Abstract
The PAX3-FKHR fusion protein is present in the majority of alveolar rhabdomyosarcoma (ARMS) associated with increased aggressiveness and poor prognosis. To better understand the molecular pathogenesis of PAX3-FKHR, we carried out the first and unbiased genome-wide identification of PAX3-FKHR binding sites and associated target genes in ARMS. The data shows that PAX3-FKHR binds to the same sites as PAX3 at both MYF5 and MYOD enhancers. The genome-wide analysis reveals that the PAX3-FKHR sites are: 1) mostly distal to transcription start sites; 2) conserved; 3) enriched for PAX3 motifs; and 4) strongly associated with genes over-expressed in PAX3-FKHR positive RMS cells and tumors. There is little evidence in our dataset for PAX3-FKHR binding at the promoters. The genome-wide analysis further illustrates a strong association between PAX3 and E-box motifs in these binding sites, suggestive of a common co-regulation for many target genes. We further provide the first direct evidence that FGFR4 and IGF1R are the targets for PAX3-FKHR. The map of PAX3-FKHR binding sites provides the frame work for the understanding of PAX3-FKHR pathogenic roles and molecular targets to allow a systematic evaluation of agents against this aggressive RMS.
Introduction
The paired box transcription factor (PAX) family consists of 9 highly related members with critical roles in organogenesis and tissue specification during development, including skeletal muscle, pancreas, central nervous system, kidney, and immune system (1). In various cell model systems, PAX genes have been implicated in the maintenance of the multipotent state, the initiation of differentiation programs, cell migration, proliferation and survival. Mutations and gene rearrangement of PAX genes are frequently associated with human diseases and cancers (2–4). In particular, PAX3 and PAX7 are required during early embryogenesis for the development of the neural-crest and skeletal muscle (2, 5), and gene rearrangements with FKHR are frequently implicated with rhabdomyosarcoma (RMS) (6).
RMS is the most common soft tissue sarcoma in children (7) that consists of alveolar (ARMS) and embryonal (ERMS) subtypes. The majority of ARMS are associated with specific chromosome translocations that result in PAX3-FKHR (FOXO1A) or PAX7-FKHR fusion protein, consisting of the N-terminal PAX3/PAX7 DNA binding domain and the C-terminal FKHR transactivation domain (8, 9). The fusion protein was suggested as a more potent transactivator than PAX3 in vitro (10, 11) and in vivo (12). Conditional activation of PAX3-FKHR was shown to collaborate with the inactivation of Ink4a/Arf or p53 in facilitating the development of murine ARMS (13). The PAX3-FKHR translocation is not only the most common translocation in ARMS but also associated with increased treatment failure and mortality rate (14). Thus, it is important to identify the direct transcription targets of PAX3-FKHR. Nearly all published studies on PAX3-FKHR targets and their cis-acting sequences focused on promoter elements immediately adjacent to the transcription start sites, even though the role of PAX3-FKHR binding sites as promoters has not been verified. In fact, for the best studied targets of PAX3, the myogenic determination genes MYF5 and MYOD, the cis-acting sequences responsible for PAX3 regulation are distal enhancers (15–18). As the identification of the direct effectors of PAX3-FKHR may have pivotal roles in delineating its molecular pathogenic mechanism and in identifying new targets of therapy, a genome-wide analysis of the direct targets of PAX3-FKHR is the next necessary step.
To elucidate the targets of PAX3-FKHR, chromatin immunoprecipitation and DNA sequencing (ChIP-seq) were performed with a novel antibody specific for PAX3-FKHR fusion protein. Our results show that the majority of binding sites are conserved distal regulatory elements, enriched for PAX3 and E-box/MYF motifs, and strongly associated with genes up-regulated by PAX3-FKHR. The dataset of PAX3-FKHR binding sites has a high degree of accuracy shown in the examples of MYF5 and MYOD enhancers, with a resolution sufficient for rapid identification and confirmation of specific recognition sequences. The identification of the PAX3-FKHR binding sites and associated genes enables rapid identification and validation of its targets, which would enhance our understanding of myogenic development, the molecular pathogenesis of RMS, and the evaluation of targeted agents for this cancer.
Materials and methods
Cell lines and reagents
All human RMS cell lines and Rh1 Ewing’s sarcoma cell line were maintained in RPMI-1640 with 10% fetal bovine serum. PAX3-FKHR-HA and PAX3 and PAX3-FKHR expression plasmids were plasmid was kindly provided by Drs. Michael Anderson and Fred Barr. shRNA plasmids targeting FKHR were obtained from Open Biosystems with the target sequences: s2, GCTTAGACTGTGACATGGAAT; s4, CAGGACAATAAGTCGAGTTAT. Lentiviruses for shRNA vectors were generated. For RNAi experiments, cells were infected with the lentiviruses for 3 days with at least 50% cells infected.
Quantitative PCR analysis
Total and immunoprecipitated DNA were used for qPCR on GAPDH promoter and MYF5 enhancer with primers listed in Table S7 and SYBR Green PCR kit (Qiagen). For quantitative expression analysis, we used cells 3 days after infected with lentiviruses carrying scramble or shRNA vectors against FKHR portion of the PAX-FKHR. Total RNA was isolated with Trizol (Invitrogen) and the cDNA was generated using SuperScript III (Invitrogen). Quantitative PCR analysis was performed with SYBR Green PCR kit in triplicates with primers listed in Table S7 to determine the relative levels of specific transcripts, normalized against that of GAPDH. All qPCR assays were reproduced at least three times.
Antibodies and immunoblots
Cell lysate preparation and immunoblots were done as previously described (20). A monoclonal antibody against PAX3-FKHR (PFM2) was generated against a fusion region peptide (Supplementary Methods). Ascites fluid was obtained for PFM2 and IgG fraction was isolated. Antibodies against PAX3, FKHR, MYCN, MET, ERK, AKT and actin were from Cell Signaling Technology, and the antibody against IGF1R-α (C20) was from Santa Cruz Biotech.
Luciferase reporter assay for PAX3-FKHR enhancer
PAX3-FKHR binding sites were amplified from human genomic DNA using primers listed in Table S7. They were cloned into the SalI and BamHI sites of the pGL3-promoter vector (Promega) that contains SV40 promoter upstream of firefly luciferase reporter gene. Mutant enhancers were generated with site-directed mutagensis kit (Strategene) with primers listed in Table S7. All mutations were confirmed by DNA sequencing. Enhancer assays were performed with Dual Luciferase Assay System (Promega) to adjust for differences in transfection efficiency. Reporter co-transfection experiments were done with the ratio of reporter to activator plasmids at 1:1. All reporter assays were reproduced at least three times.
Statistical analysis
All quantitative experiments, including qPCR for immunoprecipitated DNA, RT-qPCR for specific gene expression, and luciferase analysis were performed in triplicates. Data analysis were performed with Prism (GraphPad) and shown in charts as mean ± SEM. Impact on gene expression patterns was tested based on the null hypothesis that the number of genes associated to binding sites within the set of differentially expressed genes was not larger than expected by chance when compared to the set of all annotated genes. P values were calculated using the hypergeometric distribution.
Results
Genome-wide mapping of PAX3-FKHR binding sites in RMS
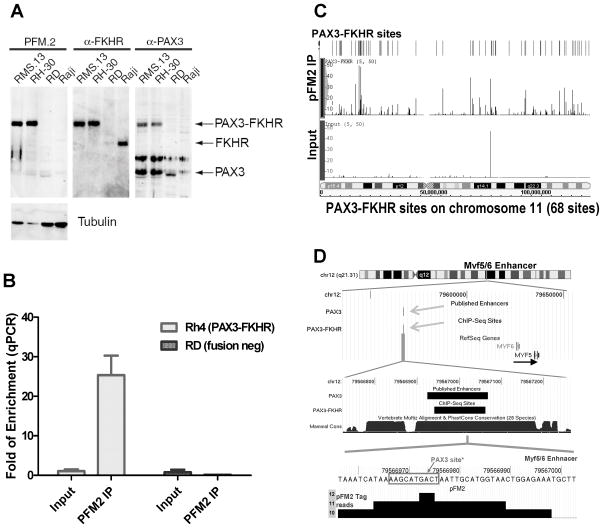
To identify the targets of PAX3-FKHR, a novel monoclonal antibody PFM2 was generated using a peptide corresponding to the fusion region. The antibody is specific to PAX3-FKHR (Fig. 1A). The precipitation with PFM2 in PAX3-FKHR+ Rh4 cells resulted in 20–40 fold enrichment of the MYF5 enhancer over the input DNA, the best known target of PAX3 (Fig. 1B). In contrast, parallel ChIP with fusion negative RD cells gave no enhancer enrichment (Fig. 1B). Both the ChIP and input DNA were sequenced to obtain 3.96 and 4.06 million aligned sequence tags with similar chromosome distribution (Supplementary Fig. 1, Supplementary Table 1). While the input DNA tags were evenly distributed, distinct sequence tag clusters were identified with PFM2 ChIP DNA shown in an example of chromosome 11 (Fig. 1C). Altogether, a total of 1463 statistically significant putative PAX3-FKHR binding sites were identified in the human genome with an average size of 186 bp, at about 1 site per 2 million base pairs (mbp) (GEO no. GSE19063, see Supplementary Methods for a detailed description of selection criteria and data access). The chromosome distribution of the binding sites is closely correlated with the length of the chromosome (Fig. S1, r2=0.83), similar to that of estrogen receptor and p53 (Supplementary Fig. 1, Supplementary Table 1) (21, 22). The ChIP-seq result was highly reproducible with 80% (1166 sites) identified in a repeat experiment (data not shown).
Figure 1.
ChIP-seq analysis of PAX3-FKHR binding sites in rhabdomyosarcoma cells. A, immunoblots show that PFM2 recognizes PAX3-FKHR protein in RMS cell lines positive for the translocation (RMS13 and Rh30). B, quantitative PCR analysis shows that PFM2 selectively immunoprecipitates the MYF5 enhancer from PAX3-FKHR+ Rh4 cells, not from the negative RD cells. C, distribution of PAX3-FKHR binding sites in chromosome 11. Peak height represents the number of sequence tags. D, a PAX3-FKHR site mapped within the MYF5 enhancer. The maximum signal aligns with a known PAX3 recognition site at this PAX3-dependent enhancer.
The specificity of the binding sites was very good, as there was no overlap between the 1463 sites in Rh4 and the 223 sites obtained from fusion negative RD cells via ChIP-seq, which also gave 4 million aligned sequences (data not shown). The specificity of the binding sites was verified with the best characterized PAX3-dependent enhancer located at −58/−56 kb distal to MYF5 (Fig. 1D). The binding site identified by ChIP-seq is defined in a 108 bp region, within the previously identified 145 bp minimal PAX3-responsive MYF5 enhancer (17), demonstrating that PAX3-FKHR binds to the identical site in RMS cells as PAX3 does during development. The maximum of the tag density was located at the boxed PAX3 binding sequence AAGCATGACT at the enhancer previously shown to be critical for PAX3-dependent activation (17, 23). This result shows that the dataset may have sufficient resolution for identifying specific recognition sequences.
PAX3-FKHR binding at conserved distal regulatory elements
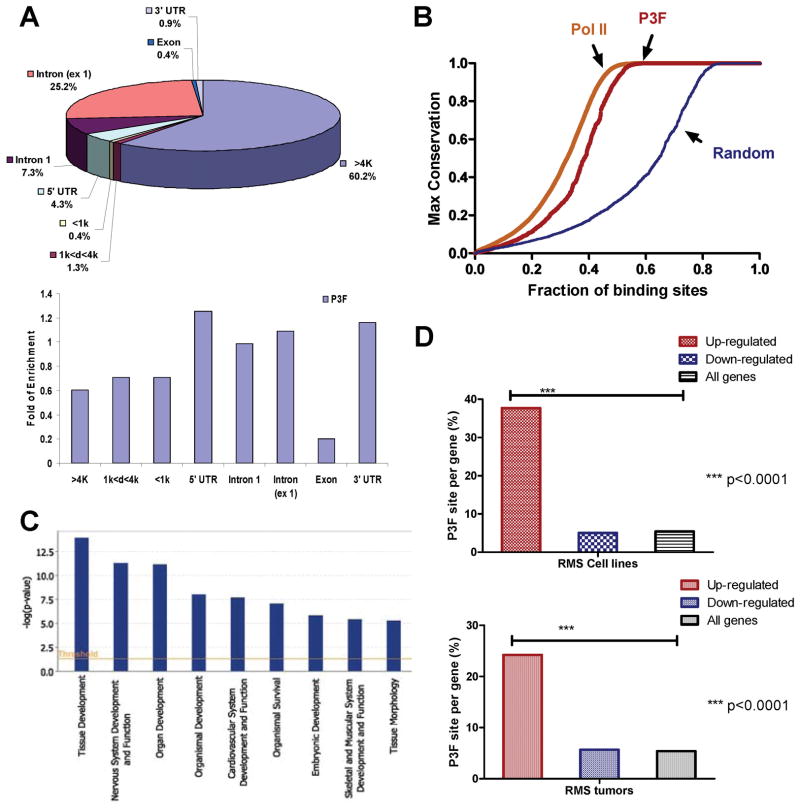
The analysis of the binding sites showed that the vast majority of the sites were either more than 4 kb from the transcription start sites outside of the genes (60.2%), or in the introns (32.5%). Only 0.4% of the binding sites were located within 1 kb upstream of the initiation sites (Fig. 2A). In addition, there was no binding site enrichment within either a 1 kb or 4 kb window upstream of the start sites (Fig 2A). The data suggests that PAX3-FKHR binds primarily to distal sites. The enrichment analysis further revealed reduced bindings sites in the exons (Fig. 2A), consistent with distinctive functions for the two. Maximal conservation analysis shows that there is a significant conservation amongst these PAX3-FKHR binding sites in vertebrates, in contrast to that of randomly selected sequences. The degree of conservation is similar to that of RNA pol-II binding sites (Fig. 2B), again indicating the functionality of these PAX3-FKHR sites. The analysis of the 1463 binding sites identifies 1072 genes either overlapping or located proximal to them (Supplementary Table 2). Gene ontology analysis shows a bias towards developmental processes, including those of the nervous, skeletal, and muscular systems (Fig. 2C), consistent with the known roles of PAX3 in early development and its expression in those systems. Thus, the binding sites are conserved and identify potential targets of PAX3 during development.
Figure 2.
PAX3-FKHR binding sites as putative enhancers. A, distribution of PAX3-FKHR sites relating to the transcription start sites, exons, and introns. Enrichment or suppression PAX3-FKHR sites are shown. Unit of 1 represents the average of number of sites across the genome. B, degree of conservation of PAX3-FKHR sites compared with that of Pol-II ChIP-seq sites and randomly generated sequence tags. C, gene ontology analysis of genes associated with PAX3-FKHR sites with Ingenuity IPA. D, the association of PAX3-FKHR sites with genes up-regulated by PAX3-FKHR in RD cells (Table S3), and genes up-regulated in a panel of PAX3/7-FKHR positive RMS tumors (Table S4).
Inspection of the PAX3-FKHR sites provides a direct link between PAX3/PAX3-FKHR and genes implicated in skeletal, muscular development and RMS (Table 1). These genes are involved in growth, survival and oncogenesis, cell migration and tumor metastasis, as well as genes important for myogenic differentiation. All their binding sites were reconfirmed in the repeat experiment. Despite their roles of in muscle development and cancer, many were not previously known to be direct targets of either PAX3 or PAX3-FKHR, and in most cases, the PAX3-FKHR dependent regulatory elements were not defined.
Table 1A.
Selected Genes with PAX3-FKHR Binding Sites
| ID | Entrez Gene Name | Location | Type | Drugs |
|---|---|---|---|---|
| Self-renewal, growth, survival, oncogenesis | ||||
| ALK | anaplastic lymphoma receptor tyrosine kinase | Plasma Membrane | receptor kinase | PF-02341066 |
| FGFR2 | fibroblast growth factor receptor 2 | Plasma Membrane | receptor kinase | TKI-258, AZD2171, brivanib, PHA-739358 |
| FGFR4 | fibroblast growth factor receptor 4 | Plasma Membrane | receptor kinase | |
| IGF1R | insulin-like growth factor 1 receptor | Plasma Membrane | receptor kinase | CP-751871, AMG479, R1507, MK-0646, AVE1642 |
| KDR | kinase insert domain receptor, VEGFR2 | Plasma Membrane | receptor kinase | bevacizumab, sunitinib, vatalanib, sorafenib, vandetanib |
| MEIS1 | Meis homeobox 1 | Nucleus | transcription factor | |
| MYCN | v-myc myelocytomatosis viral related oncogene, neuroblastoma derived | Nucleus | transcription factor | |
| SPRY1 | sprouty homolog 1, antagonist of FGF signaling (Drosophila) | Plasma Membrane | FGFR4 ligand | |
| Migration, metastasis | ||||
| CXCR4 | chemokine (C-X-C motif) receptor 4 | Plasma Membrane | GPCR | JM 3100 |
| MET | met proto-oncogene (hepatocyte growth factor receptor) | Plasma Membrane | receptor kinase | PF-02341066 |
| Myogenic differentiation | ||||
| EYA2 | eyes absent homolog 2 (Drosophila) | Nucleus | phosphatase | |
| EYA4 | eyes absent homolog 4 (Drosophila) | Cytoplasm | phosphatase | |
| MEOX1 | mesenchyme homeobox 1 | Nucleus | transcription factor | |
| MEOX2 | mesenchyme homeobox 2 | Nucleus | transcription factor | |
| MYF5/6 | myogenic factor 6 (herculin) | Nucleus | transcription factor | |
| MYOD1 | myogenic differentiation 1 | Nucleus | transcription factor | |
| PRRX1 | paired related homeobox 1 | Nucleus | transcription factor | |
| Other potential targets | ||||
| ABAT | 4-aminobutyrate aminotransferase | Cytoplasm | enzyme | valproic acid |
| ADRA1D | adrenergic, alpha-1D-, receptor | Plasma Membrane | GPCR | paliperidone, risperidone, antazoline, acetaminophen, epinephrine, acetaminophen |
| ADRA2A | adrenergic, alpha-2A-, receptor | Plasma Membrane | ||
| ADRA2C | adrenergic, alpha-2C-, receptor | Plasma Membrane | ||
| DHFR | dihydrofolate reductase | Unknown | enzyme | pyrimethamine, trimethoprim, iclaprim, methotrexate, |
PAX3-FKHR sites associated with genes up-regulated by PAX3-FKHR in RMS
To establish the association between the binding sites and genes regulated by PAX3-FKHR, correlative analysis was performed with the genes up and down-regulated by PAX3-FKHR when introduced to the RD cells via retroviral transduction (24). There is 7-fold enrichment of PAX3-FKHR binding sites in the genes up-regulated by PAX3-FKHR at 37.7%; whereas no enrichment of PAX3-FKHR sites was seen for genes down-regulated by PAX3-FKHR at 5% (Fig. 2D, Supplementary Table 3). Hypergeometric distribution analysis shows that the association between genes with PAX3-FKHR sites and those up-regulated by PAX3-FKHR is statistically significant (p<0.0001). In addition to the association with syngeneic RMS cells with exogenously introduced PAX3-FKHR, there is a strong correlation between the binding sites and the up-regulated genes in a panel of 160 RMS tumors (25). Of the 363 genes with increased expression in PAX3/7-FKHR positive RMS, 24.2% have the binding sites (Fig. 2D, Supplementary Table 4). In contrast, of the 122 genes with decreased expression, only 5.7% have the binding sites. Again, hypergeometric distribution analysis shows such association is significant (p<0.0001). Thus, PAX3-FKHR sites are strongly associated with genes up-regulated by PAX3-FKHR in vitro in RMS cells and in RMS tumors with PAX3/7-FKHR translocations.
PAX3-FKHR binds MYOD core enhancer at a newly defined PAX3 recognition sequence and regulates other limb development genes
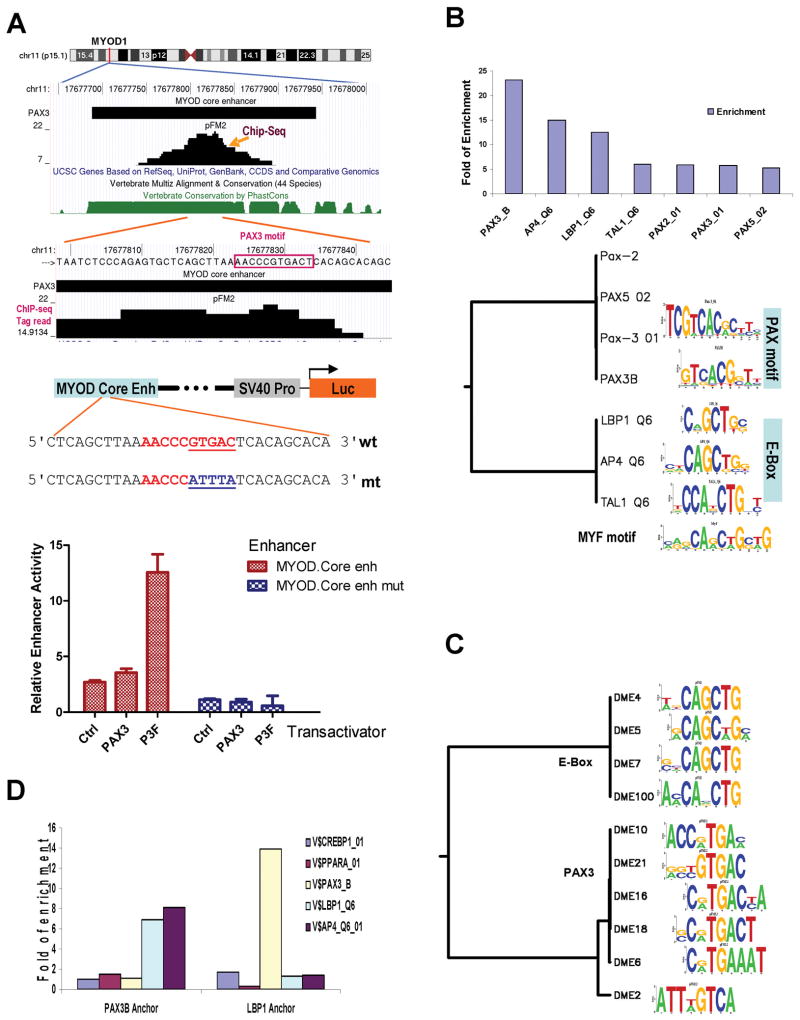
MYOD is the master controller of skeletal muscle development (26). PAX3 regulation of MYOD is mediated via the MYOD core enhancer (15, 27). Our genome-wide analysis of PAX3-FKHR sites shows a binding site in a region of 161 bp, located within the previously mapped 258 bp MYOD core enhancer (Fig. 3A). Interestingly, sequence examination at the binding signal peak identified a novel PAX3 recognition sequence AACCCGTGAC (box). To confirm the role of this PAX3 recognition sequence, an enhancer reporter was constructed with this MYOD enhancer and a mutant was then derived by site-directed mutagenesis to inactivate the PAX3 recognition sequence. Enhancer analysis showed that this particular PAX3 recognition sequence was responsible for PAX3 and PAX3-FKHR-dependent activation of the core enhancer (Fig. 3A). Thus, our data enabled the identification of a novel recognition sequence necessary for PAX3 and PAX3-HKFR-mediated activation of MYOD core enhancer. In addition to MYF5 and MYOD, we identified novel PAX3-FKHR binding sites in several additional transcription factors important for muscle and limb development and potentially for ARMS including homeobox genes MEOX1, MEOX2, and PRRX1 (Table 1). Selected sites were validated by enhancer assays, site directed mutagenesis, and shRNA depletion of PAX3-FKHR (Supplementary Figs. 2, 3).
Figure 3.
PAX3 recognition sequence in the MYOD core enhancer and the co-enrichment of PAX3 and E-box motifs in the binding sites. A, the identification of a novel PAX3 recognition sequence at the MYOD core enhancer and site-direct mutagenesis study of the site in transfected A204 RMS cells. B, enrichment of transcription factor motifs in PAX3-FKHR sites and clustering the top enriched motifs. C, results of a de novo motif search in which 9 of the top 10 motifs were shown to be related to either E-box or PAX3 motifs (see Table S6 for details). D, co-enrichment of PAX3 and E-box motifs among PAX3-FKHR sites. Either PAX3B or LBP1 was anchored, and the relative enrichment of other sites within 100 bp of the anchor is shown.
Genome-wide co-enrichment of PAX3 and E-box motifs within PAX3-FKHR site
There is limited knowledge on how PAX3 functions in regulating gene expression. It is a weak transactivator and may require other elements in regulating gene expression. Genetic hierarchy analysis of skeletal muscle differentiation reveals that MYOD expression requires both PAX3 and MYF5 (28). Analysis of the MYOD core enhancer shows several E-box motifs that are required for its expression in the skeletal muscle lineage (27). We asked if the presence of both PAX3 and E-box motifs was more a common property of PAX3-FKHR binding sites. Transcription factor motif analysis of PAX3-FKHR sites showed that PAX3_B was the most enriched motif at 21.7 fold, together with several related paired box motifs (Supplementary Table 5 and Fig. 3B). Interestingly, several other motifs were also enriched: AP4 by 12.9 fold and LBP1 by 11.1 fold, sharing a common CAGCTG E-box motif. Thus, motif analysis of PAX3-FKHR sites reveals a genome-wide enrichment of PAX3 and E-box motifs. To confirm the results, de novo motif analysis of the ChIP-Seq data was performed with DME (29). Of the top 10 output motifs, at least 5 were PAX3 or highly related motifs, and 4 others were E-box related motifs using Transfac database (Fig. 3C, Supplementary Table 6). Thus, motif and de novo analysis of PAX3-FKHR sites reveal a genome-wide selective enrichment of PAX3 and E-box motifs.
We asked whether the enrichment was due to co-localization of these two motifs adjacent to each other. When PAX3_B was used as the anchor, both LBP1 and AP4 motifs (E-box) were enriched by 7–8 fold within 100 bp of the anchor (Fig. 3D). Neither the PAX3_B site, nor controls (CREBP1, or PPAR-α) were enriched adjacent to the PAX3_B anchor. Similarly, when LBP1 was used as the anchor, only PAX3_B motif was enriched by 14 fold. Thus, the genome-wide analysis of PAX3 and E-box motifs shows a strong co-localization of them at PAX3-FKHR binding sites. These observations suggest a broad role of PAX3 and MYF/E-box proteins in regulating the expression of many targets in addition to MYOD.
FGFR4 is a direct target of PAX3-FKHR
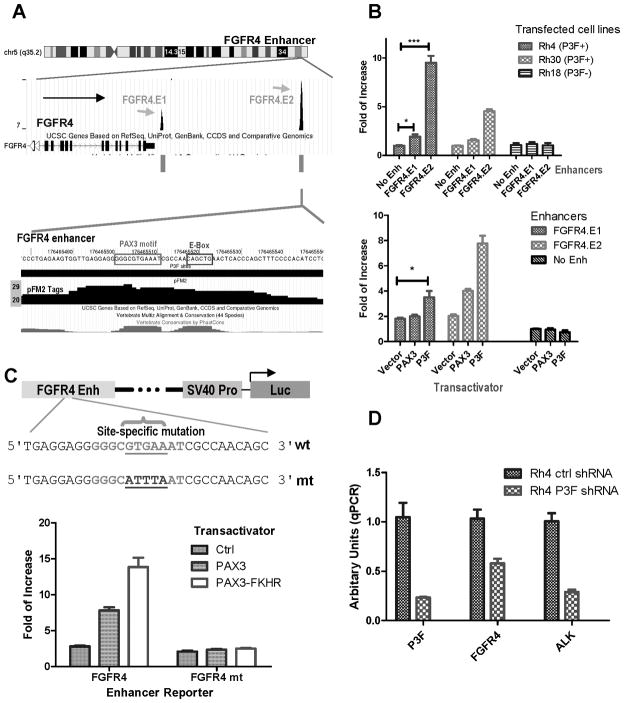
The FGF signaling pathway has been implicated in vivo in promoting cell proliferation and differentiation during myogenesis (30). FGFR4 is expressed during myogenic differentiation under the control of PAX3 via a downstream enhancer (31). The importance of this pathway has recently been emphasized by the identification of activating mutations of FGFR4 in 7% of RMS (19) that promotes metastasis in xenograft mouse. The expression level of FGFR4 was associated with ARMS and poor survival (19). Our results revealed two binding sites downstream of FGFR4 (Fig. 4A). Both putative FGFR4 enhancer sequences were cloned into the enhancer reporter and used for enhancer analysis. The results showed that the enhancer activities were detected in PAX3-FKHR+ Rh4 and Rh30 cells, but not in the negative Rh18 cells (Fig. 4B). The distal FGFR4.E2 gave significantly greater activity than that of FGFR4.E1. In co-transfection experiments into RD cells, PAX3-FKHR was able to induce both E1 and E2 activity (Fig. 4B), of which E2 is a stronger enhancer.
Figure 4.
Fine mapping of a PAX3-FKHR-dependent enhancer for FGFR4 gene. A, ChIP-seq results identified two PAX3-FKHR binding sites downstream of FGFR4. The peak of the FGFR4.E2 binding signal is associated with a conserved PAX3 sequence. B, enhancer activity both for both binding sites in PAX3-FKHR (P3F) positive and negative cells; and with co-transfection PAX3 or PAX3-FKHR expression plasmid in RD cells. C, site-direct mutagenesis of PAX3 recognition sequence FGFR4.E2 enhancer resulted in the loss of trans-activation by PAX3 or PAX3-FKHR in RD cells. D, RT-qPCR data showing down-regulation of PAX3-FKHR with a lentiviral shRNA (s2) vector led to reduced ALK and FGFR4 mRNA in Rh4.
The examination of FGFR4.E2 reveals PAX3 and E-box recognition sequences that are both conserved in vertebrates (Fig. 4A). A mutant in the putative PAX3 recognition site resulted in the complete inactivation of PAX3-FKHR-dependent induction (Fig. 4C). The data show that the PAX3-FKHR binding site downstream of FGFR4 is a PAX3-FKHR-dependent enhancer.
To determine if the expression of FGFR4 in RMS cells is dependent on PAX3-FKHR, Rh4 cells were infected with a lentivirus containing a shRNA (s2) targeting PAX3-FKHR. RNA was isolated and analyzed for PAX3-FKHR and FGFR4 mRNA levels. The results showed that both PAX3-FKHR and FGFR4 mRNA levels were reduced with this shRNA (P3F.s2, Fig. 4D). Thus, PAX3-FKHR is responsible for the elevated FGFR4 mRNA level in a fusion positive RMS cell line.
It is worth noting that our study may provide more accurate data on PAX3-FKHR-dependent regulation for MET. In contrast to a previous report showing that the MET promoter was regulated by PAX3 (32), our data identified two PAX3-FKHR binding sites in the introns of MET. Enhancer reporter assay showed both were activated by PAX3 and PAX3-FKHR (Table 1; Supplementary Fig. 4). As these sequences are selectively bound by PAX3-FKHR in RMS cells, the results indicate that MET is regulated by PAX3 or PAX3-FKHR via intronic enhancers.
Identification of PAX3-FKHR-dependent enhancers at ALK and MYCN
Of the genes contained proximal or internal binding sites for PAX3-FKHR, Ingenuity Pathway (Ingenuity Systems) analysis revealed the strongest disease association with developmental, skeletal and muscular disorders, of which MYCN was implicated (Supplementary Fig. 5). In additional to be amplified in neuroblastoma, MYCN was shown to be amplified in about 20% of both RMS associated with adverse outcome (33). However, its expression was significantly higher in ARMS than ERMS. Moreover, the increase MYCN expression was associated with worse prognosis only in ARMS, not ERMS. Our ChIP-seq data showed a binding site located at 96.8 kb downstream of transcription start that could serve PAX3-FKHR dependent enhancer (Table 1). Further, protein analysis in a panel of RMS cell lines showed highest levels of MYCN only in PAX3-FKHR fusion positive RMS cells (Fig. 5C) and it is down-regulated with a shRNA targeting PAX3-FKHR (Fig. 5D), suggesting a role of PAX3-FKHR in the expression of MYCN in RMS.
Figure 5.
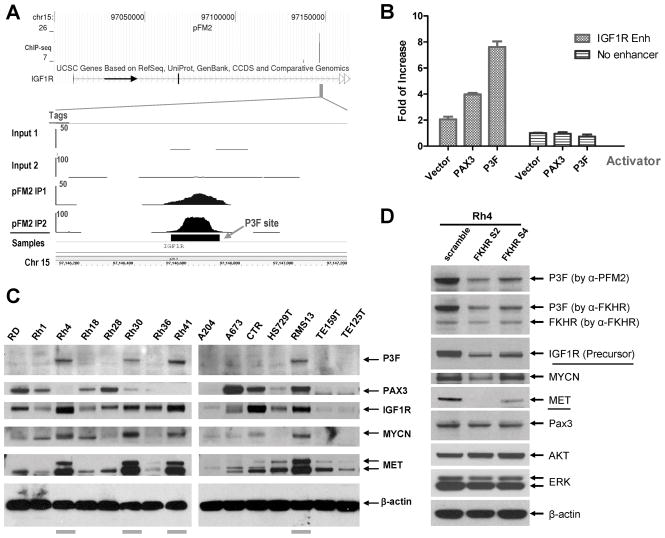
IGF1R as a target for PAX3-FKHR transactivation. A, identification of a PAX3-FKHR binding site in the 2nd intron of IGF1R. ChIP-seq results of input and PFM2 IP DNA from two experiments are shown. B, PAX3 and PAX3-FKHR-dependent activation of the IGF1R enhancer in co-transfected RD cells. C, immunoblots of IGF1R, MYCN, MET, and PAX3-FKHR in a panel of RMS cell lines. D, shRNA lentiviral vectors against PAX3-FKHR results in the down-regulation of IGF1R, MYCN, and MET in Rh4.
Although originally identified in translocations in lymphoma, activating mutations in ALK was also recently associated with neuroblastoma (34–37). Our ChIP-seq data again showed a strong PAX3-FKHR site in the 3rd intron of ALK, which was a very potent PAX3-FKHR dependent enhancer (Table 1). Infecting Rh4 cells with a lentivirus containing a shRNA (s2) for PAX3-FKHR led to significant reduction of ALK mRNA (Fig. 4D). Thus, ALK expression can also be regulated by PAX3-FKHR.
PAX3-FKHR directly up-regulates IGF1R that may serve as a biomarker for anti-IGF1R therapy
PAX3 is known to be important for the proliferation and survival of muscle progenitor cells (38, 39). While it is not known how PAX3 accomplishes these functions, it is possible that PAX3-FKHR targets genes important for growth and survival, and for the development of RMS. Ingenuity Pathway analysis of PAX3-FKHR targets also revealed IGF1R in the developmental disorder network (Supplementary Fig. 5). The IGF1R has a PAX3-FKHR binding site in the second intron (Fig. 5A). When cloned into the enhancer reporter, it exhibited specific enhancer activity that was dependent on either PAX3 or PAX3-FKHR (Fig. 5B). The analysis of IGF1R protein levels in RMS cell lines showed an apparent correlation with PAX3-FKHR fusion protein, in which all four cell lines with the fusion protein had high levels of IGF1R (Fig. 5C). To demonstrate the requirement of PAX3-FKHR for elevated IGF1R expression, Rh4 cells were infected with two shRNA lentiviral vectors targeting PAX3-FKHR. The results showed that in addition to down-regulating PAX3-FKHR, these RNAi vectors also reduced IGF1R protein level, similar to that of MET (Fig. 5D), whereas the levels of ERK and AKT are constant. The results indicate that PAX3-FKHR can directly up-regulate IGF1R.
Discussion
In this study, we present a high resolution whole genome map of PAX3-FKHR binding sites in ARMS. To our knowledge, this is the first such map for a PAX protein. Understanding of how PAX3-FKHR acts to drive ARMS tumorigenesis depends on developing a comprehensive catalog of the genes which are directly regulated by this aberrant oncogenic transcription factor. Previous expression array analysis on PAX3-FKHR regulated genes provided the information on the genes that were differentially expressed (40–42). However, that approach has significant limitations due to its inability to identify direct targets and to link the genes with specific cis-acting sequences for follow up investigations. Promoter analysis, while used frequently for the study of putative PAX3-FKHR regulated genes, is entirely constrained to sequences immediately adjacent to the transcription start. Development studies of PAX3 targets reveals that PAX3 regulates myogenic initiating genes via distal enhancers (15–18, 31). While our PAX3-FKHR binding site results show concise binding on the PAX3-dependent enhancers of MYF5 and MYOD, which were identified with transgenic animal models, our data failed to provide support for nearly all previous reports on the characterization of PAX3-FKHR-mediate gene regulation as they mostly focused on limited promoter sequences (43–45). In one instance, we show two intronic enhancers for MET, not at the previously described MET promoter. PAX3 motifs are rather degenerate and our data shows that only about 1 in 1000 of predicted PAX3 motifs in the genome were actually bound by PAX3-FKHR (Supplementary Table 5). We believe that the traditional plasmid-based report assay is necessary but not sufficient to establish the roles of the regulatory sequences and the binding of PAX3-FKHR to these sequences in vivo is essentially. Overall, in our genome-wide analysis, only 0.4% of the binding sites are within 1 kb upstream of the transcription start. The binding sites are conserved distal elements, enriched for PAX3 motifs, present in genes implicated in the developmental processes, and associated with genes induced by PAX3-FKHR in RMS cells and tumors. Our whole genome map of PAX3-FKHR binding sites nominates numerous candidates for a role in the transforming activity of ARMS, and strongly suggests that the action of this fusion oncogene is effected through a network of pathways affecting myoblast differentiation, migration, proliferation and survival (Table 1). Thus, the map of PAX3-FKHR binding sites should provide the framework for future studies of its downstream targets and pathogenesis mechanism.
Our study also provides a genomic overview in understanding PAX3 or PAX3-FKHR-mediated gene regulation. It was previously established that MYF5 (E-box protein) and PAX3 were genetically required for proper MYOD expression (28). Our genome-wide analysis of PAX3-FKHR sites demonstrates co-localization of PAX3 and E-box motifs at these sites, suggesting potential co-regulation of many target genes by PAX3 and MYF-like E-box proteins. The roles of such co-regulation in development and cancer are questions for future investigations.
The mechanism of PAX3-FKHR action in ARMS can also be viewed as a form of aberrant muscle development. Sustained expression of other developmental transcription factors as a result of PAX3-FKHR is a likely to be an important aspect of ARMS pathogenesis. In addition to the myogenic E-box factors, we also identified a number of homeobox genes as PAX3-FKHR targets. Our data showed that PAX3-FKHR binds to the enhancers of MEOX1, MEOX2 and PRRX1 and is responsible for their expression. Mesenchyme homeobox genes 1 and 2 (MEOX1, MEOX2) are expressed in somites that give rise to connective tissue, cartilage, muscle and axial skeleton. Both genes were implicated in axial skeleton and limb muscle development (46). PAX3 and MEOX2 were co-expressed in most migrating myoblasts and MEOX2 was absent from limbs in homozygous Pax3Sp mice (47). Similarly, PRRX1 is implicated in limb development. Mice homozygous for mutant Prrx1 died soon after birth and exhibited defects of skeletogenesis (48). PRRX1 was expressed at a significantly reduced level in homozygous Pax3Sp mice (49). Thus, our work identified new developmental transcription factors driven by PAX3-FKHR in ARMS (and likely by PAX3 in normal development). Determining which of these transcriptional regulators are important in establishing the ARMS phenotype and how they act are important questions for future research.
Identification of the genes and the pathways directly regulated by PAX3-FKHR provides an opportunity to better understand the molecular pathogenesis of ARMS, to mine new therapeutic targets, and to provide biomarkers for the development of novel agents. In this connection, our study identifies ALK, FGFR4, IGF1R and MYCN as direct targets for PAX3-FKHR that may have important roles in RMS pathogenesis. Previous studies showed that MYCN amplification and over-expression was associated with adverse prognosis in ARMS (33) and it was downstream of PAX3-FKHR (50). Our data identifies a PAX3-FKHR-dependent regulatory sequence in MYCN. In addition, our data indicates ALK as a target of PAX3-FKHR in RMS, opening the possibility of follow up investigations on roles of ALK in RMS. Finally, the comprehensive map of PAX3-FKHR targets provided by this study will facilitate the search for therapeutic approaches for RMS. While it is currently not possible to target the PAX3-FKHR itself, selectively targeting one or more critical proteins regulated by PAX3-FKHR such as IGF1R or FGFR4 may improve the outcome of patients with this aggressive cancer. Our results provide the first evidence on the regulation of FGFR4 by PAX3-FKHR in ARMS that was recently suggested to contribute to in vivo tumor growth and lung metastasis (19). Our data further demonstrates that PAX3-FKHR regulates IGF1R expression in RMS. We previously showed that its elevated expression was directly correlated with RMS sensitivities to a therapeutic antibody against IGF1R, and inhibiting IGF1R resulted in profound reduction of AKT signaling both in vitro and in vivo (20). Thus, the systematic identification of PAX3-FKHR targets provides a blueprint for the evaluation of targeted therapeutics against signaling pathways driven by the essential causative genetic aberration in this tumor.
Supplementary Material
Table 1B.
Verification of PAX3-FKHR Binding Site via Enhancer Assay
| Fold Induciton by | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Enh No. | Binding Intensity | None | PAX3 | PAX3- FKHR | Rel. Location | Dist. Tx Start bp | Dist. Tx End bp |
| Vector | None | NA | 1.0 | 1.0 | 0.9 | NA | NA | NA |
| ALK | 1 | high | 2.6 | 10.8 | 19.5 | 3rd Intron | −262,835 | 464,601 |
| FGFR4 | 1 | low | 1.5 | 1.9 | 3.7 | 3' downstream | −8,913 | −839 |
| FGFR4 | 2 | high | 1.9 | 5.7 | 8.3 | 3' downstream | −16,273 | −8,199 |
| IGF1R | 1 | Med | 1.8 | 4.0 | 4.8 | 2nd Intron | −136,375 | 171,486 |
| MET | 1 | Low | 3.9 | 10.2 | 7.4 | 3rd Intron | −35,329 | 61,711 |
| MET | 2 | High | 1.3 | 2.5 | 3.8 | 18th Intron | −83,277 | 13,763 |
| MYCN | 1 | Med | 0.9 | 3.8 | 5.4 | 3' downstream | −96,819 | −92,786 |
| MEOX1 | 1 | Med | 1.2 | 2.4 | 5.4 | 5' upstream | 994 | 20,274 |
| MEOX2 | 1 | High | 0.8 | 0.8 | 3.8 | 1 intron | −798 | 73,218 |
| PRRX1 | 1 | Med | 0.6 | 2.1 | 18.2 | 1st intron | −4,226 | 67,742 |
Acknowledgments
We thank Drs. Fred Barr and Michael Anderson for PAX3 and PAX3-FKHR plasmids, Andrew Smith for assistance with DME analysis, Zhigang Kang, and Sean Davis for technical assistance.
Grant support
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. This project was also funded in part with federal funds from NIH, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Blake JA, Thomas M, Thompson JA, White R, Ziman M. Perplexing Pax: from puzzle to paradigm. Dev Dyn. 2008;23710:2791–803. doi: 10.1002/dvdy.21711. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–73. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 3.Robson EJ, He SJ, Eccles MR. A PANorama of PAX genes in cancer and development. Nat Rev Cancer. 2006;61:52–62. doi: 10.1038/nrc1778. [DOI] [PubMed] [Google Scholar]
- 4.Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;731:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol. 2007;3306–7:530–3. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;2040:5736–46. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- 7.Kramer S, Meadows AT, Jarrett P, Evans AE. Incidence of childhood cancer: experience of a decade in a population-based registry. J Natl Cancer Inst. 1983;701:49–55. [PubMed] [Google Scholar]
- 8.Galili N, Davis RJ, Fredericks WJ, et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;53:230–5. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 9.Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;32:113–7. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- 10.Fredericks WJ, Galili N, Mukhopadhyay S, et al. The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol. 1995;153:1522–35. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennicelli JL, Advani S, Schafer BW, Barr FG. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene. 1999;1830:4348–56. doi: 10.1038/sj.onc.1202812. [DOI] [PubMed] [Google Scholar]
- 12.Relaix F, Polimeni M, Rocancourt D, Ponzetto C, Schafer BW, Buckingham M. The transcriptional activator PAX3-FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes Dev. 2003;1723:2950–65. doi: 10.1101/gad.281203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller C, Arenkiel BR, Coffin CM, El-Bardeesy N, DePinho RA, Capecchi MR. Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev. 2004;1821:2614–26. doi: 10.1101/gad.1244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorensen PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children's oncology group. J Clin Oncol. 2002;2011:2672–9. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 15.Goldhamer DJ, Faerman A, Shani M, Emerson CP., Jr Regulatory elements that control the lineage-specific expression of myoD. Science. 1992;2565056:538–42. doi: 10.1126/science.1315077. [DOI] [PubMed] [Google Scholar]
- 16.Goldhamer DJ, Brunk BP, Faerman A, King A, Shani M, Emerson CP., Jr Embryonic activation of the myoD gene is regulated by a highly conserved distal control element. Development. 1995;1213:637–49. doi: 10.1242/dev.121.3.637. [DOI] [PubMed] [Google Scholar]
- 17.Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;2017:2450–64. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadchouel J, Tajbakhsh S, Primig M, et al. Modular long-range regulation of Myf5 reveals unexpected heterogeneity between skeletal muscles in the mouse embryo. Development. 2000;12720:4455–67. doi: 10.1242/dev.127.20.4455. [DOI] [PubMed] [Google Scholar]
- 19.Taylor JGt, Cheuk AT, Tsang PS, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. 2009;11911:3395–407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao L, Yu Y, Darko I, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;6819:8039–48. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;3811:1289–97. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 22.Wei CL, Wu Q, Vega VB, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;1241:207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Buchberger A, Freitag D, Arnold HH. A homeo-paired domain-binding motif directs Myf5 expression in progenitor cells of limb muscle. Development. 2007;1346:1171–80. doi: 10.1242/dev.02798. [DOI] [PubMed] [Google Scholar]
- 24.Davicioni E, Finckenstein FG, Shahbazian V, Buckley JD, Triche TJ, Anderson MJ. Identification of a PAX-FKHR gene expression signature that defines molecular classes and determines the prognosis of alveolar rhabdomyosarcomas. Cancer Res. 2006;6614:6936–46. doi: 10.1158/0008-5472.CAN-05-4578. [DOI] [PubMed] [Google Scholar]
- 25.Davicioni E, Anderson MJ, Finckenstein FG, et al. Molecular classification of rhabdomyosarcoma--genotypic and phenotypic determinants of diagnosis: a report from the Children's Oncology Group. Am J Pathol. 2009;1742:550–64. doi: 10.2353/ajpath.2009.080631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tapscott SJ, Weintraub H. MyoD and the regulation of myogenesis by helix-loop-helix proteins. J Clin Invest. 1991;874:1133–8. doi: 10.1172/JCI115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucharczuk KL, Love CM, Dougherty NM, Goldhamer DJ. Fine-scale transgenic mapping of the MyoD core enhancer: MyoD is regulated by distinct but overlapping mechanisms in myotomal and non-myotomal muscle lineages. Development. 1999;1269:1957–65. doi: 10.1242/dev.126.9.1957. [DOI] [PubMed] [Google Scholar]
- 28.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;891:127–38. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 29.Smith AD, Sumazin P, Zhang MQ. Identifying tissue-selective transcription factor binding sites in vertebrate promoters. Proc Natl Acad Sci U S A. 2005;1025:1560–5. doi: 10.1073/pnas.0406123102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;165:525–32. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Lagha M, Kormish JD, Rocancourt D, et al. Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program. Genes Dev. 2008;2213:1828–37. doi: 10.1101/gad.477908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein JA, Shapiro DN, Cheng J, Lam PY, Maas RL. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci U S A. 1996;939:4213–8. doi: 10.1073/pnas.93.9.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson D, Lu YJ, Gordon T, et al. Relationship between MYCN copy number and expression in rhabdomyosarcomas and correlation with adverse prognosis in the alveolar subtype. J Clin Oncol. 2005;234:880–8. doi: 10.1200/JCO.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;4557215:971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 35.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;4557215:975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janoueix-Lerosey I, Lequin D, Brugieres L, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;4557215:967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 37.Mosse YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;4557215:930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Relaix F, Montarras D, Zaffran S, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;1721:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;4357044:948–53. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 40.Khan J, Bittner ML, Saal LH, et al. cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci U S A. 1999;9623:13264–9. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber TD, Barber MC, Tomescu O, Barr FG, Ruben S, Friedman TB. Identification of target genes regulated by PAX3 and PAX3-FKHR in embryogenesis and alveolar rhabdomyosarcoma. Genomics. 2002;793:278–84. doi: 10.1006/geno.2002.6703. [DOI] [PubMed] [Google Scholar]
- 42.Begum S, Emami N, Cheung A, Wilkins O, Der S, Hamel PA. Cell-type-specific regulation of distinct sets of gene targets by Pax3 and Pax3/FKHR. Oncogene. 2005;2411:1860–72. doi: 10.1038/sj.onc.1208315. [DOI] [PubMed] [Google Scholar]
- 43.Epstein JA, Song B, Lakkis M, Wang C. Tumor-specific PAX3-FKHR transcription factor, but not PAX3, activates the platelet-derived growth factor alpha receptor. Mol Cell Biol. 1998;187:4118–30. doi: 10.1128/mcb.18.7.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarnowski M, Grymula K, Reca R, et al. Regulation of expression of stromal-derived factor-1 receptors: CXCR4 and CXCR7 in human rhabdomyosarcomas. Mol Cancer Res. 81:1–14. doi: 10.1158/1541-7786.MCR-09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Wang C. Identification of a new class of PAX3-FKHR target promoters: a role of the Pax3 paired box DNA binding domain. Oncogene. 2007;2611:1595–605. doi: 10.1038/sj.onc.1209958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Candia AF, Hu J, Crosby J, et al. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;1164:1123–36. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- 47.Mankoo BS, Collins NS, Ashby P, et al. Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature. 1999;4006739:69–73. doi: 10.1038/21892. [DOI] [PubMed] [Google Scholar]
- 48.Martin JF, Bradley A, Olson EN. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 1995;910:1237–49. doi: 10.1101/gad.9.10.1237. [DOI] [PubMed] [Google Scholar]
- 49.Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997;1242:505–14. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- 50.Mercado GE, Xia SJ, Zhang C, et al. Identification of PAX3-FKHR-regulated genes differentially expressed between alveolar and embryonal rhabdomyosarcoma: focus on MYCN as a biologically relevant target. Genes Chromosomes Cancer. 2008;476:510–20. doi: 10.1002/gcc.20554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.