Abstract
Objective
The purpose of this study was to examine the effects of aerobic exercise training (AEXT) on dipping status in pre-hypertensive and stage-1 hypertensive individuals. A secondary purpose was to evaluate whether AEXT alters oxidative stress and endothelial biomarkers correlated to dipping status.
Methods
Twenty-three subjects underwent 24-h ambulatory blood pressure monitoring at baseline and after 6 months of AEXT. AEXT consisted of training at 70% VO2max 3 days/week for 6 months. Total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein (LDL)-cholesterol, oxidized LDL (ox-LDL), triglycerides, urinary and plasma nitric oxide end-products, superoxide dismutase and 8-iso-PGF2α were measured before and after AEXT. Statistically, ANOVA and linear regression were used.
Results
Before and after AEXT, there were no significant differences between dippers and non-dippers in any of the biomarkers except for total cholesterol following AEXT. In a sub-analysis following AEXT, 14 subjects retained their original dipping status, five subjects changed from dippers to non-dippers and four subjects changed from non-dippers to dippers. Significant differences existed between these groups in changes in total and LDL-cholesterol, ox-LDL, 8-iso-PGF2α and % Dip.
Conclusions
Changes in cholesterol levels but not oxidative stress or endothelial biomarkers were related to changes in BP variables following AEXT in dippers and non-dippers.
Keywords: Aerobic exercise, ambulatory blood pressure monitoring, dipper, non-dipper, hypertension, oxidative stress
Introduction
A large number of studies have shown 24-h ambulatory blood pressure monitoring (ABPM) to be more strongly correlated to target organ damage and cardiovascular morbidity than clinical blood pressure (BP) measurements (1–3). In healthy populations, it is common to observe a decline in BP during sleep known as the dipping phenomenon. In contrast, non-dippers are classified as people in whom the decline in nocturnal BP is attenuated or absent with BP falling less than 10% during sleep compared with awake values (4). The classification of dippers and non-dippers is of clinical importance as non-dippers may have more pronounced target-organ damage, higher incidence of cardiovascular complications and greater mortality (3–6).
The mechanisms responsible for the lack of a nocturnal decrease in BP in non-dippers are not fully understood. Endothelial impairment and oxidative stress may contribute to the non-dipping phenomenon (7). Factors associated with endothelial dysfunction include a reduction in nitric oxide (NO) production and release (8), in addition to reduced antioxidant protection (9).
A study by Ino-Oka et al. (10) found that the autonomic nervous system did not account for all cases of non-dipper hypertension. In their study, subjects wore a digital Holter ECG during their ABPM session. The frequency of a subject's cardiac R-R interval was used as an index of sympathetic and parasympathetic nerve tone. Autonomic dominant hypertension was defined as patients in which the sympathetic to parasympathetic ratio was highly correlated to BP. Hypertensives without any correlations were described as “irregular type”. The irregular-type hypertensives were all non-dippers. Thus, the mechanism underlying BP regulation in these non-dippers does not appear to be the autonomic nervous system. Oxidative stress and endothelial dysfunction have been linked to increased BP (11) and may account in part, for non-dipping hypertension that cannot be explained by autonomic regulation.
Aerobic exercise training (AEXT) reduces BP in hypertensive patients (12,13). However, limited and conflicting evidence exists regarding the effect of AEXT on dippers vs. non-dippers. Nami et al. (14) found that a 3-month AEXT program failed to reduce BP in non-dipper hypertensives. In contrast, Park et al. (15) found that non-dippers responded to acute bouts of aerobic exercise, and demonstrated reductions in nocturnal systolic BP. The investigators suggested that responses to acute aerobic exercise in non-dippers are reflective of the BP responses that would occur with chronic aerobic training.
The primary purpose of this study was to examine the effects of AEXT on dipping status in pre-hypertensive and stage-1 hypertensive individuals. A secondary purpose of the study was to evaluate whether the non-pharmacological intervention of chronic exercise alters oxidative stress and endothelial biomarkers and to determine if the changes were correlated to dipping status.
Materials and methods
The study group consisted of men and women between the ages of 50 and 75 years who were sedentary (regular aerobic exercise <2 days per week and <20 min per session), pre-hypertensive (BP> 120/80 mmHg) or stage-1 hypertensive (BP <160/90 mmHg), non-diabetic, non-smokers, and free of cardiovascular, renal, liver and lung disease. Excessive obesity could impede a subject's ability to exercise train. Thus, for inclusion in the study participants were required to have a body mass index (BMI) <37 kg/m2 and have no medical conditions in which vigorous exercise would be contraindicated. Moreover, women were required to be postmenopausal (absence of menstrual cycle for >2 years) and had to maintain their hormone replacement therapy (HRT) regime, be it on or off HRT, for the duration of the study. Participants were recruited via mailed brochures, as well as through advertisements in both local newspapers and on the radio. Upon response to either, the participants were contacted by telephone to assess their eligibility. Each participant gave written informed consent following the explanation of study protocols. The protocol was approved by the University of Maryland, College Park, Institutional Review Board.
Screening
To ensure the eligibility of all qualified participants, two screening visits were completed prior to inclusion in the study. Screening visit one consisted of blood sampling following a 12-h overnight fast to assess blood chemistries. Participants also underwent a 2-h 75-g oral glucose tolerance test to assess diabetes status. Any individual who exhibited evidence of renal (i.e. estimated creatinine clearance <60 ml/min) or liver disease, had a hematocrit <35%, or who had triglyceride levels >400 mg/dl, fasting blood glucose > 126 mg/dl, or 2-h glucose levels >200 mg/dl were excluded from the study. Screening visit two required all qualified participants to undergo a physician-administered physical examination to confirm that participants displayed no evidence of cardiovascular, pulmonary or other chronic diseases that would prohibit participation in exercise training. Following completion of the physical examination, participants performed a maximal graded treadmill test. The test was terminated when participants could no longer continue or when signs or symptoms of cardiovascular disease were manifested. Any participants who exhibited signs or symptoms of cardiovascular disease or who had >2 mV ST-segment depression during the graded exercise test were excluded from the study.
Dietary stabilization
Participants underwent dietary stabilization for 6 weeks. They were instructed by a registered dietitian on the American Heart Association (AHA) Dietary Guidelines for Healthy American Adults, a diet formerly known as the AHA Step 1 diet. This diet consisted of ~55% of total daily calories from carbohydrates, 15% from protein, and <30% from fat, with saturated fat ≤10% of total calories, sodium content ≤3–4g/day, and cholesterol intake <300 mg/day. Participants met with the dietitian two times per week at which time body weight and BP were recorded for each visit. Participants were required to remain within 5% of their study entry body weight for the duration of the study. In addition, participants who exhibited BP consistently less than 120/80 mmHg or greater than 159/99 mmHg during the stabilization period were excluded from the study. Finally, any participant taking one or two anti-hypertensive medications were tapered off of their medication pending written approval from the participant's personal physician. These participants underwent baseline testing 4 weeks after medications were stopped.
Baseline testing
Following completion of the 6-week dietary stabilization, participants underwent baseline testing, which included a second maximal graded treadmill test, 24-h urine collection, blood sampling, casual BP measurements and 24-h ABPM. The second maximal graded treadmill test was administered under the supervision of the study physician. Each test began at 70% of the peak heart rate attained during the participant's initial screening exercise test and treadmill grade increased 2% every 2 min until test termination. Electrocardiogram (ECG), BP and oxygen consumption (VO2) were measured continuously throughout the test and participants were deemed to have achieved true maximal VO2 (VO2max) based upon standard criteria (16). The objective of this second test was to determine participants' cardiovascular fitness and to develop individualized exercise prescriptions for the exercise training intervention. measurements and 24-h ABPM. The second maximal graded treadmill test was administered under the supervision of the study physician. Each test began at 70% of the peak heart rate attained during the participant's initial screening exercise test and treadmill grade increased 2% every 2 min until test termination. Electrocardiogram (ECG), BP and oxygen consumption (VO2) were measured continuously throughout the test and participants were deemed to have achieved true maximal VO2 (VO2max) based upon standard criteria ( 16). The objective of this second test was to determine participants' cardiovascular fitness and to develop individualized exercise prescriptions for the exercise training intervention.
Casual BP measurement
Casual BP was measured in all participants on 3 separate days according to the JNC VII guidelines (17). Briefly, participants were fasted for ≤ 12 h before measurement. They sat quietly in a seated position with feet flat on the floor for 15–20 min and BP was measured at least twice until systolic and diastolic values were within 4 mmHg. When these criteria were met, the average BP was recorded. The average of the three separately recorded BP values was used as the outcome variable in data analyses.
Blood sampling
In the morning following a 12-h overnight fast, blood samples for plasma NO end-products, nitrate and nitrite (NOx) and lipoprotein analyses were drawn into EDTA tubes and blood samples for superoxide dismutase (SOD) analysis were drawn into heparinized tubes. Samples were centrifuged at 3000 rpm for 20 min at 4°C, at which point they were transferred to plastic microtubes (1.5 ml) and stored at −80°C until assay.
Twenty-four-hour ambulatory BP monitoring
Participants underwent 24-h ABPM using a noninvasive monitor (SpaceLabs Medical Inc., Model 90219, Redmond, WA) beginning on the morning of each participant's typical day, with the exclusion of Friday through Sunday. BP measurements were obtained at 30-min intervals during the day (06:00–22:00 h) and 60-min intervals at night (22:00–06:00 h). Prior to each session, the monitor was calibrated against a conventional sphygmomanometer. Participants were instructed not to exercise prior to or during the 24-h ABPM period and to pause momentarily and maintain their body position during each BP measurement. Throughout the duration of the 24-h recording period, participants were required to record their activity and emotional status at the time of each BP measurement. Upon completion of the 24-h ABPM period, data was transferred from the monitor to a laboratory computer for analysis using the SpaceLabs analysis software package. Values outside of the normal physiological range were automatically edited by the analysis software (BP≥260/150 mmHg, pulse pressure > 150 mmHg and heart rate > 200 beats/min). Furthermore, BP values that differed by > 15 mmHg from any other BP readings within a 1-h time frame were manually edited if they could not be explained by changes in activity or emotional status, as noted in the participant's activity log. After AEXT, participants were given their activity log from their baseline ABPM test and instructed to emulate their baseline daily activity during the ABPM test after AEXT. All final testing was performed 24–36 h after an exercise training session in order to exclude the effects of acute aerobic exercise on BP.
Classification of dippers and non-dipper
Non-dippers were classified as those who showed a reduction in mean arterial pressure (MAP) of less than 10% between day and night (4). MAP was used in classification of dippers and non-dippers as it fully represents the perfusion pressure seen by organs. The percentage dip (% Dip) in MAP was calculated as: (daytime MAP–night-time MAP) × (100/daytime MAP). BP values between 05:00 and 23:59 h were used as the daytime values and BP values between 00:00 and 05:00 h were used as night-time values. These time intervals were selected after visual inspection of ABPM data to confirm that all participants were indeed sleeping during the 00:00 and 05:00 h night-time period (18). If the subject recorded a waking period related to BP measurement, the measure was edited from analysis. However, this was a rare occurrence (<2% of all BP values).
Twenty-four-hour urine collection
Urinary NOx were measured from a 24-h urine collection on the same day as 24-h APBM. Because NO has a short half-life and is rapidly oxidized to the stable end-products nitrate and nitrite, all obtained NOx values provide an index of systemic NO production under conditions of a controlled low nitrate diet (19,20). To eliminate the potential effect of dietary nitrate intake on urinary nitrate levels, participants were required to follow a low nitrate diet for the 2 days preceding, as well as on the day of urine collection. Subjects were instructed to exclude the following food groups: cured meats, malts, vegetables raw or cooked including potatoes, melons, fish, imported cheese and any fermented foods.
Measurement of urinary NOx
As an index of systemic NO production, urinary NOx (uNOx) levels were measured using a colorimetric assay based on the Griess reaction. Prior to conducting the assay, urine samples were centrifuged at 3000 rpm for 15 min at 4°C. The first step of the assay involved the reduction of nitrate to nitrite using nitrate reductase (Aspergilus species). Samples were then incubated with the Griess reagents, acidified sulfanilamide (1% sulfanilamide in 5% phosphoric acid) and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, which react with nitrate to produce a magenta-colored azo dye. Absorbance was read at 541 nm using an Emax Maxline Microplate Reader (Sunnyvale, CA) with comparisons made to a blank containing only the Griess reagent. Nitrite concentrations were then determined using a standard curve of known concentrations of sodium nitrite (NaNO2) with the obtained values representing the total amount of uNOx end-products (nitrate + nitrite). To control for inter-assay variability, baseline and final urine samples were assayed on the same plate in duplicate with inter-assay and intra-assay coefficients of variation (CVs) calculated to be 4 and 13%, respectively.
Measurement of plasma NOx
Plasma NOx (pNOx) levels were measured in order to have a second index of endogenous NO production (19). pNOx was measured using the colorimetric assay based on the Griess reaction described above. Prior to performing the assay, all samples were filtered (Ultrafree-MC, Millipore Corporation, Billerica, MA) and centrifuged for 50 min at 9000 rpm at 4°C to remove protein components that interfere with NOx quantification. To control for inter-assay variability, baseline and final plasma samples were assayed on the same plate in duplicate. The intra- and inter-assay CVs were 3.3 and 4.9% respectively.
Measurement of lipid levels
Plasma total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol and triglycerides levels were determined using conventional methods (21,22). Plasma ox-LDL levels were measured using a competitive ELISA (Mercodia, Uppsala, Sweden) that utilizes the murine monoclonal antibody 4E6. This antibody is directed against a conformational epitope in LDL that is generated from substitution of at least 60 lysine residues in apoB with aldehydes (23). This number corresponds to the minimal number of substituted lysines required for scavenger receptor- mediated uptake of ox-LDL. The intra-assay and inter-assay CVs were 18.9 and 15.6%, respectively.
Measurement of urinary F2-isoprostanes
Twenty-four-hour urine was collected to measure urinary 8-iso-PGF2α, a major F2-isoprostane and a reliable marker of in vivo oxidative stress (24). Urinary levels of 8-iso-PGF2α were measured as previously described (25). In brief, an antibody was raised in rabbits by immunization with 8-iso-PGF2α coupled to BSA at the carboxylic acid by the 1,1-carbonyldiimmidazole method (25). Urinary samples that were stored at −80°C and later transported to Sweden under dry ice were kept frozen at −70°C until analysis. Unextracted urine samples were used in the assay. The levels of 8-iso-PGF2α were corrected for urinary creatinine values.
Measurement of SOD activity
SOD activity was measured using a commercially available assay kit according to the manufacturer's instructions (Cayman Chemicals, Ann Arbor, MI). This assay utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of SOD enzyme needed to exhibit 50% dismutation of the superoxide radical. Baseline and final samples were assayed on the same plate and all samples were assayed in duplicate. The inter-assay and intra-assay CV were 7 and 22% respectively. All stored samples used for measurement were assayed within 1 year of collection.
Exercise intervention
Participants underwent a 24-week AEXT program following the completion of baseline testing. Participants engaged in AEXT under direct supervision of study personnel three times per week, beginning with 20 min of exercise per session at 50% of VO2max. Training duration was then increased by 5 min each week until 40 min of exercise at 50% of VO2max was reached, at which point training intensity was increased by 5% each week until 70% of VO2max was achieved. Following 10 weeks of exercise training, a fourth unsupervised exercise session was incorporated into the exercise program. During the 24-week AEXT intervention, participants were required to record their exercise heart rate, exercise duration and mode of exercise for all supervised and unsupervised sessions in printed logs to ensure adherence to the exercise training program. Furthermore, participants were also required to complete food frequency checklists every 2 months to monitor dietary intake and to ensure adherence to the prescribed AHA diet.
Final testing
Upon completion of the AEXT intervention, all participants underwent the same testing as performed during the baseline testing period. Participants were required to continue exercise training until all final tests were completed. Blood draws and tests were conducted 24–36 h after an exercise session. Only participants with at least 70% adherence to the exercise training intervention were included in the final statistical analysis.
Statistical analysis
Data are expressed as means±the standard error of the mean (SEM). Variables for dipper and non-dipper groups were compared using analysis of variance (ANOVA). Pearson correlation coefficients were used to examine relationships between outcome variables and BP variables. Linear regression was used to assess the portion of variation in outcome variables that was accounted for by changes in BP. Change variables were calculated as: (final value–baseline value). A value of p0.05 was considered significant. Differences in sample sizes between baseline and final testing are related to subject attrition during the exercise intervention. Differences in sample sizes within an outcome variable are related either to issues related in acquiring blood samples or to issues related to assay.
Results
The study included 23 pre-hypertensive (n=14) and stage-1 hypertensive (n=9) adults who were classified as dipper (n=11) or non-dipper (n=12) according to their nocturnal decline in BP during a 24-h ABPM period at baseline. The dipper group was composed of six pre-hypertensive and five stage-1 hypertensive adults. The non-dipper group was composed of eight pre-hypertensive and four stage-1 hypertensive adults. The clinical characteristics of dipper and non-dipper groups at baseline are presented in Table I . The groups showed similar values for age, gender, weight and BMI. There were no statistically significant differences between dippers and non-dippers with respect to average 24-h SBP, DBP or MAP. Likewise, average daytime SBP and DBP were also similar between groups. At night-time, however, non-dippers exhibited higher average SBP and DBP; though these values did not reach statistical significance. The % Dip observed between day and night BP was statistically significant for SBP, DBP, and MAP with non-dippers having a significantly smaller % Dip. The % Dip was calculated as: % Dip=(daytime BP–night-time BP)×(100/daytime BP). No differences between dippers and non-dippers were found for all remaining clinical characteristics at baseline with the exception of casual SBP, which was significantly higher in dippers (137.2±2.8 mmHg dippers vs. 128.9±2.8 mmHg non-dippers, p=0.04).
Table I.
Blood pressure measurements of dipper and non-dipper groups.
| Baseline |
|||
|---|---|---|---|
| Variable | Dippers (n= 11) | Non-dippers (n= 12) | p-value |
| Gender (M/F) | 5/6 | 6/6 | - |
| Age (years) | 58.3± 1.2 | 58.8 ± 2.1 | 0.82 |
| Weight (kg) | 85.3± 3.2 | 85.1 ± 4.5 | 0.97 |
| BMI (kg/m2) | 28.9± 1.0 | 29.3 ± 1.4 | 0.78 |
| Avg. 24-h SBP (mmHg) | 129.0± 3.7 | 127.6 ± 2.7 | 0.76 |
| Avg. 24-h DBP (mmHg) | 80.4± 2.4 | 80.0 ± 2.2 | 0.91 |
| Avg. 24-h MAP (mmHg) | 97.7± 2.6 | 96.0 ± 2.3 | 0.63 |
| Avg. day SBP (mmHg) | 134.1± 4.0 | 129.3 ± 2.8 | 0.32 |
| Avg. day DBP (mmHg) | 84.1± 2.6 | 81.6 ± 2.2 | 0.46 |
| Avg. night SBP (mmHg) | 116.1± 3.7 | 121.7 ± 2.7 | 0.23 |
| Avg. night DBP (mmHg) | 69.8± 2.3 | 75.3 ± 2.5 | 0.13 |
| Dip MAP (%) | 15.4± 0.8 | 6.9 ± 0.8 | 0.001 |
BMI, Body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; MAP, Mean arterial pressure.
Values are expressed as means ± SEM.
p< 0.05.
Baseline values for oxidative stress biomarkers and endothelial function biomarkers are presented in Table II . Baseline measures of total cholesterol, LDL-cholesterol, ox-LDL, HDL-cholesterol, triglycerides, VO2max, 8-iso-PGF2α, uNOx, pNOx and SOD were not significantly different between dippers and non-dippers (Table II).
Table II.
Outcome variables of dipper and non-dipper groups.
| Baseline |
|||
|---|---|---|---|
| Variable | Dippers | Non-Dippers | p-value |
| Total cholesterol (mg/dl) | 198.6± 14.1 (n= 10) | 179.6 ± 10.4 (n= 11) | 0.28 |
| LDL-cholesterol (mg/dl) | 116.7± 11.9 (n= 10) | 107.7 ± 8.0 (n= 11) | 0.53 |
| Ox-LDL (U/l) | 165.3± 22.1 (n= 10) | 154.7 ± 30.3 (n= 10) | 0.78 |
| HDL-cholesterol (mg/dl) | 57.3± 7.4 (n= 10) | 42.6 ± 2.7 (n= 11) | 0.07 |
| Triglycerides (mg/dl) | 99.5± 17.6 (n= 10) | 112.1 ± 18.8 (n= 11) | 0.63 |
| VO2max (ml/kg/min) | 25.6± 1.3 (n= 11) | 25.9 ± 1.1 (n= 12) | 0.85 |
| 8-Isoprostane F2α (nmol/mmol creatinine) | 0.30± 0.04 (n= 11) | 0.26 ± 0.02 (n= 12) | 0.31 |
| uNOx (μmol/l) | 0.003± 0.00003 (n= 8) | 0.003 ± 0.0005 (n= 10) | 0.40 |
| pNOx (μmol/l) | 18.1± 1.0 (n= 10) | 16.7 ± 1.2 (n= 9) | 0.40 |
| SOD (U/ml) | 0.6± 0.1 (n= 10) | 0.6 ± 0.1 (n= 12) | 0.86 |
LDL, Low-density lipoprotein; Ox-LDL, Oxidized low-density lipoprotein; HDL, High-density lipoprotein; uNOx, Urinary nitrates/nitrites; pNOx, Plasma nitrates/nitrites; SOD, Superoxide dismutase.
Values are expressed as means ± SEM.
p< 0.05.
In a separate sub-analysis, subjects were re-categorized according to their ABPM that occurred during final testing. At final testing, subjects were instructed to repeat the same daily activities that they did during the baseline 24-h ABPM period. Three groups emerged. There was a group of subjects (n=14) that did not change their dipping status following AEXT. These subjects remained dippers or remained non-dippers. Five subjects changed from dipper to non-dipper, and four subjects changed from non-dipper to dipper. Change values for BP variables, oxidative stress biomarkers and endothelial function biomarkers were assessed relative to these new groups (Table III). Changes in night-time BP were significant for SBP, DBP and MAP between the groups. Changes in % Dip were significant for SBP, DBP and MAP (Table III). The group that changed from non-dipper to dipper with AEXT demonstrated decreases in BP across all above variables. In contrast, we also observed that the group that changed from dipper to non-dipper increased their BP values.
Table III.
Change variables for participants that did not change their dipping status, those that changed from dipper to non-dipper, and those that changed from non-dipper to dipper.
| Change variable | No change | Dippers to non-dippers | Non-dippers to dippers | p-value |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | − 3.2± 3.0 (n= 12) | 17.0 ± 9.0(n= 2) | − 24.3± 15.5 (n= 3) | 0.02 |
| LDL-cholesterol (mg/dl) | − 3.5± 2.2 (n= 12) | 15.0 ± 11.0 (n= 2) | − 22.7± 19.4 (n= 3) | 0.04 |
| Ox-LDL (U/l) | − 25.2± 16.8 (n= 10) | − 4.1± 29.4 (n= 4) | − 102.6± 39.1 (n= 3) | 0.09 |
| HDL-cholesterol (mg/dl) | 2.4± 2.1 (n= 12) | 2.0 ± 2.0 (n= 2) | 6.7 ± 2.9 (n= 3) | 0.62 |
| Triglycerides (mg/dl) | 9.0± 10.5 (n= 12) | − 10.0± 20.0 (n= 2) | − 35.7± 11.9 (3) | 0.15 |
| VO2max (ml/kg/min) | 8.2± 1.4 (n= 8) | 1.7 ± 5.2 (n= 4) | 2.2 ± 5.9 (n= 2) | 0.27 |
| 8-Isoprostane F2α(nmol/mmol creatinine) | 0.05± .04 (n=14) | − 0.06± 0.04 (n= 5) | 0.24 ± 0.10 (n= 4) | 0.01 |
| uNOx (μmol/l) | − 0.002± 0.0003 (n= 11) | − 0.002± 0.0004 (n= 4) | − 0.003± 0.0006 (n= 3) | 0.25 |
| pNOx (μmol/l) | − 0.1± 0.9 (n= 10) | − 0.3± 1.6 (n= 5) | − 1.3± 2.5 (n= 3) | 0.87 |
| SOD (U/ml) | − 0.01± 0.05 (n= 13) | 0.2 ± 0.2 (n= 4) | 0.2 ± 0.2 (n= 4) | 0.33 |
| % Dip MAP (%) | − 0.07± 0.8(n= 14) | 8.8 ± 1.5 (n= 5) | − 7.2 ± 2.8 (n= 4) | 0.001 |
LDL, Low-density lipoprotein; Ox-LDL, Oxidized low-density lipoprotein; HDL, High-density lipoprotein; uNOx, Urinary nitrates/nitrites; pNOx, Plasma nitrates/nitrites; SOD, Superoxide dismutase; MAP, Mean arterial pressure.
Values are expressed as means ± SEM.
Changes in total cholesterol and LDL-cholesterol were significantly different between the groups. Again, the subjects that changed from non-dippers to dippers decreased their values, while the subjects that changed from dippers to non-dippers increased their values. Also, the difference in ox-LDL changes approached significance (p=0.09), with the largest reductions in ox-LDL observed in subjects that changed from non-dippers to dippers, and the smallest reductions observed in subjects that changed from dippers to non-dippers. Finally, the change in 8-iso-PGF2α levels following AEXT was significantly different between the groups. However, the non-dipper to dipper change group demonstrated an increase in 8-iso-PGF2α levels while the dipper to non-dipper change group decreased their 8-iso-PGF2α levels.
For all subjects, the change in total cholesterol correlated significantly with the change in % Dip of MAP (Figure 1). As total cholesterol levels decreased with training, the % Dip in MAP also increased. Subjects in the group that changed from non-dipper to dipper exhibited large increases in % Dip of MAP and large decreases in total cholesterol. Subjects in the group that changed from dipper to non-dipper demonstrated decreases in % Dip MAP, and increases in total cholesterol.
Figure 1.
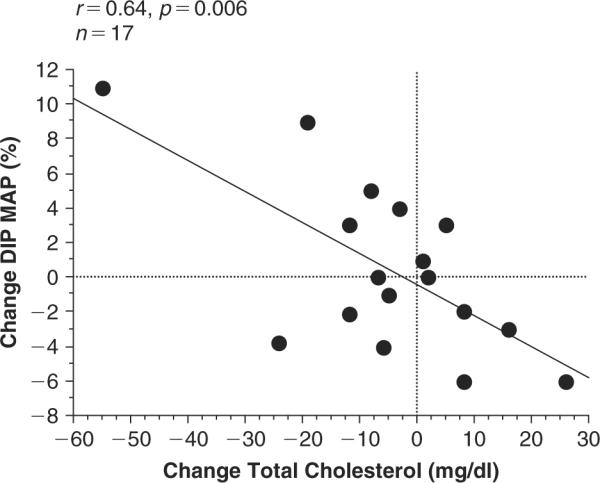
Relationship between the change in total cholesterol and the change in % Dip in mean arterial pressure (MAP). r=0.64 and p 0.006.
In Figure 2 , the change in LDL-cholesterol also showed significant relationship to the change in % Dip of MAP. As LDL-cholesterol levels decreased with training, the % Dip in MAP increased. Associations seen in Figure 1 are similar to the associations seen in Figure 2 for the change groups. Subjects in the group that changed from non-dipper to dipper exhibited large increases in % Dip of MAP and large decreases in LDL-cholesterol. Subjects in the group that changed from dipper to non-dipper demonstrated decreases in % Dip MAP, and increases in LDL-cholesterol.
Figure 2.
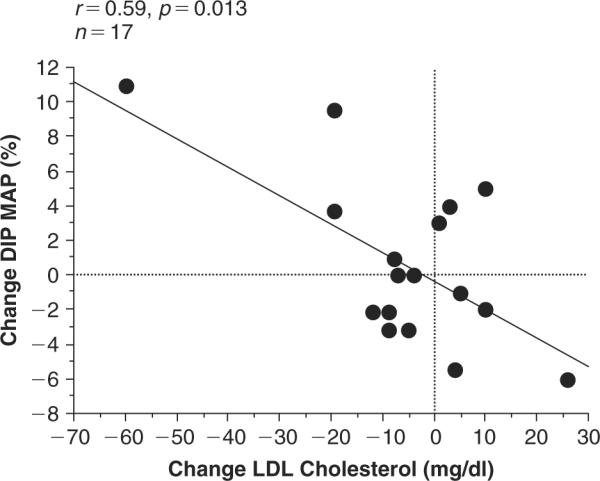
Relationship between the change in LDL-cholesterol and the change in % Dip in mean arterial pressure (MAP). r=0.59 and p 0.013.
There was one subject in the non-dipper to dipper change group whose data appeared to influence the results disproportionately. However, this subject's data was validated and remained in statistical analyses because of their overall response to AEXT. The subject consistently showed relatively large responses as 24-h average, day, night and % Dip decreased for SBP DBP, and MAP. Also, total cholesterol, LDL-cholesterol, ox-LDL and triglycerides all demonstrated a large decrease, and their HDL-cholesterol and SOD levels demonstrated a large increase with AEXT.
Discussion
The dip in BP seen during sleeping hours may be a result of a number of factors such as autonomic nervous system control, function of the local endothelium, or a combination of these two. For people who do not exhibit a night-time dip in BP, irregularities in circadian rhythm or endothelial function could be fundamental to vascular health. Thus, finding non-pharmacological ways to influence non-dippers and mitigate their risk for target end-organ damage is critical.
The present study is the first to assess multiple biomarkers of NO production and oxidative stress in relation to dipping status. It is also the first study to assess these biomarkers in response to AEXT in participants classified as dippers or non-dippers. In the present investigation, we studied a population of 50–75-year-old pre-hypertensive and stage-1 hypertensive individuals and classified them into dipper or non-dipper groups based on their 24-h ABPM profile. Biomarkers were measured under standardized conditions before and after 6 months of AEXT.
Of the many oxidative stress biomarkers, thiobarbituric acid-reactive substances (TBARS) and uNOx have been previously examined in non-dipper populations. In contrast to Pierdomenico et al. (26), who observed a significantly higher level of oxidative stress in non-dippers as measured by the lipid perioxidation measure TBARS, we saw no significant differences in 8-iso-PGF2α levels (a measure of lipid peroxidation) between the groups at baseline.
We also report a lack of association between dipping status and uNOx levels at baseline. The results of our study are in opposition to Higashi et al. (7) who found uNOx levels to be lower in non-dipping hypertensives. Perhaps the discrepancy between the present study and the Higashi et al. study could be related to the population studied and protocol used. Higashi et al. included smokers, and only withheld antihypertensive agents for at least 2 weeks prior to data collection. The current study excluded smokers and also tapered medication such that the subjects were off of their medication over a month before any baseline testing was conducted. Therefore, smoking and medication use could have influenced uNOx levels in the study by Higashi et al. To our knowledge, no other studies have assessed oxidative stress or endothelial function biomarkers following AEXT in dippers and non-dippers.
The primary purpose of our study was to determine how AEXT influenced dipping status, biomarkers of oxidative stress and endothelial function in subjects who were classified as dippers or non-dippers based on their 24-h ABP. The rationale for this was to identify potential differences in AEXT-induced changes in dippers and non-dippers. However, no correlations between any of the oxidative stress biomarkers and dipping status prior to or following AEXT were found. Therefore, subjects were re-classified after AEXT based on our criteria using the 24-h ABP profile that was obtained after AEXT and a separate analysis was performed. Fourteen subjects retained their original dipping status. However, five subjects became non-dippers who were previously dippers, and four subjects became dippers who were previously non-dippers.
In this sub-analysis, we found that total cholesterol showed the greatest reduction in subjects that had a beneficial change from non-dipper to dipper, while subjects that became non-dippers following AEXT increased their total cholesterol. The subjects who remained either non-dipper or dipper (i.e. did not change dipping status) after AEXT, only minimally reduced their total cholesterol levels. These patterns were also true for LDL-cholesterol and ox-LDL. Following AEXT the group that experienced the beneficial status change of non-dipper to dipper showed a large decrease in LDL and ox-LDL levels, while the group that changed from dipper to non-dipper increased their LDL-cholesterol levels and only minimally changed their ox-LDL levels. This difference among groups was significant for LDL-cholesterol changes and approached significance for ox-LDL changes with AEXT. The differences in how these three groups changed their cholesterol levels with AEXT were significant, thus suggesting AEXT-induced changes in total cholesterol levels may be linked to changing ones dipping status with AEXT.
Changes in biomarkers of endothelial function demonstrated inconsistency for people who changed from non-dippers to dippers following AEXT. Changes in uNOx, pNOx, and SOD did not correlate with change in dipping status, and the change in 8-iso-PGF2α levels did not correlate to groups as expected. 8-iso-PGF2α levels were higher in the beneficial non-dipper to dipper group, and the dipper to non-dipper group reduced their levels below baseline values. Given that the non-dipper to dipper change group was associated with a greater levels of 8-iso-PGF2α following AEXT, it demonstrates the need for more investigation into dipping status and endothelial dysfunction. Perhaps other biomarkers of oxidative stress are more appropriate to characterize these changes in a non-dipper population.
LDL-cholesterol and ox-LDL have been shown to have adverse effects on endothelial cell function such as uncoupling of eNOS (27), increased ROS (28), and transcriptional regulation of eNOS (29). While decreases in cholesterol levels highlight the known inverse relationship between endothelial NO production and cholesterol (30–32), we did not observe concomitant beneficial changes between biomarkers of endothelial function and cholesterol levels in relation to dipping status. Though exercise-induced reduction of cholesterol levels may have decreased reactive oxygen species (ROS) production (33), and increased NO bioavailability, we did not observe such changes in these variables. However, restoration of endothelial function may have contributed to the beneficial switch the group that changed from non-dipper to dipper experienced as demonstrated by their change in BP. The group that changed from non-dipper to dipper increased their % Dip MAP while also decreasing their total cholesterol levels and LDL-cholesterol levels. Hence, even though our biomarkers of endothelial function did not demonstrate beneficial changes in relation to the observed changes in total cholesterol, LDL-cholesterol and ox-LDL levels, we observed changes in dipping status that were related to the subject's change in cholesterol levels. In accordance, the group that changed from dipper to non-dipper increased their total cholesterol and LDL-cholesterol levels and decreased % Dip MAP.
There are some limitations of our study worth noting. As with virtually all studies reporting on blood biomarkers in dippers and non-dippers, we were not able to obtain blood samples throughout the 24-h ABPM period. Secondly, it is somewhat difficult to compare the present results with the results of all studies on dipping status because the method for categorizing subjects as dippers and non-dippers is not standardized (34). In the present study, we used well known published methods to categorize subjects (4). The methods used account for sleep-awake pattern and have been clinically reproducible (18,35). Finally, in the sub-analysis following AEXT, the sample sizes for the groups were small. However, the change in dipping status following an intervention is significant and carries clinical relevance.
In conclusion, AEXT was beneficial in many regards in the present study. However, AEXT did not beneficially affect all participants in regard to dipping status. We initially observed changes in BP that occurred with AEXT in participants that were categorized as dipper or non-dipper were not significantly correlated to the baseline levels of, or changes in, biomarkers of oxidative stress and endothelial function and therefore did not explain dipping status in pre- and stage-1 hypertensives. However, 39% of our study population changed dipping status. We observed a relationship between change in total cholesterol level, change in LDL-cholesterol level, and change in ox-LDL-cholesterol level to the change in dipping status with AEXT. Therefore, to simply assess dipping status only at baseline prior to an intervention may not provide a complete picture as interventions will likely not influence dipping status similarly for all participants.
Acknowledgements
This work was supported by NIH/NIA grant #AG19640 (P.I. Michael D. Brown) and NIH/NIA grant #AG15384, #AG17474 and #AG00268 (P.I. James M. Hagberg).
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Devereux RB, Pickering TG, Harshfield GA, Kleinert HD, Denby L, Clark L, et al. Left-ventricular hypertrophy in patients with hypertension – Importance of blood-pressure response to regularly recurring stress. Circulation. 1983;68:470–476. doi: 10.1161/01.cir.68.3.470. [DOI] [PubMed] [Google Scholar]
- 2.Khattar RS, Swales JD, Banfield N, Dore C, Senior R, Lahir A. Prediction of coronary and cerebrovascular morbidity and mortality by direct continuous ambulatory blood pressure monitoring in essential hypertension. Circulation. 1999;100:1071–1076. doi: 10.1161/01.cir.100.10.1071. Erratum. Circulation. 1999;100:1760. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood-pressure – An independent predictor of prognosis in essential-hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. Erratum. Hypertension. 1995;25:462. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, et al. Circadian blood-pressure changes and left-ventricular hypertrophy in essential-hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring – Unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241. doi: 10.1161/HYPERTENSIONAHA.107.087262. [DOI] [PubMed] [Google Scholar]
- 6.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients – Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 7.Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Sasaki S, et al. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and non-dippers. J Am Coll Cardiol. 2002;40:2039–2043. doi: 10.1016/s0735-1097(02)02535-4. [DOI] [PubMed] [Google Scholar]
- 8.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Rad Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Rodford JL, Torrens C, Siow RC, Mann GE, Hanson MA, Clough GF. Endothelial dysfunction and reduced anti-oxidant protection in an animal model of the developmental origins of cardiovascular disease. J Physiol. 2008 doi: 10.1113/jphysiol.2008.156976. Epub July 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ino-Oka E, Yumita S, Sekino H, Ohtaki Y, Takahashi T, Inooka H, et al. The effects of physical activity and autonomic nerve tone on the daily fluctuation of blood pressure. Clin Exp Hypertens. 2004;26:129–136. doi: 10.1081/ceh-120028550. [DOI] [PubMed] [Google Scholar]
- 11.Touyz R. Molecular and cellular mechanisms regulating vascular function and structure – Implications in the pathogenesis of hypertension. Can J Cardiol. 2000;16:1137–1146. Can J Cardiol. 2000;16:1451. [PubMed] [Google Scholar]
- 12.Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension – An update. Sports Med. 2000;30:193–206. doi: 10.2165/00007256-200030030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 14.Nami R, Mondillo S, Agricola E, Lenti S, Ferro G, Nami N, et al. Aerobic exercise training fails to reduce blood pressure in nondipper-type hypertension. Am J Hypertens. 2000;13:593–600. doi: 10.1016/s0895-7061(99)00265-4. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Jastremski CA, Wallace JP. Time of day for exercise on blood pressure reduction in dipping and nondipping hypertension. J Hum Hypertens. 2005;19:597–605. doi: 10.1038/sj.jhh.1001901. [DOI] [PubMed] [Google Scholar]
- 16.American College of Sports Medicine . ACSM's Guidlines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Baltimore, MD: 2000. [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Dov IZ, Ben-Arieh L, Mekler J, Bursztyn M. Blood pressure dipping is reproducible in clinical practice. Blood Press Monit. 2005;10:79–84. doi: 10.1097/00126097-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine – what does this measure tell us about the activity of the endogenous nitric oxide system? Curr Opin Nephrol Hypertens. 1998;7:59–62. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes PM, Leone AM, Francis PL, Struthers AD, Moncada S. The L-arginine-nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun. 1995;209:590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 21.Friedewa WT, Fredrick DS, Levy RI. Estimation of concentration of low-density lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg-2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 23.Allain CC, Poon LS, Chan CSG. Enzymatic determination of total serum-cholesterol. Clin Chem. 1974;20:859. [PubMed] [Google Scholar]
- 24.Basu S. The enigma of in vivo oxidative stress assessment: isoprostanes as an emerging target. Scand J Food Nutrition. 2007;51:48–61. [Google Scholar]
- 25.Basu S, Basu S. Radioimmunoassay of 8-iso-prostaglandin F2alpha: an index for oxidative injury via free radical catalysed lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids. 1998;58:319–325. doi: 10.1016/s0952-3278(98)90042-4. [DOI] [PubMed] [Google Scholar]
- 26.Pierdomenico SD, Costantini F, Bucci A, De Cesare D, Bucciarelli T, Cuccurullo F, et al. Blunted nocturnal fall in blood pressure and oxidative stress in men and women with essential hypertension. Am J Hypertens. 1999;12:356–363. doi: 10.1016/s0895-7061(98)00273-8. [DOI] [PubMed] [Google Scholar]
- 27.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 28.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 29.Ramasamy S, Parthasarathy S, Harrison DG. Regulation of endothelial nitric oxide synthase gene expression by oxidized linoleic acid. J Lipid Res. 1998;39:268–276. [PubMed] [Google Scholar]
- 30.Vanizor B, Orem A, Karahan SC, Kiran E, Erem C, Aliyazicioglu R, et al. Decreased nitric oxide end-products and its relationship with high density lipoprotein and oxidative stress in people with type 2 diabetes without complications. Diabet Res Clin Pract. 2001;54:33–39. doi: 10.1016/s0168-8227(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 31.skur-Smielecka E, Wykretowicz A, Kempa M, Furmaniuk J, Wysocki H. The influence of short-term treatment with simvastatin on the inflammatory profile of patients with hyper-cholesterolaemia. Coronary Artery Disease. 2001;12:143–148. doi: 10.1097/00019501-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Yashiro A, Nakashima Y, Nanri H, Ikeda M, Kuroiwa A. Plasma nitrite/nitrate level is inversely correlated with plasma low-density lipoprotein cholesterol level. Clin Cardiol. 1997;20:361–365. doi: 10.1002/clc.4960200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JS, Lee T, Chow SE. Role of exercise intensities in oxidized low-density lipoprotein-mediated redox status of monocyte in men. J Appl Physiol. 2006;101:740–744. doi: 10.1152/japplphysiol.00144.2006. [DOI] [PubMed] [Google Scholar]
- 34.Henskens LH, Kroon AA, van Oostenbrugge RJ, Haest RJ, Lodder J, de Leeuw PW. Different classifications of nocturnal blood pressure dipping affect the prevalence of dippers and nondippers and the relation with target-organ damage. J Hypertens. 2008;26:691–698. doi: 10.1097/HJH.0b013e3282f4225f. [DOI] [PubMed] [Google Scholar]
- 35.Dimsdale JE, von KR, Profant J, Nelesen R, ncoli-Israel S, Ziegler M. Reliability of nocturnal blood pressure dipping. Blood Press Monit. 2000;5:217–221. doi: 10.1097/00126097-200008000-00004. [DOI] [PubMed] [Google Scholar]


