Acta. 1986;852:100–105. doi: 10.1016/0005-2728(86)90061-7. [DOI] [PubMed] [Google Scholar]
- Tuller G, Nemec T, Hrastnik C, Daum G. Yeast. 1999;15:1555–1564. doi: 10.1002/(SICI)1097-0061(199910)15:14<1555::AID-YEA479>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Uribe S, Ramirez J, Peña A. J Bact. 1985;161:1195–1200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K, Bertoli E, Griffiths DE. Biochem J. 1975;146:401–407. doi: 10.1042/bj1460401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y-H, Lu CY, Lee H-C, Pang C-Y, Ma Y-S. Ann New York Acad Sci. 1998;854:155–170. doi: 10.1111/j.1749-6632.1998.tb09899.x. [DOI] [PubMed] [Google Scholar]
- Wei Y-H, Lu CY, Wei CY, Lee H-C. Chin J Physiol. 2001;44:1–11. [PubMed] [Google Scholar]
- Yang S, Ma H-W, Yu L, Yu CA. J Biol Chem. 2008;283:28767–28776. doi: 10.1074/jbc.M803013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Egawa T, Yeh S-R, Yu L, Yu CA. Proc Natl Acad Sci USA. 2007;104:4864–4869. doi: 10.1073/pnas.0607812104. [DOI] [PMC free article] [PubMed] [Google Scholar]