Summary
Toxoplasma gondii is a widespread protozoan parasite that causes water and foodborne infections in humans. The parasite infects intestinal enterocytes but also spreads by migration across the epithelial layer and entry into the submucosa. Within the lamina propria, innate immune responses lead to initial parasite control, although the infection disseminates widely and persists chronically despite adaptive immunity. Inflammatory monocytes exit the bone marrow and home to the lamina propria where they express antimicrobial effector functions that control infection. Ablation of the signals for recruitment of inflammatory monocytes in the mouse results in uncontrolled parasite replication, extensive infiltration of neutrophils, intestinal necrosis and rapid death. Inflammatory monocytes play a pivotal role in mucosal immunity against T. gondii, and likely other enteric pathogens.
Introduction
Toxoplasma gondii has become a model for studying innate and adaptive immunity in rodents due to the robust type I immune response that is induced upon primary infection [1]. Control of both acute and chronic infection depends on production of IL-12 that drives induction of IFN-γ, which activates effector mechanisms in both hematopoietic and nonhematopoietic cells [2–4]. Macrophages [5], neutrophils [6,7], and dendritic cells (DCs) [8,9] have been shown to produce IL-12 in response to parasites or antigen, although in vivo studies indicate that DCs may be the most source of this cytokine during infection [10]. IL-12 induction largely depends on signaling through the common adaptor Myd88 [11,12]. The dominant receptor thus far identified for triggering this pathway is TLR-11, which recognizes profilin in the mouse [13], although this pathway is absent in humans. Alternative means of activating DC cells include detection of a secretory cyclophillin by CCR5 on DC cells [14]. In response to IL-12, IFN-γ is produced by NK, NKT, and both CD4 and CD8 T-lymphocytes, which collectively activate effector mechanisms in a variety of cell types [1].
Although a myriad of in vitro studies have elucidated many of the cellular mechanisms capable of parasite control, defining the role of such specific responses in vivo is much more difficult. Often localized responses in either specialized resident cells, or newly recruited cells are paramount to the control of infection. These responses may not be easily mimicked by purely in vitro models. Newer methods for generating lineage-specific gene knockouts or knockins, combined with in vivo systems for evaluating pathogen control have opened a new window on these questions. Here we discuss new findings on the cellular control of T. gondii in the mouse model that reveal an unexpected role for inflammatory monocytes in mucosal immunity following the natural oral route of infection.
Infection and transmission
Toxoplasma gondii is a widespread protozoan parasite that infects virtually all types of warm-blooded vertebrates. The infection is transmitted by cats, where sexual developmental stages replicate in intestinal epithelial cells leading to shedding of oocysts in the feces, thus contaminating soil and water [15]. Ingestion of oocysts results in infection in a wide range of intermediate hosts, which support asexual growth of the parasite that rapidly disseminates throughout the body [15]. The primary infection is characterized by fast growing tachyzoites, which infect a wide range of nucleated cells in a rapidly lytic cycle. In response to ensuing innate and adaptive immune responses, the parasite differentiates into a slow-growing tissue stage referred to as bradyzoites, which reside in tissue cysts within long-lived cells. Transmission between intermediate hosts is facilitated by the ability of tissue cysts to cause oral infection upon ingestion by other intermediate hosts, a trait that distinguishes T. gondii from its closest relatives [16]. Asexual propagation has lead to a highly clonal population structure and distinct lineages present dramatically different phenotypes in terms of acute virulence in laboratory mice [17]. Humans are largely an accidental host and infections are normally subclinical, yet severe disease can occur in the immune compromised and due to congenital infection [15].
Oral model for toxoplasmosis
Following ingestion, the parasite invades enterocytes in the small intestine where it can replicate efficiently. However these cells are short lived and rapidly sloughed into the lumen; hence, systemic infection requires a mechanism to cross the epithelial barrier. Toxoplasma gondii accomplishes this by a process of active invasion, avoiding extensive disruption of the endothelium in the process [18]. The force for tissue migration is derived from a novel process of cell motility that also powers cell invasion [19]. Within the lamina propria, the parasite encounters leukocytes and can either invade these cells or enter directly into the lymphatic and circulatory systems [18]. Early in infection, CD11c+ and CD11b+ leukocytes in the gut are infected and CD11b+ cells are involved in dissemination to distant sites, including the brain [20]. Unlike some bacterial pathogens, there appears to be little involvement of Peyer’s patches and dendritic cells that express the chemokine receptor CCR6 are not required for control of the infection [21]. Within the gut, important contributions are made to innate immunity by epithelial cells, NK and NKT cells, and intraepithelial enterocytes within the lamina propria, as reviewed previously [22]. Although oral infection leads to a localized response that normal resolves without severe pathology, following high dose infection, or in models that combine susceptible mice (C57BL/6) with inflammatory parasite lineages (the type II strain ME49), a severe form of ileitis results in necrosis and death of the animal [23]. Gut pathology is driven by an overly aggressive type 1 response that ultimately leads to a breach of the intestinal barrier, triggering responses to leakage of bacterial components from the lumen [24]. This may explain evidence that TLR2, TLR4, and TLR9 are important in vivo, despite the fact that no strong parasite-derived ligands have been identified for these receptors [25].
Monocyte subsets and recruitment
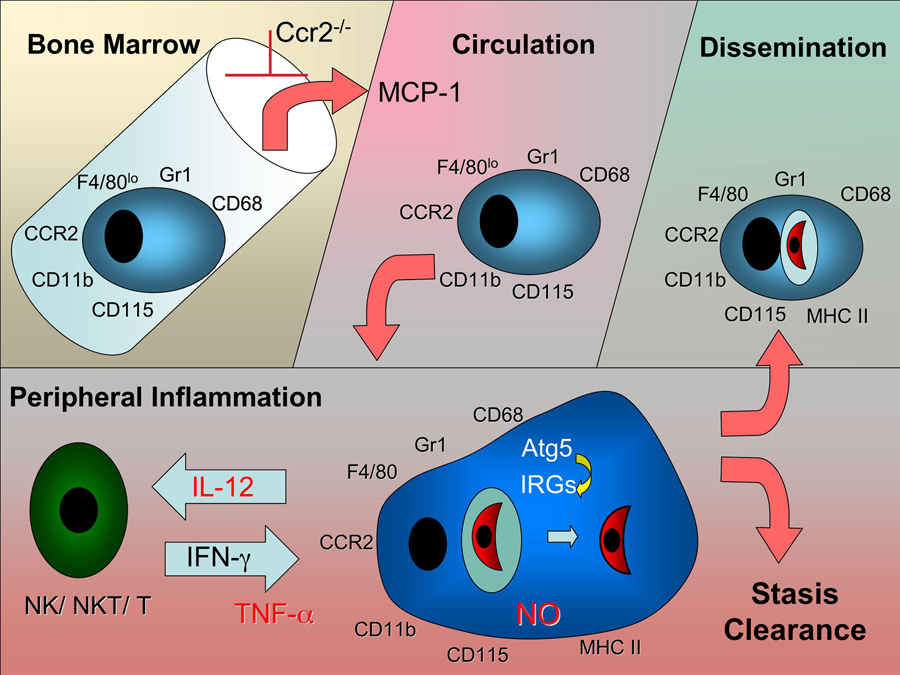
Monocytes and macrophages can adopt a wide range of phenotypes, thus allowing fine tuning of effector functions to local environments. Studies in the mouse model reveal that inflammatory monocytes exit the bone marrow, circulate in the blood, and home to sites of infection [26] (Fig. 1). These cells express the chemokine receptor CCR2 in addition to CD11b and the granulocyte marker Gr1 [27] (Fig. 1). In contrast, tissue macrophages express CX3CR1 but have low levels of CCR2 and Gr1 [27]. The granulocyte marker Gr1 was originally characterized by mAb RB6-8C5,which later turned out to recognize both Ly-6G (abundant on neutrophils but absent on monocytes) and Ly-6C (abundant on monocytes, and also expressed at lower levels on neutrophils) [28]. Ly-6C+ inflammatory monocytes have been shown to be important in the control of Listeria in the spleen, where they differentiae into TipDC cells that also express CD11c, produce TNF-α, and express inducible nitric oxide synthase (iNOS) [29]. Inflammatory monocytes home in response to monocyte chemo-attractant protein (MCP)-1 (CCL2) as well as several related chemokines. Ccr2−/− mice are unable to recruit inflammatory monocytes due to a failure of these cells to exit the bone marrow and hence these mice are extremely susceptible to listeriosis [30]. This surprising finding revealed that this chemokine-receptor pairing is needed for bone marrow exit, rather than peripheral homing, suggesting that other signals control recruitment to the site of infection. Ly-6C+ monocytes have also been implicated in spread of Listeria to the brain [31], mucosal responses to Salmonella in the mouse [32], control of rodent malarias in the spleen [33], and development of experimental autoimmune encephalomyelitis [34].
Figure 1.
Trafficking of inflammatory monocytes during infection with T. gondii. Inflammatory monocytes express Gr1 and a variety of monocyte lineage markers including CD11b, F4/80, and CD68 [21]. They originate from myeloid precursors in the bone marrow and exit from this site in response to circulating MCP-1 and related chemokines produced in response to inflammation [56]. Exit from the bone marrow is blocked in Ccr2−/− mice, which still upregulate the number of Gr1+ monocytes in response to infection [30]. Inflammatory monocytes circulate in the blood and home to sites of inflammation where they upregulate MHC II ,and secrete IL-12 and TNF-α [21]. Production of NO by inflammatory monocytes blocks replication of intracellular T. gondii [46]. In response to IFN-γ, or T cells, moncytes upregulate immunity related GTPases (IRGs), which are recruited to the parasite-containing vacuole in a Atg5-dependent manner, resulting in clearance [47]. Inflammatory monocytes may also contribute to control of infection following dissemination.
Inflammatory monocytes control toxoplasmosis in mice
Needle inoculation of low virulence isolates of type II strains of T. gondii into the peritoneal cavity of mice results in rapid recruitment of inflammatory monocytes [35]. Infection with the highly virulent type I RH strain induces recruitment of similar inflammatory monocytes, as well as high numbers of neutrophils [35]. Monocyte recruitment relies in induction of MCP-1 and to requires the chemokine receptor CCR2; in their absence mice rapidly succumbed to nonlethal challenge with a normally avirulent strain of T. gondii [36]. Although this model bypasses the natural oral route of infection, it nonetheless provides ready access to inflammatory monocytes elicited by infection and hence has been useful to define the phenotype of these cells. Monocytes elicited by T. gondii infection express CD11b, F4/80, CD68, CD115, and Gr1 but remain CD11c negative [35]. These cells are equipped with several effector mechanisms including production of IL-12, secretion of TNF-α, upregulation of MHC class II, and induction of iNOS expression [35]. When harvested and plated in vitro, inflammatory monocytes are also able to restrict the growth of T. gondii, in part through production of nitric oxide (NO) [35]. Hence, they likely play an important role in local control of parasite proliferation. Consistent with this, parasite levels in the peritoneum and other tissues rise uncontrolled in Mcp-1−/− or Ccr2−/− mice compared to wild type mice [36]. Remarkably, this lack of parasite control exists despite normal levels of systemic IL-12 and IFN-γ. Thus, deficiency in inflammatory monocytes results in lack of control of parasite replication rather than impacting the induction of Th1 cytokines. This contrasts to the situation with ablation of dendritic cells using diptheria toxin under the control of the CD11c promoter, which results in lower levels of IL-12 and hence reduced IFN-γ responses [10].
Oral challenge of Mcp-1−/− or Ccr2−/− mice with tissue cysts bearing bradyzoites of T. gondii also reveals a profound defect in control of infection [21]. In this case, Gr1+ monocytes are recruited to the lamina propria of the small intestine villi, the site of primary parasite infection [21]. Within the vllius, Gr1+ monocytes lie directly beneath the basement membrane where they encounter invading parasites that cross the epithelial layer. Gr1+ monocytes in the gut express iNOS, secrete IL-12, and produce TNF-α, mediators that have been implicated in control of infection (Fig. 1). In their absence, parasite numbers are unrestricted, resulting in extensive tissue damage, necrosis, and rapid death [21]. In the gut, inflammatory monocytes express CD11b, F4/80 but fail to express CD11c, distinguishing them from the above mentioned TipDC cells that are important in Listeria infection. In contrast to the situation with wild type mice where neutrophil infux is minimal, large numbers of neutrophils are recruited to sites of active parasite proliferation in the absence of CCR2 [21]. Similar to the situation with Listeria, inflammatory monocytes fail to exit the bone marrow in Ccr2−/− mice, although their numbers are greatly upregulated following infection (Fig. 1)[21]. Adoptive transfer of Gr1+ monocytes obtained from either the bone marrow or the peritoneal cavity of mice separately challenged i.p. with T. gondi, results in protection of Ccr2−/− mice [21]. Remarkably, Gr1+ monocytes isolated from Ccr2−/− mice also home to the site of infection and are protective when adoptively transferred [21], again indicating a second signal is required for peripheral homing.
Other studies on the role of monocytes following oral challenge of T. gondii in Ccr2−/− mice found less impact of infection on intestinal pathology, presumably due to a lower virulence challenge that leading less severe intestinal symptoms [37]. However, mice that survived past the initial phase of infection went on to develop more severe infection in the central nervous system (CNS) that lead to death within ~ 30 days [37]. Increased susceptibility in these mice was not associated with decreased type 1 cytokine induction, but with failure to upregulate iNOS expression in the CNS [37], suggesting a role for inflammatory monocytes in systemic protecting against infection (Fig. 1). Collectively, these studies reveal an essential role of Gr1+ monocytes in protecting against microbial infection in the mucosa and also in peripheral organs including immunological privileged sites such as the CNS.
Mechanisms of macrophage control
Although a variety of cytokines contribute to control of toxoplasmosis [1], the pivotal role of IFN-γ stems from its ability to activate a number of downstream effector mechanisms that control stasis and killing of intracellular parasites. Induction of reactive oxygen intermediates, NO production, and degradation of nutrients have been implicated in control of T. gondii following IFN-γ activation of cells [1]. Responsiveness to IFN-γ is of critical importance within monocytes/macrophages as shown by lineage specific expression of a dominant negative receptor that blocks IFN-γ signaling [38]. Recent studies indicate that in the murine system, the primary mechanism of control is the immunity related GTPases (IRGs) that are strongly upregulated in response to IFN-γ [39]. The IRG protein family is highly expanded in the mouse, but is more limited in other vertebrates and largely absent in humans [40]. In the murine system, IRGs are essential for control of a variety of intracellular pathogens including T. gondii [39]. IRGs cycle between GDP-bound non-active forms and GTP-bound forms that oligomerize and are loaded onto pathogen-containing vacuoles [41]. This mechanism overcomes the fact that the parasite resides in a non-fusogenic vacuole that normally lies outside the endosomal-lysosomal system and that fails to recruit host proteins [19]. By a process that is not well understood, IRG proteins strip the vacuole membrane and destroy the parasite with in the cytosol [42–44]. This extremely efficient mechanism is responsible for removal of a majority of avirulent parasites, although virulent lineages are remarkably resistant to clearance [45,46]. In activated macrophages, homeostasis of IRGs depends on the autophagy protein Atg5 [47]. In the absence of Atg5, IRGs form aggregates that are not recruited to the PV, leading to failure to clear intracellular parasites and susceptibility to infection in vivo [47]. The precise molecular mechanism controlled by Atg5 is uncertain, however this pathway does not appear to involved classical macro-autophagy. Direct digestion of parasite antigens within the cytosol could provide an explanation for the potent TAP-dependent presentation of antigens via MHC class I [1].
Role for neutrophils in pathology
A number of studies have documented the recruitment of neutrophils following infection with T. gondii, however these responses are typically encountered with high doses of parasites [48], using needle inoculation into sites that are not the normal route of infection [49], or using highly virulent lineages that rapidly lead to death and which are not effectively controlled by the immune system [50,51]. In contrast, nonlethal challenge by the natural oral route leads to little influx of neutrophils into the lamina propria of the small intestine, which is the primary site of initial infection [21]. Furthermore, despite evidence that neutrophils produce IL-12 in response to T gondii [6,7], disruption of their recruitment in CXCR2−/− mice leads to reduced IFN-γ and TNF-αlevels, but only mild susceptibility to acute infection in mice [52]. Hence, there has been some controversy about whether neutrophil recruitment is beneficial or detrimental to the host. Initial attempts to address this question utilized ablation treatments with mAb RB6-8C5 [53]; however, as mentioned above this antibody reacts with both Ly-6C and Ly-6G and hence depletes both neutrophils and monocytes [28]. In contrast, treatment with the Ly-6G specific mAB 1A8 selectively ablates neutrophils in vivo [28]. Treatment of mice with mAb 1A8 revealed that selective depletion of neutrophils has little effect in survival against T. gondii, while ablation of neutrophils and monocytes has a profound effect [54]. This phenotype mimics that of Ccr2−/− mice where the lack of monocyte recruitment results in lack of parasite control. Importantly, in the absence of inflammatory monocytes the ensuing pathology is associated with high levels of neutrophil influx to the small intestine [54]. Hence, during primary response to T. gondii infection, inflammatory monocytes are essential for control while neutrophils are largely involved in adverse pathological events [21,54].
Conclusions and Future Directions
Inflammatory monocytes are recruited in response to T. gondii infection in the gut and there they express antimicrobial activities important in control of infection. These include a potent system for direct destruction of the intracellular vacuole via recruitment of IRG proteins. Although the depletion of inflammatory monocytes in Ccr2−/− mice is consistent with a critical role of monocytes, these mice are also deficient in recruitment of mature T cells, which can also contribute to mucosal pathology [55]. Hence, lineage specific depletion of CCR2 would be extremely beneficial for separating these distinct roles. Myeloid depletion of Atg5 has shown a role for this protein in recruitment of IRGs to the parasite-containing vacuole, yet this has not been tested during the natural oral route of infection, nor have other Atg gene products been examined. The process whereby Atg5 influences recruitment of IRGs has also not been defined, although it almost certainly involves a non-autophagy pathway. Finally the role of Ly-6C+ monocytes in dissemination to distant cites such as the brain, or the possibility that they contribute to heightened surveillance in the gut during chronic infection, have yet to be addressed. Toxoplasmosis in the murine model offers an excellent opportunity to define the role of these processes that are likely also important for control of other enteric infections.
Acknowledgements
We are grateful to Oliver Liesenfeld and Eric Pamer for helpful discussions. Supported by NIH grants AI059176 and AI036629 (to L.D.S.) and the German DFG (to I.R.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics in publishing: general statement
The authors comply with the Ethics in Publishing.
Conflicts of interest
The authors have no conflicts of interest.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiol. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 2.Gazzinelli RT, Heiny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient mice. Proc. Nat. Acad. Sci. (USA) 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 4.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-g- and tumor necrosis factor (TNF)-a- dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 1999;189:1083–1091. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 6.Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- 7.Bliss SK, Zhang Y, Denkers EY. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J Immunol. 1999;163:2081–2088. [PubMed] [Google Scholar]
- 8.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sousa CR, Yap G, Schulz O, Rogers N, Schito M, Aliberti J, Hieny S, Sher A. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–647. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J. Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Kim L, Butcher BA, Lee CW, Uematsu S, Akira S, Denkers EY. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J. Immunol. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 12.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: My D88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 13.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 14.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nature Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 15.Dubey JP. Toxoplasmosis of animals and humans. Boca Raton: CRC Press; 2010. [Google Scholar]
- 16.Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- 17.Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: Clonal expansion driven by infrequent recombination and selective sweeps. Ann. Rev. Microbiol. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 18.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 2002;195:1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 20.Courret N, Darche S, Songio P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c and CD11b expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2005;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunay IR, DaMatta RA, Fux B, Presti R, Greco A, Colonna M, Sibley LD. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. Although macrophages were previously thought to play a role in resistance in vivo, their involvement in mucosal responses was underappreciated until this work demonstrated their importance in control of acute toxoplasmosis in the mouse.
- 22.Buzoni-Gatel D, Schulthess J, Menard LC, Kasper LH. Mucosal defenses against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell. Microbiol. 2006;8:535–544. doi: 10.1111/j.1462-5822.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 23.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J. Infect. Dis. 2002;185:S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 24.Heimesaat MM, Berewill S, Fischer D, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 25.Denkers EY. Toll-like receptor initiated host defense against Toxoplasma gondii. J. Biomed. Biotechnol. 2010;2010:737125. doi: 10.1155/2010/737125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. Provides a comprehensive overview of monocyte lineages and their phenotypes in the mouse.
- 27.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principle subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 28. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leuk. Biol. 2007;83:64–70. doi: 10.1189/jlb.0407247. Previous studies using mAb RB6 to deplete neutrophils have been complicated by the unintended depletion of inflammatory monocytes. This study resolves this problem using a new mAb to Ly-6G that selectively depletes neutrophils.
- 29.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 30.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 31.Drevets DA, Dillon MJ, Schawang JS, Rooijen NV, Ehrchen J, Sunderkotter C, Leenen PJM. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into brain during systemic infection of mice. J. Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 32.Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lyphoid tissue during oral Salmonella infection. J. Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 33.Sponaas AM, Freitas do Rosario AP, Voisine C, Mastelic B, Thompson J, Koernig S, Jarra W, Renia L, Mauduit M, Potocnik AJ, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- 34.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mordue DG, Sibley LD. A novel population of Gr-1+ activated macrophages induced during acute toxoplasmosis. J. Leukoc. Biol. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 36.Robben PR, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp. Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benevides L, Milanezi CM, Yamauchi LM, Silva JS, Silva NM. CCR2 receptor is essential to activate microbicdal mechanisms to control Toxoplasma gondii infection in the central nervous system. Am. J. Path. 2008;173:741–751. doi: 10.2353/ajpath.2008.080129. Using a less virulent challenge model this study provides evidence that Gr1+ monocytes also participate in defense against toxoplasmosis in the CNS.
- 38. Lykens JE, Terrell CE, Zoller EE, Divanovic S, Trompette A, Karp CL, Aliberti J, Flick MJ, Jordan MB. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol. 2010;184:877–885. doi: 10.4049/jimmunol.0902346. Consistent with an important role for macrophages in controlling infection in vivo, this report demonstrates that IFN-γ signaling in macrophages is critical to control T. gondii in the mouse.
- 39.Taylor GA, Feng CG, Sher A. Control of IFN-γ mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microb. Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, Glowalla E, Leptin M, Howard JC. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, Jaiser F, Zerrahn J, Martens S, Howard JC. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J. 2008;27:2495–2509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJP, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. Plos Pathogens. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFN-gamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. This work expands on previous studies that implicated IRGs in clearance of intracellular parasites by defining the kinetics of recruitment, vacuole dissolution, and pathogen destruction using cell biological approaches in vitro.
- 45.Khaminets A, Hunn JP, Konen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong YC, Boothroyd JC, Reichmann G, et al. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Ferguson DJ, Wilson DC, Howard JC, Sibley LD, Yap GS. Virulent Toxoplasma gondii evade immunity-related GTPas-mediated paraiste vacuole disruption within primed macrophages. J. Immunol. 2009;182:3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. This report makes an unexpected connection between the autophagy protein Atg5 and recruitment of IRGs that are involved in clearance of intracellular T. gondii. The mechanism involves altered cellular trafficking and implies a unique non-autophagy role for Atg5 in pathogen resistance.
- 48.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 2000;165:4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 49.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gavrilescu LC, Denkers EY. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol. 2001;167:902–909. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- 51.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 52.Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 2001;167:6503–6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- 53.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect. Immun. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. Using selective depletion of neutrophils vs. combined depletion of neutrophils and monocytes, this study reveals that unlike inflammatory monocytes which are essential, neutrophils do not play a critical role in control of T. gondii in the mouse.
- 55.Egan CE, Craven MD, Leng J, Mack M, Simpson KW, Denkers EY. CCR2-dependent intraepithelial lymphocytes mediate inflammatory gut pathology during Toxoplasma gondii infection. Mucosal Immunol. 2009;2:527–535. doi: 10.1038/mi.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]