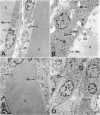
Abstract
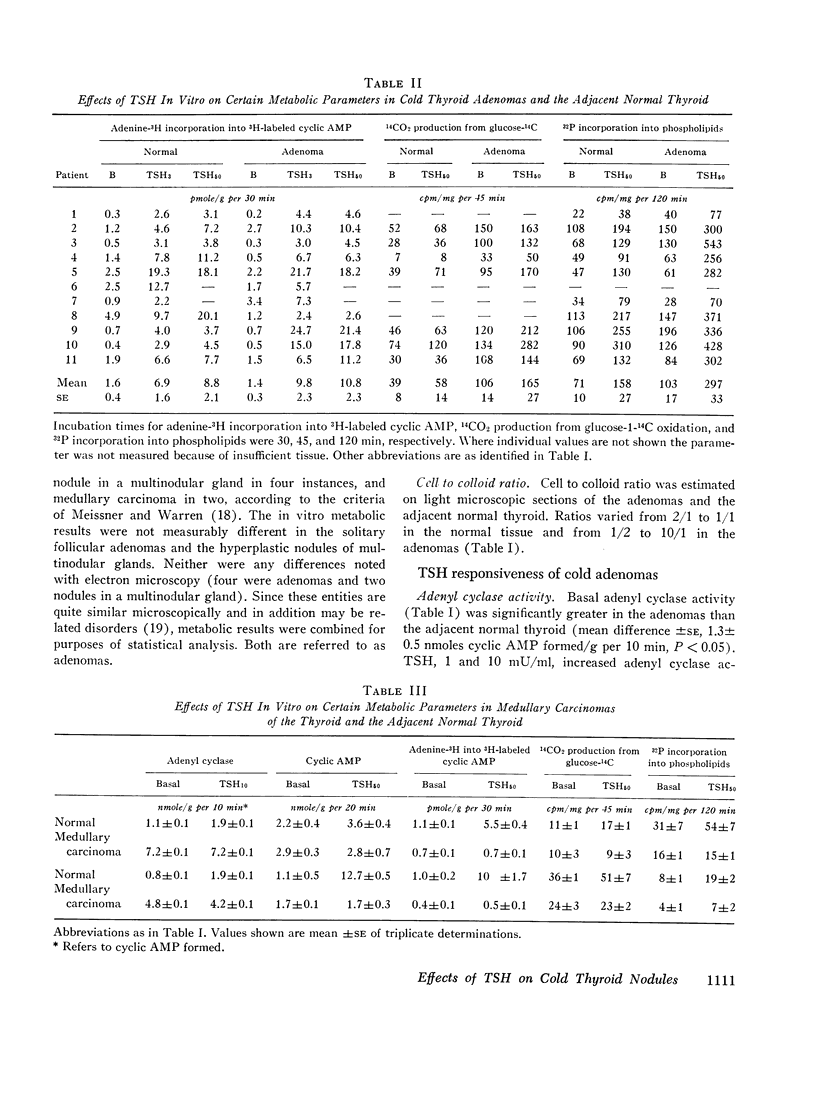
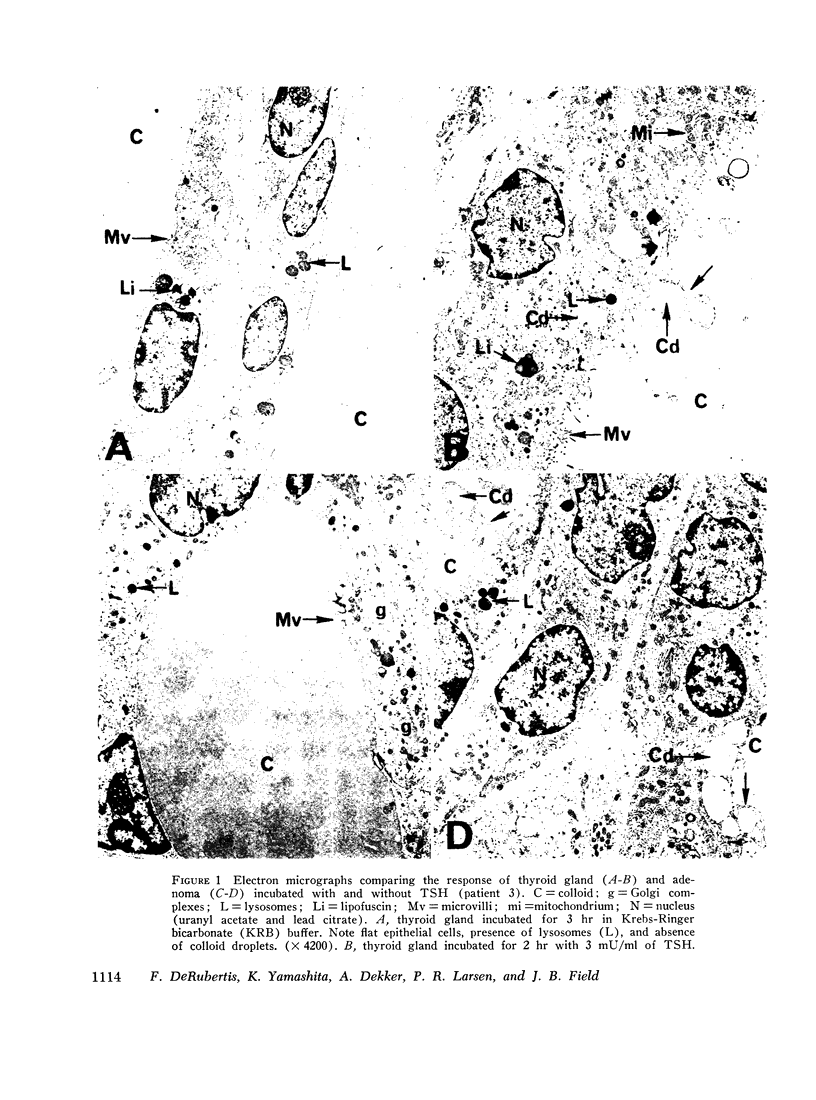
“Cold” thyroid nodules do not concentrate 131I before or after thyrotropin (TSH) administration. In an attempt to elucidate the reason for this TSH unresponsiveness, the effect of TSH in vitro on several metabolic parameters was studied in 11 “cold” thyroid adenomas, 2 medullary carcinomas, and in the surrounding normal thyroid tissue. Basal adenyl cyclase activity, glucose-1-14C oxidation, and 32P incorporation into phospholipids were significantly greater in the adenomas than in the adjacent normal thyroid; basal cyclic 3′,5′-adenosine monophosphate (cyclic AMP) concentration and adenine-3H incorporation into 3H-labeled cyclic AMP were not different. In adenomas as well as normal thyroid, all parameters responded significantly to in vitro TSH stimulation. The response to TSH of adenyl cyclase activity and 32P incorporation was enhanced in adenomas compared with that of the adjacent normal thyroid. These differences were not explained by an increased cellularity of the adenomas. Medullary carcinomas did not respond to TSH in any of the above parameters.
The studies demonstrate an intact, TSH-responsive adenyl cyclase-cyclic AMP system in the adenomas and, accordingly, imply the presence of receptor sites for TSH on the cells of the adenoma. The failure of such nodules to concentrate 131I may be owing to a subsequent impairment in the expression of cyclic AMP action on iodine metabolism.
Full text
PDF






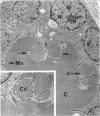
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn C. S., Rosenberg I. N. Iodine metabolism in thyroid slices: effects of TSH, dibutyryl cyclic 3',5'-AMP, NaF and prostaglandin E-1. Endocrinology. 1970 Feb;86(2):396–405. doi: 10.1210/endo-86-2-396. [DOI] [PubMed] [Google Scholar]
- BAKKE J. L., LAWRENCE N. The effect of thyroid stimulating hormone upon the iodide collecting mechanism of thyroid tissue slices. Endocrinology. 1956 May;58(5):531–545. doi: 10.1210/endo-58-5-531. [DOI] [PubMed] [Google Scholar]
- Dekker A., Field J. B. Correlation of effects of thyrotropin, prostaglandins and ions on glucose oxidation, cyclic-AMP, and colloid droplet formation in dog thyroid slices. Metabolism. 1970 Jun;19(6):453–464. doi: 10.1016/0026-0495(70)90097-1. [DOI] [PubMed] [Google Scholar]
- FIELD J. B., PASTAN I., JOHNSON P., HERRING B. Stimulation in vitro of pathways of glucose oxidation in thyroid by thyroid-stimulating hormone. J Biol Chem. 1960 Jul;235:1863–1866. [PubMed] [Google Scholar]
- HALMI N. S., SCRANTON J. R., TURNER J. W. Kinetic analysis of enhanced TSH-effect on thyroidal iodide transport in hypophysectomized rats fed a low iodine diet. Endocrinology. 1963 Mar;72:501–502. doi: 10.1210/endo-72-3-501. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Field J. B. A method for determination of 3',5'-cyclic adenosine monophosphate based on adenosine triphosphate formation. J Lab Clin Med. 1969 Oct;74(4):682–690. [PubMed] [Google Scholar]
- Kaneko T., Zor U., Field J. B. Stimulation of thyroid adenyl cyclase activity and cyclic adenosine 3',5'-monophosphate by long-acting thyroid stimulator. Metabolism. 1970 Jun;19(6):430–438. doi: 10.1016/0026-0495(70)90094-6. [DOI] [PubMed] [Google Scholar]
- Klinck G. H., Oertel J. E., Winship T. Ultrastructure of normal human thyroid. Lab Invest. 1970 Jan;22(1):2–22. [PubMed] [Google Scholar]
- MAAYAN M. L., ROSENBERG I. N. Effect of injection of thyrotropin upon deiodination of diiodotyrosine by rat thyroid and liver. Endocrinology. 1963 Jul;73:38–44. doi: 10.1210/endo-73-1-38. [DOI] [PubMed] [Google Scholar]
- Nève P., Dumont J. E. Time sequence of ultrastructural changes in the stimulated dog thyroid. Z Zellforsch Mikrosk Anat. 1970;103(1):61–74. doi: 10.1007/BF00335401. [DOI] [PubMed] [Google Scholar]
- Oka H., Field J. B. Effects of ions on TSH stimulation of P-32 incorporation into thyroid slice phospholipid. Am J Physiol. 1966 Dec;211(6):1357–1360. doi: 10.1152/ajplegacy.1966.211.6.1357. [DOI] [PubMed] [Google Scholar]
- PERLMUTTER M., SLATER S. L., ATTIE J. Method for preoperative differentiation between the benign and the possibly malignant solitary nontoxic thyroid nodule. J Clin Endocrinol Metab. 1954 Jun;14(6):672–673. doi: 10.1210/jcem-14-6-672. [DOI] [PubMed] [Google Scholar]
- PERLMUTTER M., SLATER S. L. Which nodular goiters should be removed; a physiologic plan for the diagnosis and treatment of nodular goiter. N Engl J Med. 1956 Jul 12;255(2):65–71. doi: 10.1056/NEJM195607122550202. [DOI] [PubMed] [Google Scholar]
- Pastan I. The effect of dibutyryl cyclic 3',5'-AMP on the thyroid. Biochem Biophys Res Commun. 1966 Oct 5;25(1):14–16. doi: 10.1016/0006-291x(66)90632-2. [DOI] [PubMed] [Google Scholar]
- Pastan I., Wollman S. H. Colloid droplet formation in dog thyroid in vitro. Induction by dibutyrl cyclic-AMP. J Cell Biol. 1967 Oct;35(1):262–266. doi: 10.1083/jcb.35.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG I. N., ATHANS J. C., ISAACS G. H. STUDIES ON THYROID IODINE METABOLISM. Recent Prog Horm Res. 1965;21:33–72. [PubMed] [Google Scholar]
- STANBURY J. B., CHAPMAN E. M. Congenital hypothyroidism with goitre. Absence of an iodide-concentrating mechanism. Lancet. 1960 May 28;1(7135):1162–1165. doi: 10.1016/s0140-6736(60)91042-4. [DOI] [PubMed] [Google Scholar]
- Salabè G. B., Grasso R., Ceccarini E., Baschieri L. Diiodotyrosine dehalogenating activity in thyroid cold nodules. Metabolism. 1968 Mar;17(3):271–279. doi: 10.1016/0026-0495(68)90130-3. [DOI] [PubMed] [Google Scholar]
- TENENHOUSE A., ARNAUD C., RASMUSSEN H. THE ISOLATION AND CHARACTERIZATION OF THYROCALCITONIN. Proc Natl Acad Sci U S A. 1965 Apr;53:818–822. doi: 10.1073/pnas.53.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG W. STIMULATORY EFFECT OF THYROTROPIN ON SYNTHESIS OF THYROXINE BY ISOLATED THYROID CELLS. Endocrinology. 1964 Feb;74:304–306. doi: 10.1210/endo-74-2-304. [DOI] [PubMed] [Google Scholar]
- Tonoue T., Tong W., Stolc V. TSH and dibutyryl-cyclic-AMP stimulation of hormone release from rat thyroid glands in vitro. Endocrinology. 1970 Feb;86(2):271–277. doi: 10.1210/endo-86-2-271. [DOI] [PubMed] [Google Scholar]
- Valenta L., Jirasek J. E. Histochemistry of thyroid tumors. Significance of two enzymes and PAS-positive materials. Arch Pathol. 1967 Sep;84(3):215–223. [PubMed] [Google Scholar]
- Valenta L., Kyncl F., Niederle B., Jirousek L. Soluble proteins in thyroid neoplasia. J Clin Endocrinol Metab. 1968 Apr;28(4):442–450. doi: 10.1210/jcem-28-4-442. [DOI] [PubMed] [Google Scholar]
- Vander J. B., Gaston E. A., Dawber T. R. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968 Sep;69(3):537–540. doi: 10.7326/0003-4819-69-3-537. [DOI] [PubMed] [Google Scholar]
- Velenta L., Lissitzky S., Aquaron R. Thyroglobulin-iodine in thyroid tumors. J Clin Endocrinol Metab. 1968 Apr;28(4):437–441. doi: 10.1210/jcem-28-4-437. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Wollman S. H. Changes in fine structure and acid phosphatase localization in rat thyroid cells following thyrotropin administration. J Cell Biol. 1965 Jun;25(3):593–618. doi: 10.1083/jcb.25.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B., Raghupathy E., Tonoue T., Tong W. TSH-like actions of dibutyryl-cAMP on isolated bovine thyroid cells. Endocrinology. 1968 Oct;83(4):877–884. doi: 10.1210/endo-83-4-877. [DOI] [PubMed] [Google Scholar]
- Wolff J., Berens S. C., Jones A. B. Inhibition of thyrotropin-stimulated adenyl cyclase activity of beef thyroid membranes by low concentration of lithium ion. Biochem Biophys Res Commun. 1970 Apr 8;39(1):77–82. doi: 10.1016/0006-291x(70)90760-6. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Preparation of thyroid plasma membranes containing a TSH-responsive adenyl cyclase. Biochem Biophys Res Commun. 1970 Jul 13;40(1):171–178. doi: 10.1016/0006-291x(70)91062-4. [DOI] [PubMed] [Google Scholar]
- Zor U., Kaneko T., Lowe I. P., Bloom G., Field J. B. Effect of thyroid-stimulating hormone and prostaglandins on thyroid adenyl cyclase activation and cyclic adenosine 3',5',-monophosphate. J Biol Chem. 1969 Oct 10;244(19):5189–5195. [PubMed] [Google Scholar]