Abstract
Although behavioral inflexibility and Purkinje cell loss are both well established in autism, it is unknown if these phenomena are causally related. Using a mouse model, we tested the hypothesis that developmental abnormalities of the cerebellum, including Purkinje cell loss, result in behavioral inflexibility. Specifically, we made aggregation chimeras (Lc/+↔+/+) between lurcher (Lc/+) mutant embryos and wildtype (+/+) control embryos. Lurcher mice lose 100% of their Purkinje cells postnatally, while chimeric mice lose varying numbers of Purkinje cells. We tested these mice on the acquisition and serial reversals of an operant conditional visual discrimination, a test of behavioral flexibility in rodents. During reversals 1 and 2, all groups of mice committed similar numbers of “perseverative” errors (those committed while session performance was ≤ 40% correct). Lurchers, however, committed a significantly greater number of “learning” errors (those committed while session performance was between 41% and 85% correct) than both controls and chimeras, and most were unable to advance past reversal 3. During reversals 3 and 4, chimeras, as a group, committed more “perseverative”, but not “learning” errors than controls, although a comparison of Purkinje cell number and performance in individual mice revealed that chimeras with fewer Purkinje cells made more “learning” errors and had shorter response latencies than chimeras with more Purkinje cells. These data suggest that developmental cerebellar Purkinje cell loss may affect higher level cognitive processes which have previously been shown to be mediated by the prefrontal cortex, and are commonly deficient in autism spectrum disorders.
Keywords: cerebellum, chimera, lurcher, reversal learning, behavioral flexibility, PFC, mPFC, executive function, autism
Introduction
Executive function is an umbrella term for the group of closely linked, high level cognitive skills which enable the effective execution of goal-directed behaviors (Hughes, Russell, & Robbins, 1994; Pennington & Ozonoff, 1996). These skills, which include working memory, response inhibition, and behavioral flexibility, have consistently been shown to be dependent on the prefrontal cortex (PFC) in humans, non-human primates, and rodents (Dalley, Cardinal, & Robbins, 2004; Robbins & Arnsten, 2009; Robbins & Roberts, 2007). Recently, there has been considerable interest in the idea that the cerebellum, by means of its reciprocal connections to the PFC, may play a role in the performance of tasks requiring executive control. This hypothesis is supported by many functional imaging and lesion studies in humans which implicate the cerebellum in an array of executive tasks (Bellebaum & Daum, 2007; Strick, Dum, & Fiez, 2009), as well as the occurrence of cerebellar abnormalities and executive function deficits in psychiatric disorders such as autism and schizophrenia (Amaral, Schumann, & Nordahl, 2008; Andreasen & Pierson, 2008; Hill, 2004).
Behavioral flexibility, one subtype of executive function, is the ability to adapt behavior in response to changing environmental demands (Ragozzino, 2007). People with autism have considerable difficulty performing tasks requiring behavioral flexibility both in their everyday lives and on neuropsychological tests (Hill, 2004). Considering that cognitive theories of autism suggest that fundamental deficits in executive function may underlie the clinically significant symptoms of this disorder (Hill, 2004; Pennington & Ozonoff, 1996) and cerebellar neuroanatomical abnormalities are consistently found in autism (Amaral et al., 2008), determining the relationship between cerebellar abnormalities and behavioral inflexibility may provide important clues to the neural mechanisms underlying autism spectrum disorders.
We have recently reported increased activity and repetitive behavior in a mouse model designed to mimic the developmental cerebellar pathology observed in autism (Martin, Goldowitz, & Mittleman, 2010). In this study, we further characterize this lurcher chimera mouse model by assessing the impact of developmental cerebellar Purkinje cell loss on behavioral flexibility. Mice that are heterozygous for the lurcher spontaneous mutation (Grid2Lc) lose virtually all of their cerebellar Purkinje cells between the 2nd and 4th weeks of development as a result of a gain-of-function mutation in the δ2 glutamate receptor gene (Caddy & Biscoe, 1979; Zuo, De Jager, Takahashi, Jiang, Linden, & Heintz, 1997). Consequently, lurcher mutants are ataxic, displaying a characteristic swaying of the hindquarters and a jerky up and down movement. Using lurcher mutant (Lc/+) and control (+/+) mice, we created chimeric mice (Lc/+↔+/+) which lose a variable number of cerebellar Purkinje cells during development, depending on the incorporation of the wildtype lineage (Goldowitz, Moran, & Wetts, 1992).
The use of chimeras with a range of Purkinje cell loss conferred two important advantages over the simple comparison of Lc/+ and +/+ mice. First, in addition to comparing grouped performance of Lc/+, +/+, and chimeric mice, we were able to use correlational analysis to test for a relationship between Purkinje cell number and behavior. Second, because chimeric mice show no visible signs of ataxia unless they have lost greater than 90% of their Purkinje cells relative to controls (Martin, Escher, Goldowitz, & Mittleman, 2004), we were able to examine behavioral flexibility in mice with widely varying numbers of Purkinje cells while still controlling for the confounding effects of ataxia.
Following the creation of chimeras, we assessed performance of Lc/+ mutants, +/+ control mice, and Lc/+↔+/+ chimeras on the acquisition and serial reversals of an operant conditional visual discrimination, one type of reversal learning task used to assess behavioral flexibility in rodents. Reversal learning tasks measure behavioral flexibility by assessing the ability of the subject to adapt its behavior following a reversal of stimulus-reward or stimulus-response contingencies, and have been shown to depend heavily on the PFC (Clark, Cools, & Robbins, 2004; Ragozzino, 2007).
Method
Subjects
A total of 25 mice consisting of Lc/+↔+/+ chimeras (n=21), +/+ controls (n=2), and Lc/+ mutants (n=2) were tested. Following histological analysis, chimeric mice were assigned to genotypic groups (i.e., lurcher, chimera, or control) based upon Purkinje cell number. Lurcher mice (B6CBACa Aw-J/A-Grid2Lc) and controls were obtained from the Jackson Laboratory (Bar Harbor, Maine) and maintained at the University of Tennessee Animal Care Facility and the University of Memphis Animal Care Facility.
Production of Aggregation Chimeras
Using previously described methods (Martin, Goldowitz, & Mittleman, 2003), aggregation chimeras were produced by fusing two 4–8 cell embryos derived from a mating of Lc/+ with +/+ mice of the same Lc strain background and transplanted into pseudopregnant ICR host females. All surgical procedures and animal care were in accordance with National Institutes of Health guidelines for animal welfare.
Apparatus
All training and testing was carried out in six Med Associates (www.med-associates.com) mouse operant chambers (ENV-307A). Two retractable response levers (ENV-312-2M) were mounted 19 mm above the grid floor on the left and right side of the front wall of the chamber. Two visual stimuli (ENV-221M) were located centrally on the front wall at approximately the same height as the response levers. One stimulus was positioned directly above the other, and the stimuli were positioned equidistant from the two response levers. These stimuli were modified in-house such that when illuminated, the top stimulus displayed a vertical line, and the bottom stimulus displayed a horizontal line. A house light (ENV-315M) and food receptacle which allowed access to a liquid dipper (ENV-302M) were centrally located on the rear wall. The house light was positioned 19 mm below the ceiling of the chamber, and the food receptacle was positioned at approximately the same height as the response levers and stimulus lights.
Procedure
General procedures
Mice were food deprived to 85–90% of baseline weight and maintained at this weight throughout the experiment by restricted feeding. Before testing, mice were acclimated to the testing chamber and trained to lever press for reward and nose poke the food receptacle at the rear of the chamber to initiate each trial. No visual or auditory stimulus explicitly signaled the mouse to initiate each trial, but mice reliably and quickly learned to do so. Mice were tested 5–6 days per week.
Acquisition of the conditional visual discrimination
Figure 1 schematically illustrates the training procedure. Mice were required to nose poke the food receptacle at the rear of the chamber to begin the first trial. Following this, both levers extended into the chamber, and one of the two visual stimuli was illuminated. Levers remained extended and the stimulus light remained illuminated until a lever press was made. With the exception of correction trials (see below), the stimulus illuminated at trial onset was randomly chosen at the beginning of each trial, and was counterbalanced such that each of the two visual stimuli was presented an equal number of times during each session. If the vertical-line stimulus was illuminated, the mouse was required to press the right lever to receive a reward (0.02 mL of evaporated milk/sucrose solution). The reward was delivered by means of a liquid dipper and was available for 7 seconds following a correct lever press. If the horizontal-line stimulus was illuminated, the mouse was required to press the left lever to receive the reward. Training was counterbalanced such that half of the mice learned the opposite stimulus-response contingencies. Following a correct or incorrect lever press, the visual stimulus was immediately turned off and the response levers retracted. An incorrect lever press resulted in a 7 second time out during which the house light was turned off (it was on at all other times during the session) to signal that an incorrect press had occurred, and reward was omitted. A 5 second inter-trial interval with the house light on followed both reward presentation and time out, after which the mouse was required to nose poke the food receptacle to begin the next trial. Incorrect trials were followed by trials in which the same stimulus was presented repeatedly (correction trials) until the mouse made a correct choice. Sessions lasted 60 minutes or until the mouse obtained 72 rewards. Upon reaching the criterion of 85% correct responses during a single session, the mouse was moved to the serial reversal stage of the experiment.
Figure 1.
Flowchart illustrating the sequence of events which occurred over the course of each session during the acquisition of the conditional visual discrimination and subsequent reversal stages. Sessions lasted 60 minutes or until the mouse obtained 72 rewards.
Serial reversal learning
Each of the four reversal stages of the experiment was identical to the acquisition stage, with the exception that the stimulus-response contingencies were reversed relative to those of the previous stage. Mice had the opportunity to complete a total of four serial reversals.
Histology
Following the completion of behavioral testing, all chimeric mice were overdosed with anesthesia (Avertin) and transcardially perfused with physiologic saline followed by acetic acid: 95% ethanol fix (1pt:3pt) for 20 minutes. Heads were removed at the cervical vertebrae and immersion fixed overnight in the same fixative. Heads were then placed in 70% ethanol before the brains were dissected out and dehydrated with increasing concentrations of ethanol. Mid-sagittal sectioned brains were then cleared with a series of xylenes and infiltrated with paraffin, followed by embedding in paraffin blocks. Paraffin embedded brains were sectioned in the sagittal plane using a microtome set for 8 μm thickness. Every 25th section was then mounted on Superfrost+ slides. Immunocytochemistry using an anti-Calbindin antibody (Chemicon) was then performed on all slides to enable the identification of Purkinje cells. After incubation in primary antisera, slides were exposed to an anti-rabbit biotinylated secondary antibody and subsequently visualized using the ABC reaction (Vectastain kit, Vector Labs). Purkinje cells were identified by the brown reaction product of diaminobenzidine (DAB). Slides were then counterstained with cresyl violet and then dehydrated through ascending alcohol concentrations before being cleared with xylenes. Glass coverslips were then applied with Permount.
Purkinje Cell Counts
Purkinje cell nuclei were identified with the aid of a standard brightfield microscope equipped with 10x eyepieces and a 25x objective. The nuclei are easily identifiable at this magnification due to their light appearance in comparison to the surrounding darkly stained cytoplasm. Purkinje cell nuclei in every 25th section throughout the entire cerebellum were counted with the exception of the parafloccular lobe. Total numbers of Purkinje cells for the entire cerebellum were then estimated from this sampling and corrected for split nuclei using the Abercrombie correction factor (Abercrombie, 1946).
Dependent Variables
The following dependent variables were collected at each stage of the conditional visual discrimination task: (1) Sessions to criterion, (2) Trials to criterion, (3) Errors to criterion, (4) Reward retrieval latency (ms), and (5) Lever press latency (ms). As in past studies which have investigated learning following the reversal of stimulus-reward contingencies (e.g., Bussey, Muir, Everitt, & Robbins, 1997; Chudasama & Robbins, 2003), errors committed at each reversal stage were subdivided into Perseverative errors and Learning errors to assess performance at each phase of the learning process. Errors committed during sessions in which performance was well below chance levels (≤ 40% correct) were classified as Perseverative errors, suggesting that during these sessions mice continued to respond according to the response rules of the prior stage. Errors committed during sessions in which performance was at or above chance (41% – 85% correct) were classified as Learning errors, suggesting that mice had shifted away from the use of the response rules of the prior stage. We have collectively referred to all reversal sessions during which performance is ≤ 40% correct as the “perseverative phase” of the reversal stage and all sessions during which performance is between 41% and 85% correct as the “learning phase” of the reversal stage.
Statistics
Acquisition of the conditional visual discrimination
For each dependent variable, performance on the acquisition stage was analyzed using ANOVA with “Group” as a between subjects factor (control, chimera, lurcher). For these and all other ANOVAs, post hoc tests were performed using a Bonferroni correction. The two-tailed alpha level for all statistical tests was .05.
Serial reversal learning
Of the group of lurcher mice that began training for the conditional visual discrimination task (n = 8), only 1 was able to complete all 4 serial reversals. The remaining 7 mice were unable to reach criterion on reversal 3 following over two months of training at that stage. Therefore, when comparing serial reversal learning performance between groups, we conducted two separate analyses for each dependent variable. The first compared lurchers, chimeras, and controls on the first and second reversal stages. The second compared chimeras and controls on the third and fourth reversal stages. For both analyses, mixed repeated measures ANOVAs were used to examine main effects of the between subjects factor “Group” and the within subjects factor “Stage”.
Relationship between Purkinje cells and behavior
In chimeric mice, Pearson product-moment correlation coefficients were calculated to test for relationships between Purkinje cell numbers and performance on all dependent variables.
Results
Histological analysis
Consistent with our previous studies (Martin et al., 2003, 2004, 2006, 2010), experimental groups were formed following histology and according to the following criteria. Mice with approximately zero Purkinje cells (range 0 – 34) were classified as lurchers. Mice with greater than 128,000 Purkinje cells (M = 145,693, SD = 15,492, range 128,610 – 176,688) were classified as controls. Non-ataxic mice having fewer than 128,000 Purkinje cells (M = 88,710, SD = 34,883, range 29,199 – 125,493) were classified as chimeras. All mice classified as lurchers were visibly ataxic, while all mice classified as controls or chimeras showed no obvious motor impairments. As shown in Figure 2, Purkinje cell numbers in non-ataxic mice ranged from 29,199 to 176,688. Using methods similar to those of our prior studies with chimeric mice (Martin et al., 2003, 2004, 2006, 2010), the cutoff point between chimeras and controls was established by performing Purkinje cell counts of +/+ mice (n = 2) derived from our breeding colony. The cerebella of these two mice contained 128,610 and 137,375 Purkinje cells, respectively (M = 132,993). The mice derived from the chimera production process that were classified as controls (n = 5) all had Purkinje cell counts above this mean. Figure 3 graphically illustrates the differences in Purkinje cell numbers among control, chimeric and lurcher mice.
Figure 2.
Purkinje cell variability in the Lc/+↔+/+ chimeras (n = 10) and +/+ controls (n = 7) used in the conditional visual discrimination task.
Figure 3.

Four mid-sagittal sections of cerebellum shown from chimeras with varying numbers of Purkinje cellls. Sections are stained with anti-Calbindin to highlight Purkinje cells. A) Normal numbers of Purkinje cells are illustrated in this control chimera. B and C) Chimeras with 40,000 (B) and 25,000 (C) Purkinje cells are illustrated. The size of these cerebella are also reduced from the wildtype control. Arrows point to regions that lack Purkinje cells which are more prevalent and larger as the numbers of Purkinje cells decline. D) A mutant chimera cerebellum is shown that completely lacks Purkinje cells and has a small cerebellum. The anti-Calbindin staining is normal in extra cerebellar regions (such as the brainstem in this section). Scale bar = 500 μm.
Conditional Visual Discrimination and Serial Reversal Learning
The means and standard deviations of all variables are shown in Table 1. It should be noted that throughout acquisition and subsequent serial reversal learning, the variables Sessions to criterion, Trials to criterion, and Errors to criterion revealed an identical pattern of results.
Table 1.
Mean ± SD performance of control, chimeric, and lurcher mice on all stages of the conditional visual discrimination task.
| Group | Stage |
||||
|---|---|---|---|---|---|
| Acquisition | Reversal 1 | Reversal 2 | Reversal 3 | Reversal 4 | |
| Lurcher (Lc/+; n=8) | |||||
| Sessions to criterion | 26.38 (9.72) | 42.37 (12.95) | 44.00 (18.60)§ | - | - |
| Trials to criterion | 2358.75 (995.14) | 4078.75 (1177.33) | 4525.75 (1931.97)§ | - | - |
| Errors to criterion | 826.38 (296.73) | 1539.50 (423.33) | 1493.14 (556.54)§ | - | - |
| Perseverative errors | - | 299.63 (157.74) | 176.50 (69.25)‡ | - | - |
| Learning Errors | - | 1239.88 (464.28) | 1448.88 (415.14) | - | - |
| Reward retrieval latency (ms) | 2960.41 (494.42)† | 3260.02 (660.06) | 3156.17 (604.66)§ | - | - |
| Lever press latency (ms) | 14084.27 (5497.19)† | 12312.28 (3219.13) | 9295.73 (4255.43)§‡ | - | - |
| Chimera (Lc/+↔+/+; n=10) | |||||
| Sessions to criterion | 18.60 (15.01) | 15.60 (10.00) | 14.60 (5.03) | 17.80 (8.69) | 15.10 (5.55) |
| Trials to criterion | 1907.50 (1215.77) | 1803.30 (1034.80) | 1606.90 (580.55) | 1971.70 (901.59) | 1717.20 (621.41) |
| Errors to criterion | 740.90 (418.95) | 764.80 (385.09) | 602.10 (203.81) | 716.30 (288.33) | 670.90 (221.02) |
| Perseverative errors | - | 220.20 (77.74) | 110.30 (65.96)‡ | 129.60 (72.81) | 170.10 (78.04) ¥ |
| Learning Errors | - | 544.6 (389.53) | 491.8 (225.19) | 586.70 (270.53) | 500.80 (213.85) |
| Reward retrieval latency (ms) | 1436.85 (245.25) | 1301.03 (335.31) | 1361.81 (340.49) | 1366.22 (280.10) | 1372.38 (267.52) |
| Lever press latency (ms) | 5856.87 (2577.70) | 4971.008 (3239.72) | 4495.09 (2781.58)‡ | 3182.43 (1210.95) | 2825.62 (1535.83) |
| Control (+/+; n=7) | |||||
| Sessions to criterion | 13.14 (8.40) | 16.71 (7.85) | 14.00 (8.15) | 19.43 (11.86) | 22.00 (13.78) |
| Trials to criterion | 1196.71 (709.81) | 1851.29 (748.72) | 1511.71 (681.95) | 2025.43 (1151.69) | 2320.14 (1315.03) |
| Errors to criterion | 430.00 (227.24) | 763.86 (283.11) | 604.29 (228.70) | 710.29 (403.99) | 819.00 (432.39) |
| Perseverative errors | - | 265.71 (141.69) | 189.14 (72.17)‡ | 60.29 (64.44) | 85.29 (82.59) |
| Learning Errors | - | 498.14 (196.32) | 415.14 (184.44) | 650.00 (382.05) | 733.71 (388.73) |
| Reward retrieval latency (ms) | 1934.69 (477.77) | 1600.98 (391.39) | 1643.62 (484.61) | 1675.22 (507.34) | 1835.19 (694.64) |
| Lever press latency (ms) | 5234.61 (1660.87) | 3504.70 (1142.59) | 3449.96 (1664.64)‡ | 3003.43 (1268.82) | 3092.27 (1748.07) |
Note: Because 7 of 8 lurcher mice were unable to reach criterion on reversal 3, lurcher data is not presented for reversals 3 and 4.
indicates a significant difference from controls on the acqusition stage,
indicates a significant difference from controls on reversals 1 and 2,
indiciates a significant main effect of Stage on reversals 1 and 2,
indicates a significant difference from controls on reversals 3 and 4. In all cases, significance indicates p < .05
Acquisition of the conditional visual discrimination
The ability to acquire the conditional visual discrimination did not differ significantly between groups. Although lurchers and chimeras showed a non-significant trend towards committing more errors than controls (Table 1; Errors to criterion: Group, F (2, 22) = 2.84, p = .08), the number of Trials to criterion did not differ significantly among the groups (Group F (2, 22) = 2.41, p >.10). During acquisition the latency to lever press and to retrieve the reward differed significantly between groups (Lever press latency: Group, F (2, 22) = 15.02, p = .00008; Reward retrieval latency: Group, F (2, 22) = 31.82, p = .0000003). Post hoc analyses indicated that lurchers took significantly longer (in milliseconds) than chimeras and controls (Table 1; all comparisons p < .001) to lever press following stimulus onset (Lurchers, M = 14,084, SD = 5497; Chimeras M = 5857, SD = 2578; Controls M = 5235, SD = 1661) and retrieve the reward following a correct lever press (Lurchers, M = 2960, SD = 494; Chimeras, M = 1437, SD = 245; Controls, M = 1935, SD = 478).
Reversals 1 and 2
Figure 4A illustrates that perseverative responding to the previously rewarded stimulus did not differ significantly between groups on the first and second reversals (Perseverative errors: Group, F (2, 22) = 2.89, p = .08; Table 1). All mice perseverated less on the second reversal than the first (Perseverative errors: Stage, F (1, 22) = 11.98, p = .002). There was no significant Group × Stage interaction (Perseverative errors: Group × Stage, F (22, 2) = .196, p = .823).
Figure 4.
Mean ± SEM performance of control, chimeric, and lurcher mice on reversals 1–4. (A) Perseverative and (B) Learning errors committed on reversals 1 and 2. Lurcher mice committed significantly more Learning errors (those committed while session performance was between 41% and 85% correct) on reversals 1 and 2 relative to controls. (C) Perseverative and (D) Learning errors committed on reversals 3 and 4. Chimeric mice committed significantly more Perseverative errors (those committed while session performance was ≤ 40% correct) on reversals 3 and 4 relative to controls. Because the majority of lurcher mice were unable to reach criterion on reversal 3, lurcher data is not presented for reversals 3 and 4. * indicates a significant difference (p < .05) from controls. Vertical lines above each bar indicate the SEM.
Once mice entered the learning phase (41% – 85% correct responses during a session), marked learning differences appeared between groups (Learning errors: Group, F (2, 22) = 19.52, p = .00001). Specifically, lurchers committed a significantly greater number of Learning errors than chimeras and controls (both comparisons p < .0001; Figure 4B; Table 1). There was no significant effect of Stage or Group × Stage interaction (Learning errors: Stage, F (1, 22) = .069, p = .795; Learning errors: Group × Stage, F (2, 22) = .977, p = .392).
Similar to the acquisition stage, latency to lever press and to retrieve the reward during reversals 1 and 2 differed significantly between groups (Lever press latency: Group, F (2, 22) = 17.54, p = .00003; Reward retrieval latency: Group, F (2, 22) = 40.14, p = .00000005). Post hoc analyses indicated that lurchers took significantly longer to lever press and retrieve the reward than chimeras and controls on reversals 1 and 2 (all comparisons p < .001; Table 1). Lever press latency of all groups was shorter on the second reversal than the first (Lever press latency: Stage, F (1, 22) = 4.50, p = .045; Table 1).
Reversals 3 and 4
Because 7 of the 8 lurcher mice were unable to complete reversals 3 and 4, only chimeras and controls were included in the analysis of performance on these reversal stages. Although perseverative responding of chimeras and controls did not differ significantly on the first and second reversals, a significant group difference appeared during the third and fourth reversals (Perseverative errors: Group, F (1, 15) = 6.99, p = .018). Specifically, chimeras committed a greater number of Perseverative errors on reversals 3 and 4 relative to controls (Figure 4C; Table 1). There was no significant effect of Stage or Stage × Group interaction (Perseverative errors: Stage, F (1, 15) = 2.09, p = .169; Perseverative errors: Stage × Group, F (1, 15) = .117, p = .737).
As shown in Figure 4D, chimeras and controls performed similarly on the learning phase of reversals 3 and 4 (Learning errors: Group, F (1, 15) = 1.22, p = .288; Table 1). There was no significant effect of Stage or Stage × Group interaction for either variable (Learning errors: Stage, F (1, 15) = .00002, p = .988; Learning errors: Stage × Group, F (1, 15) = 1.43, p = .250). Latency to lever press and to retrieve the reward did not differ significantly between chimeras and controls on reversals 3 and 4 (Lever press latency: Group, F (1, 15) = .004, p = .950; Reward retrieval latency: Group, F (1, 15) = 3.49, p = .081).
The Relationship between Purkinje Cells and Performance
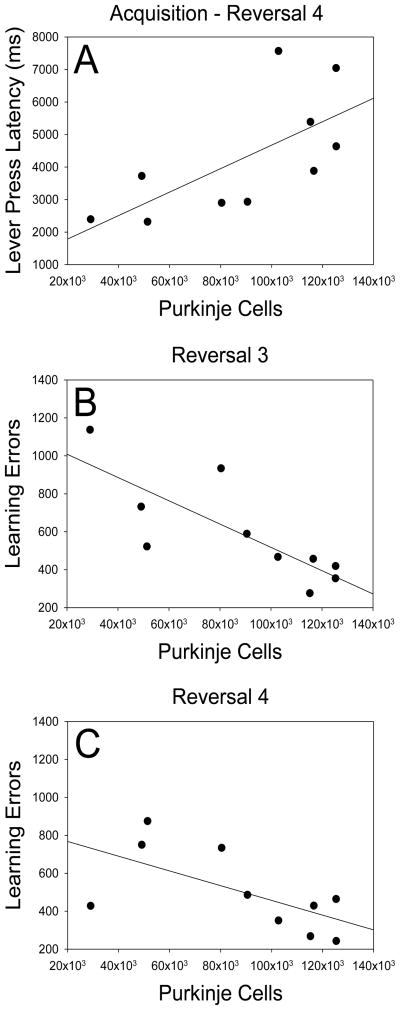
Pearson product-moment correlations indicated that Purkinje cell number in chimeric mice was significantly related to two of the seven dependent variables collected in the study. First, as indicated in Figure 5A, throughout all stages of testing we observed a significant positive correlation between Purkinje cell number and Lever press latency (r = .674, p = .033, n = 10), suggesting that mice with fewer Purkinje cells were consistently faster to lever press following stimulus onset than those with more Purkinje cells. Second, as indicated in Figure 5B and 5C, there were consistent and significant negative correlations between Purkinje cell numbers and Learning errors (those committed while session performance was between 41% and 85% correct) during reversals 3 and 4 (r = −.792, p = .006, n = 10; r = −.633, p = .049, n = 10), suggesting that once mice had stopped perseverating during stages 3 and 4, mice with more Purkinje cells reached criterion faster than those with fewer Purkinje cells. Relationships between Purkinje cells and Learning errors during reversals 1 and 2 were not significant (r = .083, p = .819, n = 10; r = −.433, p = .212, n = 10).
Figure 5.
Significant correlations in chimeric mice between Purkinje cell number, lever press latency and learning errors. (A) Lever press latency was averaged across acquisition and reversals. Throughout all stages of testing we observed a significant positive correlation between Purkinje cell number and Lever press latency (r = .674, p = .033, n = 10), suggesting that mice with fewer Purkinje cells were quicker to lever press following stimulus onset than those with more Purkinje cells. (B and C) There were consistent and significant negative correlations between Purkinje cell numbers and Learning errors (those committed while session performance was between 41% and 85% correct) during reversals 3 and 4 (r = −.792, p = .006, n = 10; r = −.633, p = .049, n = 10), suggesting that once mice had stopped perseverating during stages 3 and 4, mice with more Purkinje cells reached criterion faster than those with fewer Purkinje cells.
Discussion
Autism is partially defined by deficits in behavioral flexibility, and one of the most consistent neuropathologies in autism is developmental cerebellar Purkinje cell loss (Amaral et al., 2008; Hill, 2004; Whitney, Kemper, Bauman, Rosene, & Blatt, 2008). However, it is unknown if Purkinje cell loss during development causes behavioral flexibility deficits. Therefore, using a mouse model, we investigated the effect of complete and variable developmental loss of cerebellar Purkinje cells on conditional visual discrimination and serial reversal learning. The latter is a well known measure of behavioral flexibility. Lurcher (Purkinje cells: approximately zero), chimeric (Purkinje cells: M = 88,710, SD = 34,883) and control mice (Purkinje cells: M = 145,693, SD = 15,492) acquired a conditional discrimination of two visual stimuli and were then challenged with a series of reversals of the stimulus-response contingencies. All groups acquired the conditional visual discrimination, although lurchers were significantly slower to lever press and to retrieve the delivered reward. During reversals 1 and 2, all groups of mice committed similar numbers of Perseverative errors (those committed while session performance was ≤ 40% correct). Lurchers, however, committed a significantly greater number of Learning errors (those committed while session performance was between 41% and 85% correct). This deficit was so profound that the majority of lurchers were unable to advance past reversal 3 following extensive training.
During reversals 3 and 4, chimeras, as a group, committed more Perseverative, but not Learning errors than controls. Although the number of Learning errors committed by chimeras as a group was similar to the number committed by controls during reversals 3 and 4, comparison of Purkinje cell number and performance in individual mice revealed that chimeras with fewer Purkinje cells committed more Learning errors and lever pressed more quickly following stimulus onset than chimeras with more Purkinje cells.
Performance Deficits in Lurcher Mice
The trend towards poorer performance in lurcher mice during the acquisition stage as well as their increase in learning errors during reversals 1 and 2 is most likely related to their ataxia. Relative to controls and chimeras, lurchers consistently took significantly longer to lever press following illumination of the visual stimuli and to collect the reward following a correct lever press. We have found similar performance deficits in lurcher mice in our previous studies. In operant paradigms assessing spatial working memory, sustained attention, and breakpoint in a progressive ratio task, lurchers have consistently shown impaired performance relative to controls on components of these tasks that are clearly related to their motor abilities such as repeated head entries into the food receptacle, difficulty moving between the lever and the food receptacle, and inefficient lever pressing (Martin et al., 2004, 2006, 2010).
While it seems most parsimonious to attribute the impaired performance in lurchers to their accompanying motor deficit it should be noted that the increase in learning errors during reversals 1 and 2 can also be interpreted as a cognitive deficit. Specifically, an increase in errors restricted to the learning phase of reversals has been suggested to reflect a deficit in selective attention (Bussey et al., 1997; Chudasama & Robbins, 2003) or a deficit in the ability to maintain a novel strategy once perseveration has ceased (for review see Floresco, Zhang, & Enomoto, 2009; Ragozzino, 2007), but not a deficit in visual discrimination learning. These interpretations, however, were restricted to animals without concomitant motor deficits or ataxia. It thus remains possible that lurcher mice are demonstrating a cognitive impairment in addition to their motor deficit.
Performance Deficits in Chimeric Mice
Similar to lurchers, there was a non-significant tendency for chimeras to be impaired during acquisition of the conditional visual discrimination, although during reversals 1 and 2, chimeras and wildtype controls performed similarly. Although it is possible that chimeras had some limited impairment in conditional visual discrimination learning during acquisition, this impairment was not severe enough to significantly increase the number of trials to criterion.
In comparison to controls, chimeric mice committed significantly more Perseverative errors during reversal stages 3 and 4, a deficit that was not apparent in early reversals. Unlike lurcher mutants, chimeric mice were not ataxic. Therefore, this performance deficit cannot be attributed to gross motor deficits. Instead, it is more plausible that the Purkinje cell loss in chimeric mice resulted in a reduced ability to inhibit responding to the previously-rewarded but no-longer-correct visual stimulus. A similar deficit was recently observed in hemicerebellectomized rats while performing reversals of a four choice learning task (De Bartolo, Mandolesi, Federico, Foti, Cutuli, Gelfo, & Petrosini, 2009). Specifically, while the number of perseverative errors committed by hemicerebellectomized rats did not differ from the number committed by controls during early reversals, it was significantly higher than that of controls during later reversals. It should be noted that in the current study as well as in De Bartolo et al. (2009), the difference between controls and lesioned or chimeric animals was caused by a decrease in perseverative errors committed by controls across reversals, while levels of perseverative errors were maintained by the experimental animals (See Fig. 4A & C). Collectively these results suggest that developmental cerebellar abnormalities or acute cerebellar damage result in consistent levels of perseverative responding throughout serial reversal learning which is likely attributable to a failure to inhibit a prepotent learned response.
Relationship between Purkinje Cells and Learning Errors during Reversals 3 and 4 in Chimeric Mice
Comparisons of Purkinje cell number and performance in individual mice on reversals 3 and 4 revealed that chimeras with fewer Purkinje cells committed more Learning errors and displayed shorter latencies to lever press than chimeras with more Purkinje cells. An increase in Learning errors during reversals has been suggested to reflect deficits in either selective attention (Bussey et al., 1997; Chudasama & Robbins, 2003) or in the ability to maintain a novel strategy once perseveration has ceased (Floresco et al., 2009; Ragozzino, 2007). In the context of the current results the relationship between Learning errors and Purkinje cells indicates that mice with fewer Purkinje cells were specifically impaired in learning the new stimulus-reward contingencies, even after they had overridden the perseverative phase of responding. It also seems reasonable that in chimeric mice with comparatively fewer Purkinje cells, this learning deficit was exacerbated by the very fast latencies to respond to the visual stimuli, which itself is suggestive of an additional deficit in response inhibition (e.g., Chudasama & Robbins, 2003).
It should be noted that although there was a significant relationship between Learning errors and Purkinje cells, as a group, chimeric mice did not differ from controls in the number of learning errors. This lack of significant group difference is probably related to the makeup of the chimeric group in that only 50% of the mice in this group had a substantial reduction in the number of Purkinje cells in comparison to controls.
Possible Role of the Prefrontal Cortex in the Observed Purkinje Cell Relationships
It has been well established that the PFC is critically involved in multiple facets of executive function in both humans, non-human primates, and rodents (Dalley et al., 2004; Robbins & Arnsten, 2009; Robbins & Roberts, 2007). Recently, there has been considerable interest in the idea that the cerebellum may exert modulatory control over the executive functions subserved by the PFC. This idea has grown out of the many functional imaging and lesion studies in humans which implicate the cerebellum in an array of executive tasks (Bellebaum & Daum, 2007; Strick et al., 2009). Experimental studies in rodents support the conclusion that the cerebellum exerts a modulatory influence on functions linked to the PFC (e.g., De Bartolo et al., 2009; Lalonde & Strazielle, 2008; Martin et al., 2004).
Behavioral evidence linking the cerebellum with PFC-mediated tasks has been bolstered by a series of viral tract tracing studies in non-human primates demonstrating that the PFC and cerebellum are connected via a closed-loop circuit, providing an anatomical substrate for cerebellar control over PFC-mediated executive functions (Strick et al., 2009). Two recent studies in rats and mice provide evidence for the existence of a functional pathway connecting the medial prefrontal cortex (mPFC) to the cerebellum in rodents. First, using an electrophysiological tract tracing method, Watson, Jones, and Apps (2009) identified a pathway in rats connecting the prelimbic subdivision of the mPFC to the cerebellum. Second, we have previously reported that brief electrical stimulation of the cerebellar Purkinje cell layer resulted in transient increases in extracellular dopamine levels in the mPFC of +/+ control mice (Mittleman, Goldowitz, Heck, & Blaha, 2008). This increase was absent in Lc/+ mutant mice with a complete loss of cerebellar Purkinje cells, suggesting that Purkinje cell outputs are a critical component of this modulatory cerebellar-mPFC circuit
Because of these associations between the mPFC and the cerebellum, it is appropriate to ask if the increases we observed in perseverative and learning errors during reversal stages have previously been associated with the mPFC. Using an operant visual discrimination task, increases in learning errors during reversals have been found following excitotoxic lesions of the mPFC in mice and rats (Brigman & Rothblat, 2008; Bussey et al., 1997), and following excitotoxic lesions restricted to the infralimbic cortex of the mPFC in rats (Chudasama & Robbins, 2003). It is also possible that the neurochemical mechanisms underlying these mPFC lesion-induced deficits in reversal learning may involve dopamine transmission. Dopamine D2 receptor knockout mice and control mice treated chronically with the D2-like receptor blocker haloperidol committed significantly more errors relative to non-treated wildtype controls during the reversal stage of an attentional set shifting task performed in a U-maze (De Steno & Schmauss, 2009). The reversal learning deficit in these mice was associated with decreased expression of the transcription factor egr-2 in several areas of the PFC including the infralimbic and prelimbic subregions of the mPFC. Additionally, the magnitude of dopamine efflux in the mPFC has been linked to reversal learning in rats using an operant spatial reversal learning task (van der Meulen, Joosten, de Bruin, & Feenstra, 2007). Considered together, the (1) tract tracing studies in rodents showing anatomical connections between the cerebellum and mPFC and (2) behavioral studies in rodents linking reversal learning impairment with the mPFC suggest that a possible or perhaps likely explanation for the current findings is that the reduced number, or virtual absence of Purkinje cells in, respectively, chimeric and lurcher mice led to a disruption of dopamine transmission in the mPFC, which in turn led to deficits in the ability of these mice to flexibly adapt their behavior during reversal of the stimulus-reward contingencies.
It is also important to note that perseverative and learning errors following the reversal of stimulus-reward contingencies have also been associated with other areas of the brain including the orbitofrontal cortex, striatum, and thalamic nuclei (for review see Floresco et al., 2009; Ragozzino, 2007). Additionally, a number of studies have failed to find that lesions of mPFC result in increased errors during reversal stages (e.g., Bissonette, Martins, Franz, Harper, Schoenbaum, & Powell, 2008; Floresco, Block, & Tse, 2008). Thus, it is prudent to consider other possible mechanisms by which loss of cerebellar Purkinje cells could have affected the performance of lurcher and chimeric mice in this study. Specifically, it is possible that the cerebellum, in addition to modulating dopamine neurotransmission in the mPFC, also influences neurotransmission in other brain areas which have been shown to affect behavioral flexibility. Additional studies are needed to test this hypothesis.
Conclusion
In the current study, we assessed conditional visual discrimination and serial reversal learning performance in lurcher, control, and chimeric mice with variable degrees of developmental cerebellar Purkinje cell loss. Lurcher mice were significantly impaired during the learning phase of reversals 1 and 2. Relative to controls, chimeric mice showed enhanced perseveration during reversals 3 and 4. Although there were no group differences in learning errors between chimeras and controls during reversals 3 and 4, there was a significant negative relationship between Purkinje cells and learning errors during these reversals. These data suggest that developmental cerebellar Purkinje cell loss may affect higher level cognitive processes which have previously been shown to be partially mediated by the prefrontal cortex, and are commonly deficient in autism spectrum disorders.
Acknowledgments
This research was supported by a grant from Autism Speaks and NIH grant 1R01NS063009-01A1 to GM, CB, DG, and DH. We thank Erin Clardy and Richard Cushing for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. The Anatomical Record. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neurosciences. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of Neuroscience. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behavioural Brain Research. 2008;187:405–410. doi: 10.1016/j.bbr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1979;287:167–201. doi: 10.1098/rstb.1979.0055. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. The Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- De Bartolo P, Mandolesi L, Federico F, Foti F, Cutuli D, Gelfo F, Petrosini L. Cerebellar involvement in cognitive flexibility. Neurobiology of Learning and Memory. 2009;92:310–317. doi: 10.1016/j.nlm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- De Steno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Moran TH, Wetts R. Mouse chimeras in the study of genetic and structural determinants of behavior. In: Goldowitz D, Wahlsten D, Wimer RE, editors. Techniques for the genetic analysis of brain and behavior: Focus on the mouse. Amsterdam: Elsevier; 1992. pp. 271–290. [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Discrimination learning in Rora(sg) and Grid2(ho) mutant mice. Neurobiology of Learning and Memory. 2008;90:472–474. doi: 10.1016/j.nlm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Martin LA, Escher T, Goldowitz D, Mittleman G. A relationship between cerebellar Purkinje cells and spatial working memory demonstrated in a lurcher/chimera mouse model system. Genes, Brain, and Behavior. 2004;3:158–166. doi: 10.1111/j.1601-183x.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. The cerebellum and spatial ability: dissection of motor and cognitive components with a mouse model system. The European Journal of Neuroscience. 2003;18:2002–2010. doi: 10.1046/j.1460-9568.2003.02921.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Sustained attention in the mouse: a study of the relationship with the cerebellum. Behavioral Neuroscience. 2006;120:477–481. doi: 10.1037/0735-7044.120.2.477. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. European Journal of Neuroscience. 2010;31:544–555. doi: 10.1111/j.1460-9568.2009.07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62:544–550. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cerebral Cortex, 17 Suppl. 2007;1:i151–160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- van der Meulen JA, Joosten RN, de Bruin JP, Feenstra MG. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cerebral Cortex. 2007;17:1444–1453. doi: 10.1093/cercor/bhl057. [DOI] [PubMed] [Google Scholar]
- Watson TC, Jones MW, Apps R. Electrophysiological mapping of novel prefrontal - cerebellar pathways. Frontiers in Integrative Neuroscience. 2009;3:18. doi: 10.3389/neuro.07.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]