Abstract
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been observed in association with internalizing symptoms and is thought to be involved in the pathogenesis of depression and some anxiety disorders. This study examined basal and stress-induced cortisol concentrations in relation to internalizing and externalizing symptoms in a racially mixed community sample of 102 8–11 year-old boys. Afternoon basal cortisol concentrations were positively correlated with measures of internalizing behavior problems, social problems, and emotionality. Greater change in cortisol across a home-visit challenge task was also significantly associated with internalizing behaviors and social problems, as well as attention and thought problems. The implications of these findings and how they may relate to the pathogenesis of emotional and behavioral problems are discussed.
Keywords: children, depression, anxiety, internalizing, HPA axis, stress reactivity
Introduction
Several decades of research document alterations of hypothalamic-pituitary-adrenal (HPA) axis function in association with depression and some anxiety disorders (Holsboer, 2000; Kaufman and Charney, 2001; Hasler et al., 2004; Risbrough and Stein, 2006; Carroll et al., 2007). The HPA axis is involved in maintaining basic physiological functioning as well as coordinating neural, hormonal, and behavioral responses to stressors. Basal glucocorticoid levels follow a diurnal rhythm, with the highest concentrations in the early morning and the nadir in the late evening hours. This basal activity is necessary for normal brain growth and regulates metabolic processes needed for basic functioning. In addition, glucocorticoids are released in response to acute challenges and serve to mobilize energy resources and prepare the organism to respond to stressors (McEwen, 2007). Research on HPA axis function has examined both basal cortisol concentrations at various points in the diurnal curve and “provoked” or stress-induced changes in cortisol concentrations.
Increased basal and provoked cortisol concentrations have been shown in individuals with traits such as neuroticism and inhibition as well as internalizing symptoms (Kagan et al., 1987; Schmidt et al., 1997; de Haan et al., 1998; Smider et al., 2002; Zobel et al., 2004; Tyrka et al., 2007; Tyrka et al., 2008). It has been hypothesized that such differences are secondary to increased sensitivity to stress or lower thresholds for activation of the HPA axis and other stress-response systems. Prolonged exposure to elevated glucocorticoid concentrations may result in neurostructural changes in limbic brain regions, including neuronal cell death and inhibition of neurogenesis (Duman and Monteggia, 2006; McEwen, 2007). Thus, while acute increases in glucocorticoids can enhance memory, learning and emotional responding, prolonged or excessive elevations may lead to cognitive and affective disturbances.
Research on the association of HPA axis dysfunction in children and adolescents with internalizing disorders has yielded somewhat variable findings. Increased morning or evening cortisol concentrations have been reported in association with depression as well as other internalizing disorders in clinical samples of children and adolescents (Dahl et al., 1991; Goodyer et al., 1996; Rao et al., 1996; Gispen-de Wied et al., 2000; Goodyer et al., 2000; Goodyer et al., 2001; Mathew et al., 2003; Forbes et al., 2006). Other studies have shown increased cortisol reactivity in depressed children or adolescents in the dexamethasone suppression test or in response to a social challenge task (Tout et al., 1998; Luby et al., 2004). Two studies have shown high sensitivity but low specificity of the DST for identifying depressed children compared to children with other disorders (Targum and Capodanno, 1983; Petty et al., 1985), and two studies have found altered HPA axis function in depressed youth only when there was comorbid maltreatment. Several other investigations have not found evidence of increased basal or provoked cortisol concentrations in depressed youth (Geller et al., 1983; Ha et al., 1984; Dahl et al., 1989; Puig-Antich et al., 1989; Kutcher et al., 1991; Birmaher et al., 1992; Birmaher et al., 1996; Dorn et al., 1996; Luby et al., 2003).
Several studies have focused on broad measures of internalizing behavior problems in community samples of children and adolescents. Increased basal cortisol concentrations have been found in children with internalizing behavior problems in several investigations (Gunnar et al., 1997; de Haan et al., 1998; Tout et al., 1998; Cicchetti and Rogosch, 2001; Goodyer et al., 2003; Blair et al., 2004; Van den Bergh et al., 2008). However, other studies have found that lower cortisol activity is associated with internalizing behaviors and that higher cortisol may be linked to extroversion (Davis et al., 1999) or anger (Adam, 2006).
Challenge tasks have been designed to elicit cortisol responses with consideration of the ecological validity of the task and sometimes the context in which the task was administered. Increased salivary cortisol in children with internalizing symptoms has been shown in response to psychosocial challenge tasks (Tout et al., 1998; Luby et al., 2003) and the fear-potentiated startle paradigm (Ashman et al., 2002). Granger and colleagues (1994) used a parent-child conflict discussion laboratory task with a sample of 102 7–17 year-old clinic-referred children. While the majority of subjects had decreases in salivary cortisol concentrations in response to the task compared to pre-task cortisol levels, those who had increases had higher levels of social withdrawal, social anxiety, and social problems. Klimes-Dougan et al., (2001) also used a conflict discussion task during a home visit in their study of youths aged 11–17. As with the Granger study above, mean salivary cortisol concentrations decreased over the course of the task. Those youths who had a mild decrease in salivary cortisol over the course of the task had the lowest internalizing and externalizing scores, whereas those with higher internalizing and externalizing scores had either an increase or a strong decrease in response to the task. In addition, this study involved a social performance paradigm in which youths were instructed to talk with a shy person and then give a three-minute speech. Girls who had an increase in salivary cortisol in response to this task had higher levels of internalizing and attention problems.
In contrast to the literature demonstrating increased basal cortisol concentrations in association with internalizing behavior problems, several investigations have found children with externalizing behaviors to have low basal (Tennes and Kreye, 1985; Scerbo and Kolko, 1994; Cicchetti and Rogosch, 2001; Smider et al., 2002; Shoal et al., 2003; Shirtcliff et al., 2005) and stress-induced cortisol concentrations (van Goozen et al., 1998). Children with extroversion or externalizing behaviors may have higher thresholds for activation of stress-responsive physiological systems (Rogeness et al., 1990). On the other hand, externalizing behavioral problems are highly correlated with internalizing symptoms (Achenbach et al., 1991), and some evidence suggests that cortisol reactivity may be associated with both behavior patterns (Klimes-Dougan et al., 2001; Boyce et al., 2006), depending on contextual factors (de Haan et al., 1998; Gunnar et al., 2003) or comorbid symptoms (McBurnett et al., 1991; van Goozen et al., 1998; Cicchetti and Rogosch, 2001; Blair et al., 2004).
Most existing studies of the relationship between behavioral problems and cortisol concentrations appear to have been conducted in largely middle-class, White samples, although many studies have not provided information regarding these demographic factors. Race and ethnicity could be linked to stress reactivity and HPA axis function via physiological differences as well as disparities in stress exposure related to poverty, crime, and racism (Szanton et al., 2005). Racial differences in HPA axis function have been demonstrated in youths and adults. For example, lower morning cortisol concentrations (Bennett et al., 2004; DeSantis et al., 2007; Chong et al., 2008), lower cortisol responses to psychosocial stress (Mechlin et al., 2005; Chong et al., 2008) and higher evening cortisol values (Cohen et al., 2006; DeSantis et al., 2007) have been shown in some groups of Black youths and adults in comparison with Whites. One investigation also found higher evening cortisol concentrations among Hispanic youths in comparison to White youths (DeSantis et al., 2007). Socioeconomic status (SES) can also influence neuroendocrine activity (Lupie et al., 2001; Cohen et al., 2006). Social influences such as socioeconomic adversity or stress associated with discrimination might account for some of the racial differences in HPA axis function; however, two studies found racial differences after controlling for differences in SES (Cohen et al., 2006; DeSantis et al., 2007).
Gender is another important variable that may influence HPA axis function as well as risk for depression and other stress-related disorders. Some studies have shown sex differences in measures of salivary cortisol with boys showing lower awakening cortisol (Rosmalen et al., 2005) and lower afternoon cortisol (Klimes-Dougan et al., 2001) than girls, but others find no difference (Granger et al., 1994; Gunnar et al., 2003). Associations with behavior have also been variable with some studies showing effects in boys but not in girls (Tout et al., 1998; Shirtcliff et al., 2005; Sondeijker et al., 2008) and another similar findings for girls and boys (Smider et al., 2002).
Age and development are also important factors to consider with respect to studies of cortisol in relation to behavior. Most existing studies have been conducted in pre-school or adolescent samples. Middle childhood and early adolescence are especially critical periods to study in relation to hormone-behavior associations because this period is characterized by: 1) the emergence or worsening of some behavioral problems and gender differences in the nature of such problems; and 2) apparent maturational changes in activity of the HPA axis (Kenny et al., 1966; Kenny et al., 1966; Kiess et al., 1995; Viru et al., 1998; Walker et al., 2001; Ronsaville et al., 2006).
In this investigation we studied a sample of all boys who were aged 8–11 and from racially and ethnically diverse backgrounds to examine associations of behavioral problems with both basal and provoked cortisol concentrations. We hypothesized that internalizing behavior problems would be associated with higher basal and induced cortisol concentrations. In addition we hypothesized that externalizing problems would be linked to attenuated basal and provoked cortisol levels.
Methods
Participants
One hundred and two boys, aged 8–11 (M = 9.13, SD = 0.74) who were recruited from several New York City area public schools for a study of behavior in relation to cortisol and stress reactivity were included in this study. Flyers introducing the study were distributed to fourth-grade boys. Mothers were asked to send a card with their contact information back to the project office by mail or through the classroom if they were interested in learning more about the study. Interested parents were called and scheduled for a home visit. During the home visit (described below), informed consent was obtained from parents for their child’s participation, and boys provided assent to participate. Research teams composed of two project staff members (at least one who was of the same ethnicity as the child and at least one male) conducted in-home data collection.
The demographic characteristics of the sample of boys are as follows. Forty-six (45%) of the boys were Black, 33 (32%) were White non-Hispanic, and 23 (23%) were White Hispanic. Of the 102 boys, 53.5% lived in a two parent household (40.2% with both biological parents) and the remaining 46.5% lived with their mother or in another family configuration. Sixty-four (62.7%) mothers reported they had education beyond a high-school level and thirty-eight mothers (37.3%) did not. Family SES was scored using the Hollingshead Scale (Hollingshead, 1975). The standard scoring protocols were used for different household types. The range of possible scores for family SES was 8 to 66. This sample had an average SES score of 36.50 (SD = 13.89), which corresponds to a high school degree and employment as clerical workers, sales workers, or owners of small businesses.
Mothers were asked to rate their son’s stage of pubertal development for pubic hair growth based on drawings of Tanner stages, rated on the 1 to 5 scale, with 1 = no development and 5 = development complete. The drawings used have good validity (Morris and Udry, 1980), and correlations between parent and health examiner ratings of Tanner stages range from 0.75 to 0.87 (Brooks-Gunn et al., 1987; Dorn et al., 1990). Most boys were rated Tanner stage 1 (N=77, 76%), nineteen boys (18.6%) were rated Tanner stage 2, and six mothers did not provide ratings.
Procedure
All procedures were approved by the IRB of Teachers College at Columbia University.
Home Visit
The home visit was scheduled to occur between 3:00pm and 8:00pm and lasted approximately 90 minutes. During the visit, the boys and their parents completed self-report forms and participated in a series of challenge tasks, with sampling of saliva for cortisol assay at regular intervals
Following the informed consent and instructions, participants provided a baseline saliva sample, and four additional samples were obtained from boys after each of four tasks and at approximately 20-minute intervals. Saliva samples were collected using Salivette kits (Sarstedt, Germany) as follows: the child removed the cylinder-shaped cotton swab from the Salivette tube and placed it in his mouth. The participant was instructed to roll the swab in his mouth, without chewing it, for a two-minute period. The child was then asked to return the swab to the Salivette tube and close it securely. Saliva samples were stored in a laboratory freezer at −25 degrees Celsius until they were assayed for cortisol. The series of challenging tasks began with a cognitive puzzle task (Object Assembly task from the WISC) (Wechsler, 1991). Second, the boys participated in a cold pressor challenge with a series of two exposures (Fanurik et al., 1993). Third, there was a physical assessment involving measurements of height, weight, and vital signs. The final challenge involved a parent-child disagreement task (Klimes-Dougan et al., 2001), in which boys and their mothers discussed two topics upon which they disagreed. The disagreement topics were identified using the Issues Checklist (Clingempeel et al., 1992), a commonly used measure of assessing disagreements between parents and children ages 9–16.
Boys completed a measure of their own affect and behavior during the home visit and mothers and boys independently completed additional surveys during the 3 consecutive days following the home visit. At the end of the home visit, instructions and materials for diurnal salivary cortisol collection were reviewed and practiced. Boys received a gift (T-shirt) for their participation and mothers were paid $75.
Basal Salivary Cortisol Sampling
Mothers were asked to assist in the collection of a morning and an afternoon saliva sample on two consecutive days using the procedure learned during the home visit. As morning and afternoon saliva samples were used to assess other hormones, cotton rolls were not used and boys were asked to spit into a tube to collect saliva. Boys collected a practice sample using this method at the end of the home visit. For the morning samples, mothers were instructed to collect a saliva sample from the child immediately after the child woke up at his natural waking time and before brushing his teeth or consuming food or water. Mothers were instructed to collect the afternoon samples as soon as boys returned home from school. Cortisol Assay. Samples were assayed at the Columbia-Presbyterian Reproductive Endocrinology Department in New York City, NY. Salivary cortisol concentrations were determined using radioimmunoassay procedures outlined by Kirschbaum and colleagues (Diagnostic Products Company). The lower detection limit for the assay was 0.02 µg/dl per 200 µl of saliva. Saliva samples were centrifuged at 3000 rpm for 10 minutes. All samples yielded at least 400 µl of saliva, and samples of 200 µl were assayed in duplicate. All samples from a participant were analyzed in one assay run. The intra- and inter-assay coefficients of variation observed were less than 5% and 3%, respectively.
Measures
In the current study, we selected child-report, parent-report, and teacher-report measures of internalizing and externalizing behavior problems. Measures of domains of behavior across informants demonstrated weak to moderate size correlations, indicating that each rater provided unique information regarding child symptoms and behavior in different contexts. We therefore retained the informant-specific measures and did not develop multi-agent composite scores.
Child Report of Depressive and Anxiety Symptoms
The Children’s Depression Inventory (Kovacs, 1992), composed of 27-items on a 3-point scale that measure childhood depressive symptoms over the past two weeks, was completed by the boys (α = .81).
The 7-item anxiety subscale of the Hopkins Symptom Checklist (Rickels et al., 1976) was used to measure boys’ self-reported symptoms of anxiety (α = .79).
Parent Report of Behavior Problems
Parents completed the Child Behavior Checklist (Achenbach, 2001; Achenbach and Dumenci, 2001), a widely used measure of child and adolescent problems, adaptive functioning, and competencies based on reports of behavior over the preceding 6 months. Each item is scored on a Likert scale, (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). The CBCL has excellent psychometric properties, including reliability and validity (Achenbach and Rescorla, 2001). In order to reduce the likelihood of Type I errors, we utilized the summary scales Internalizing (α = .84) and Externalizing (α = .88) rather than the individual sub-scales that make up these summary scales. We also included the additional subscales that do not directly tap internalizing or externalizing problems: Social Problems, Thought Problems, and Attention Problems (α = .70, .65, and .79, respectively (Achenbach, 2001).
Teacher report of Child Behavior
Teachers were asked to complete paper-and-pencil ratings of the children, and 61 (60%) of the teachers completed these inventories. Analysis of the demographic and behavioral measures for boys with and without completed teacher ratings did not reveal any significant differences between these two groups. The Pupil Evaluation Inventory (Pekarik et al., 1976; Bierman et al., 1993) is a 35-item scale completed by teachers, which assesses three dimensions of child behavior: Withdrawal, Aggression, and Likeability (α = .74, .90, and .72, respectively). The teacher form of the PEI has been validated with peer ratings (Pekarik et al., 1976; La Greca, 1981), and shown to be useful in classifying boys who are aggressive and rejected (La Greca, 1981; Ledingham, 1981; Ledingham and Younger, 1985). The Emotionality, Activity and Sociability Inventory (Buss and Plomin, 1984) was also completed by the boys’ teachers. Prior psychometric research on data from this sample and a similar sample of girls revealed that only the Emotionality subscale of the EAS showed adequate internal consistency in this racially diverse sample (α=.69, .73, and .82 for Black, Hispanic, and White children respectively; unpublished data), so only this scale was used in the present investigation.
Data Reduction and Statistical Analyses
Cortisol Measures
Average morning and afternoon cortisol concentrations were obtained by taking the mean of the two samples for each time point (for morning cortisol the correlation between the two days was 0.3 and for afternoon cortisol the correlation was 0.19). In the home visit, the pattern of cortisol response was consistent with that of other studies employing mildly stressful challenges with children (Klimes-Dougan et al., 2001; Boyce et al., 2006): the first saliva sample yielded the highest cortisol concentration, suggesting that anticipatory adrenocortical activation and degree of subsequent decline in activation was indicative of HPA reactivity in this context. Therefore, a change score (delta) was calculated by subtracting the value of the last cortisol sample from the first cortisol sample. In addition, the overall cortisol response to the challenge was summarized by calculating the area under the curve (AUC) for cortisol using the trapezoidal method.
In the overall sample, two outliers (defined as scores greater than three standard deviations from the mean) in the morning and afternoon basal cortisol measures were identified. For the home visit, two outliers in the change score and three outliers in the AUC data were identified. Only one participant had an outlier in more than one of the cortisol measures. In order to reduce the influence of these data points, these values were set to equal that of the next highest value. Since only 60% of the teachers completed the teacher questionnaires, for analyses of these data we defined outliers according to the mean and standard deviations for the teacher sample. This resulted in the identification of one outlier in the home visit cortisol data, two outliers in the morning cortisol data and two outliers in the afternoon cortisol data. These values were set to equal those of the next highest value for within the sample of participants with teacher data.
Analysis of Potential Covariates
Partial correlations and analysis of covariance (ANCOVA), adjusting for the time of the cortisol sample (time of the first sample in the case of the home visit), were computed to determine whether any of the cortisol measures (am, pm, and challenge protocol AUC and change score) were associated with any of the following potential covariates: age, pubertal development (initiated puberty vs. not), socioeconomic status (SES; Hollingshead score), and race (Black, White non-Hispanic, and White Hispanic).
Hypothesis testing
Partial correlations were conducted to test for associations between the cortisol variables and the measures of internalizing, externalizing, and other behavioral problems. In addition, because internalizing and externalizing symptoms are correlated but some evidence suggests that they are associated with different patterns of HPA axis abnormalities, we conducted additional partial correlations controlling for the correlated scale. For the tests of Internalizing symptoms we controlled for Externalizing symptoms, and for Externalizing we controlled for Internalizing symptoms.
Results
Preliminary Analyses
Means and standard deviations for the study measures are shown in Tables 1 and 2. The initial partial correlation analyses controlling for time of cortisol sample and testing potential covariates revealed that none of the possible covariates (age, pubertal status, SES, or race) was correlated with any of the cortisol measures (morning, afternoon, home visit AUC or home visit change score). Consistent with prior research, the CBCL Internalizing and Externalizing summary scale scores were highly intercorrelated (r=.57, p <.001).
Table 1.
Basal and Home-Visit Salivary Cortisol Measures in 8–11 Year-Old Boys
| M | SD | |
|---|---|---|
| Morning Cortisol, µg/dl, n=101 | .37 | .15 |
| Afternoon Cortisol, µg/dl, n=98 | .09 | .07 |
| Home Visit Cortisol Change Score, n=102 | .04 | .07 |
| Home Visit Cortisol AUC, n=101 | 5.65 | 3.46 |
| Time of Morning Sample | 0809h | 01:27 |
| Time of Afternoon Sample | 1715h | 02:28 |
| Time of First Home Visit Sample | 1645h | 02:00 |
Note. AUC refers to area under the curve. For sample times standard deviation shows hours:minutes.
Table 2.
Behavioral Measures.
| Measures, (Scale Range) | M | SD |
|---|---|---|
| Parent Report | ||
| CBCL Internalizing Symptoms, (0–54) | 6.78 | 5.50 |
| CBCL Externalizing Symptoms, (0–66) | 7.65 | 6.91 |
| CBCL Social Problems, (0–22) | 2.53 | 2.49 |
| CBCL Thought Problems, (0–22) | 1.71 | 2.26 |
| CBCL Attention Problems, (0–20) | 3.77 | 3.64 |
| Child Report | ||
| Child Depression Inventory (CDI), (0–52) | 5.73 | 4.62 |
| HSCL Anxiety Subscale, (1–5) | 1.52 | 0.62 |
| Teacher Report | ||
| PEI Likable, (1–5) | 3.38 | 0.95 |
| PEI Aggression, (1–5) | 1.93 | 1.06 |
| PEI Withdrawal, (1–5) | 1.85 | 0.77 |
| EAS Emotionality, (1–5) | 1.92 | 1.05 |
Note. CBCL = Child Behavior Checklist, HSCL = Hopkins Symptom Checklist, PEI = Pupil Evaluation Inventory, EAS = Emotionality, Activity, and Sociability Inventory.
Basal Cortisol Concentrations
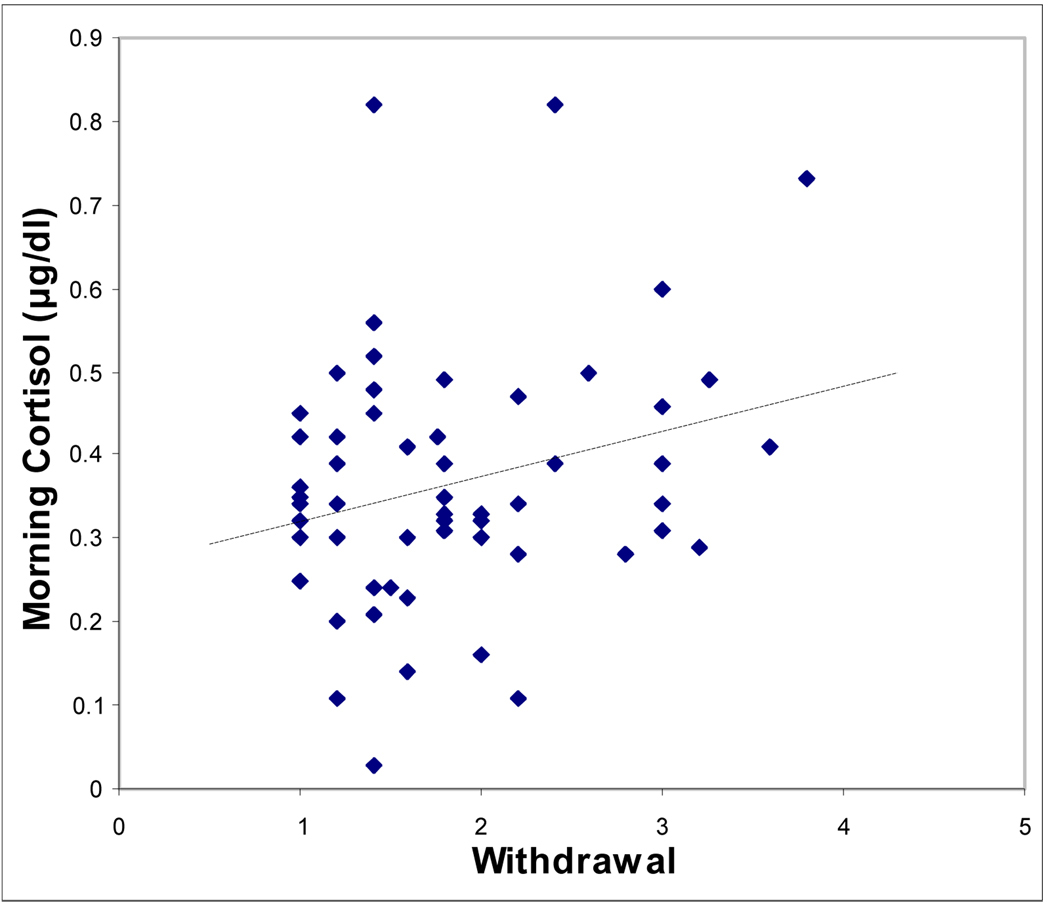
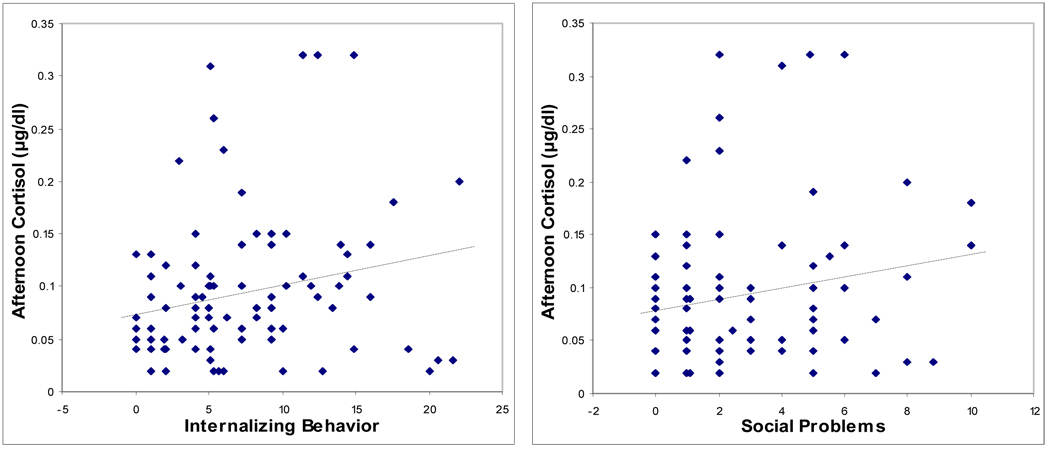
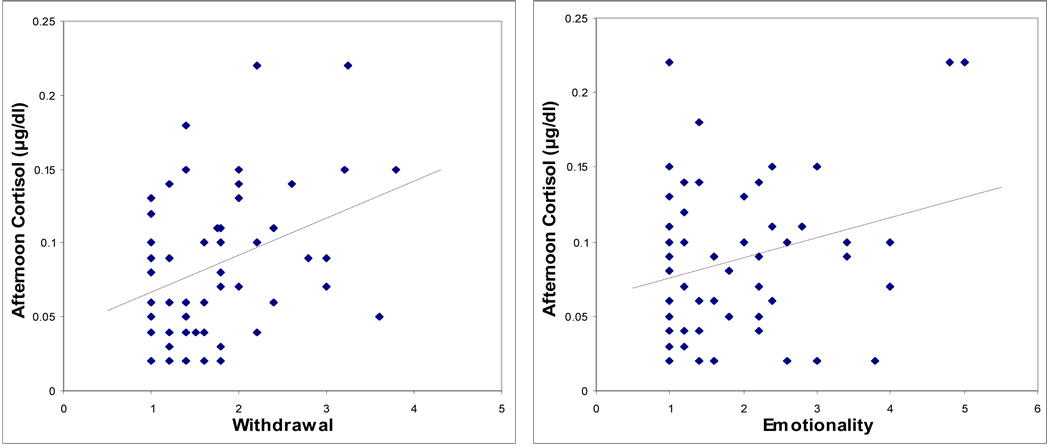
Partial correlations controlling for time of cortisol sample and testing associations between basal am and pm cortisol concentrations and the measures of boys’ emotional and behavioral symptoms are shown in Table 3. Basal am cortisol was significantly correlated with teacher-rated Withdrawal, but none of the other measures was significant. For afternoon cortisol, higher concentrations were linked to higher scores on parent-rated Internalizing symptoms and Social Problems, and teacher-rated Withdrawal and Emotionality (Table 3). Scatterplots showing the significant relationships of am cortisol and pm cortisol with the behavioral measures, unadjusted for time of sample, are shown in Figures 1 and 2, respectively.
Table 3.
Partial correlations of salivary cortisol measures and child behavior problems controlling for time of sample.
| Morning Cortisol, µg/dl |
Afternoon Cortisol, µg/dl |
Home Visit Cortisol Change Score |
|
|---|---|---|---|
| Parent Report | |||
| Internalizing Behavior | −0.01 | 0.23* | 0.27** |
| Externalizing Behavior | −0.10 | −0.04 | 0.22* |
| Social Problems | 0.06 | 0.21* | 0.25* |
| Thought Problems | 0.03 | −0.01 | 0.23* |
| Attention Problems | −0.07 | 0.18 | 0.27** |
| Child Report | |||
| Depression Inventory | −0.10 | −0.06 | −0.01 |
| Child Anxiety | −0.04 | 0.11 | 0.05 |
| Teacher Report | |||
| Withdrawal | 0.29* | 0.36** | 0.27* |
| Aggression | −0.03 | −0.02 | −0.09 |
| Emotionality | 0.20 | 0.34* | 0.13 |
Note. Parent Report Subscales are from the Child Behavior Checklist, Depression Inventory refers to the Child Depression Inventory, Child Anxiety was assessed with the Hopkins Symptom Checklist, Teacher Report measures are the Pupil Evaluation Inventory (Withdrawal, Aggression) and the Emotionality, Activity, and Sociability Inventory (Emotionality).
p < 0.05
p < 0.01
Figure 1.
Scatterplot showing relationship between teacher-report withdrawal measure and morning salivary cortisol concentration.
Note. Withdrawal is a subscale of the teacher-rated Pupil Evaluation Inventory.
Figure 2.
Scatterplots showing relationships between child behavior problems and afternoon salivary cortisol concentration.
Note. Internalizing Behavior and Social Problems are subscales of the parent-rated Child Behavior Checklist. Withdrawal is a subscale of the teacher-rated Pupil Evaluation Inventory. Emotionality is a subscale of the teacher-rated Emotionality, Activity and Sociability Inventory.
The partial correlations designed to further examine the relationship of Internalizing and Externalizing scores to the cortisol measures, showed that Internalizing was not significantly correlated with morning cortisol, even after controlling for Externalizing symptoms, and Externalizing was also not a significant predictor of morning cortisol even after controlling for Internalizing symptoms. For afternoon cortisol, the partial correlation of Internalizing symptoms became stronger after controlling for Externalizing symptoms (r = 0.29, p = 0.005). For Externalizing, after correlating for Internalizing symptoms, the correlation became significant with higher Externalizing scores associated with lower afternoon cortisol concentrations (r = −0.21, p < 0.05).
Home Visit Challenge Cortisol
The decline in cortisol concentration over the course of the challenge protocol was not statistically significant. The partial correlations controlling for time of the first sample and testing associations of the behavioral measures with cortisol AUC were not significant.
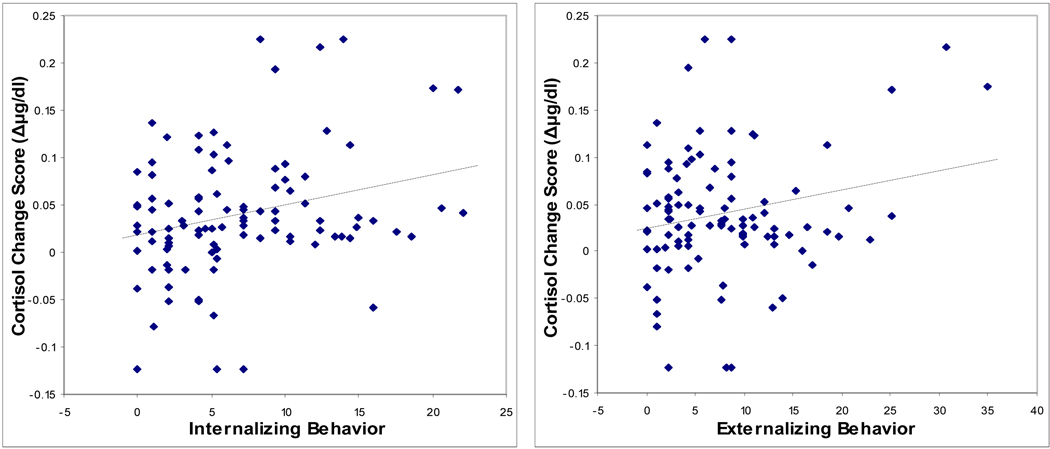
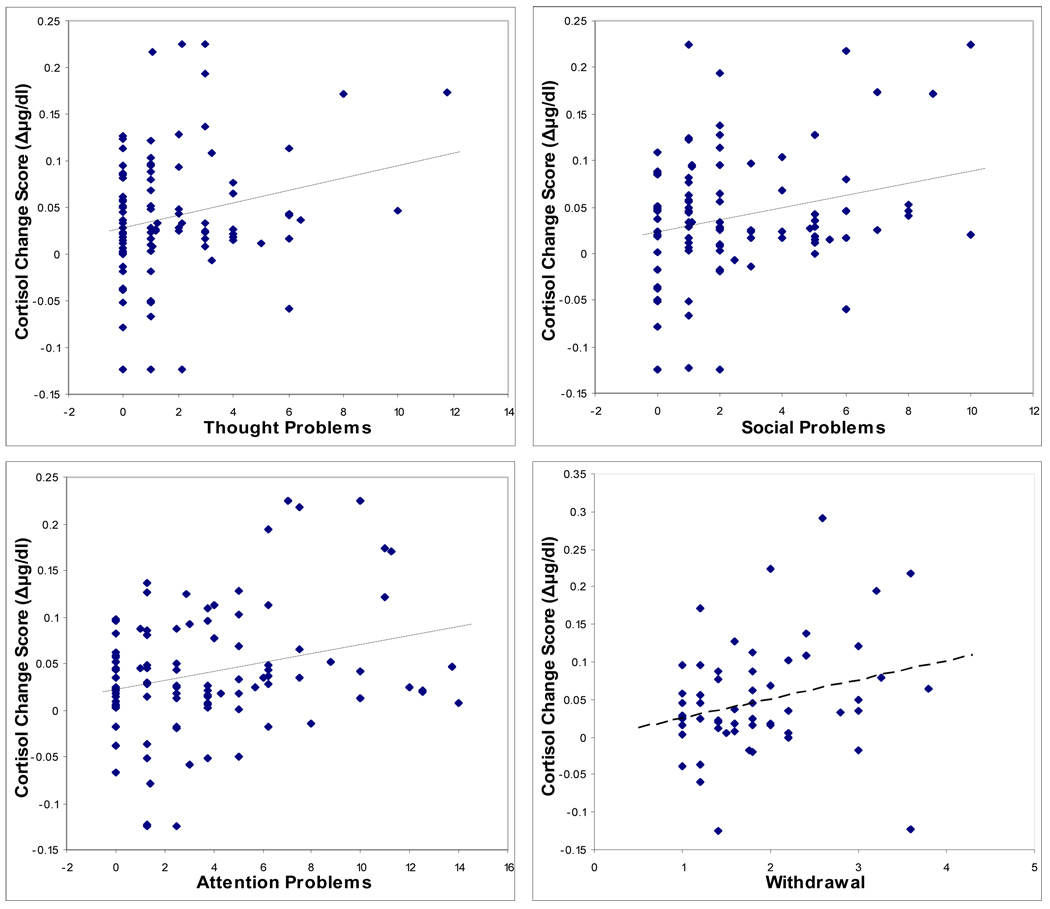
The partial correlations testing for behavioral associations with the challenge protocol cortisol change scores and controlling for the time of first sample revealed significant associations with several parent- and teacher-rated measures of child behavior (Table 3). Scatterplots showing the significant relationships between cortisol change scores and the behavioral measures, unadjusted for time of first sample, are shown in Figure 3. Greater declines in cortisol concentration over the course of the home visit protocol were associated with higher scores on the CBCL measures Internalizing symptoms, Externalizing symptoms, Social Problems, Thought Problems, and Attention Problems, as well as the teacher-rated PEI Withdrawal subscale. Further inspection of these associations indicated that the larger cortisol declines reflected numerically higher initial and lower final cortisol concentrations, but these individual time points were not significantly correlated with the behavioral measures. The partial correlation of Internalizing symptoms and home visit change score controlling for Externalizing symptoms in addition to time of sample resulted in a non-significant correlation (r = 0.19, p = 0.065). For Externalizing symptoms, the correlation with home visit cortisol change score was no longer significant after controlling for Internalizing symptoms (r = 0.05, p = 0.63).
Figure 3.
Scatterplots showing relationships between child behavior problems and home visit salivary cortisol change score.
Note. Internalizing Behavior, Externalizing Behavior, Thought Problems, Social Problems and Attention Problems are subscales of the parent-rated Child Behavior Checklist. Withdrawal is a subscale of the teacher-rated Pupil Evaluation Inventory.
Discussion
In this study we found significant associations of both basal and reactive cortisol concentrations with several measures of adjustment, particularly problems in the internalizing domain. Specifically, afternoon basal salivary cortisol concentrations were correlated with measures of mother reports of internalizing symptoms and social problems, and teacher reports of withdrawal and emotionality. In addition, morning cortisol was significantly associated with teacher reports of withdrawal. Positive associations of basal cortisol concentrations with internalizing behavior problems are consistent with the results of several prior community studies (Gunnar et al., 1997; de Haan et al., 1998; Tout et al., 1998; Cicchetti and Rogosch, 2001; Goodyer et al., 2003; Blair et al., 2004). The majority of previous investigations on this topic have studied mostly White samples of very young children or adolescents and few investigations have focused specifically on males. Our findings extend the positive association of basal cortisol concentrations with internalizing disorders to a mixed-race sample of 8–11 year-old boys from working-middle class backgrounds.
In addition to the effects for afternoon cortisol concentrations, we found an association of several measures of behavior problems with a steeper decline in cortisol concentration during the home visit challenge protocol. Previous studies of children involving similar challenge tasks have also found decreases in cortisol concentration over the course of the task (Granger et al., 1994; van Goozen et al., 2000; Klimes-Dougan et al., 2001). Similar to these prior studies, we did not have a baseline period of low cortisol activity followed by a challenge-induced increase and subsequent recovery, so that we cannot conclude that the findings are reflective of cortisol stress reactivity. However, only the change score was correlated with the behavioral measures, and not individual cortisol measures or the area under the cortisol curve. The larger declines reflected numerically higher initial and lower final cortisol concentrations, but these individual time points were not significantly correlated with the behavioral measures. Thus, our home visit findings cannot be accounted for by an initial HPA axis activation during an “anticipation phase” with subsequent habituation or inability to maintain a sufficient cortisol response to cope with the challenge (Moss et al., 1995; Moss et al., 1999; Shoal et al., 2003). However, it is worth noting that the home visit occurred at approximately the same time of day as the afternoon basal saliva sample and that the two cortisol measures had similar behavioral correlates. Thus, while our home visit findings regarding HPA axis responses to challenge do not clearly reflect stress or anticipation responses, they appear to be indicative of consistent associations of behavior with different measures of HPA axis activity.
Our association of behavioral problems with declines in salivary cortisol across the home visit challenge differs with findings of two prior studies that used conflict-discussion paradigms in children and adolescents (Granger et al., 1994; Klimes-Dougan et al., 2001). In both studies, although most children had decreases in cortisol over the course of the task, behavior problems were associated with increases in cortisol in response to the task (Granger et al., 1994; Klimes-Dougan et al., 2001). However, in the study by Klimes-Dougan and colleagues, those youths with higher internalizing and externalizing scores showed either an increase or a strong decrease in response to their challenge task. In contrast to our study, these investigations included older children and youths with preclinical or clinical levels of behavioral problems; such characteristics may account for the difference in the findings.
In this study, we also found different patterns of externalizing symptoms with cortisol concentrations after controlling for internalizing symptoms. Previous research has highlighted the correlation of internalizing and externalizing symptoms and some evidence suggests that exaggerated cortisol activity may be associated with both behavior patterns (Klimes-Dougan et al., 2001; Boyce et al., 2006) while other studies indicate that externalizing symptoms may be linked to attenuated HPA axis activity (Tennes and Kreye, 1985; Scerbo and Kolko, 1994; van Goozen et al., 1998; Cicchetti and Rogosch, 2001; Smider et al., 2002; Shoal et al., 2003; Shirtcliff et al., 2005). In this study, Externalizing became a significant negative predictor of afternoon cortisol only after controlling for Internalizing symptoms (which were positively associated with both Externalizing and afternoon cortisol). For the home visit, while Externalizing symptoms was positively correlated with the cortisol change score initially, after controlling for Internalizing symptoms there was no longer an association of Externalizing with the change score. These findings indicate the importance of accounting for the large degree of shared variance between measures of internalizing and externalizing behaviors, and that a negative association of externalizing with cortisol measures may have been masked in prior studies that did not account for effects of internalizing behaviors.
It is important to note that this was a community sample that was not selected on the basis of behavioral problems, and accordingly, scores on the behavioral measures and cortisol values were in the normative range. While these findings are generally consistent with those seen in clinical samples and could thus reflect a precursor to the development of depression and anxiety disorders, it is also possible that these associations reflect normative functioning that is categorically distinct from that seen with internalizing disorders.
It is also of note that we did not find significant effects of race or socioeconomic status in this study. The lack of racial differences could be due to the modest sample size for each group. It is also possible that socioeconomic effects or social effects related to race may have a greater impact later in adolescence.
Limitations of this study include the modest sample size and the cross sectional nature of the present findings. In addition, the study was limited to boys and to a restricted age range (and associated restricted range of pubertal status) in order to reduce variability, so we were not able to examine gender or broad age effects. Our measures of basal cortisol concentration were limited to morning and afternoon samples so that we can not address other points in the diurnal curve. In addition, while parents were instructed to collect the morning samples immediately following awakening, we did not collect information regarding time of awakening which influences morning cortisol values. Thus it is possible that the lack of association with morning cortisol concentrations was due to variability in the time elapsed between awakening and collecting the morning sample. We did not elicit a robust stress response to a specific challenge task as would have been done in a laboratory-based study; hence, the home-visit cortisol concentrations reflect fairly normative changes in response to a typically occurring challenge (i.e., meeting new people and anticipating new activities). Finally, we used Salivette cotton swab to obtain saliva during the home visit challenge task but not for the morning and afternoon basal samples. However, when saliva volume recovered from the Salivette swab is adequate, as was the case in the current study, cortisol recovery is not compromised (Harmon et al., 2007).
In conclusion, our findings extend the association between childhood internalizing behaviors and cortisol activity to a racially diverse community sample and highlight this association for boys aged 8–11, an age group that has previously been under-studied. In addition, we provide support for the association of externalizing behaviors with attenuated cortisol concentrations. These associations may reflect alterations of psychological sensitivity to stressors or other stimuli, or conversely, effects of excess cortisol activity on brain circuits involved in these behavioral patterns. Prospective studies of behavioral adjustment, stressful life experiences, and HPA axis function could shed light on this issue by elucidating the temporal relations among these processes.
Acknowledgements
We thank the staff of the Boys Health and Development Project and the boys and their families who devoted their time and energy to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accortt EE, Freeman MP, Allen JJ. Women and major depressive disorder: clinical perspectives on causal pathways. J Womens Health (Larchmt) 2008;17(10):1583–1590. doi: 10.1089/jwh.2007.0592. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. Child Behavior Checklist for Ages 6 to 18. Burlington: University of Vermont Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Achenbach TM, Dumenci L. Advances in empirically based assessment: revised cross-informant syndromes and new DSM-oriented scales for the CBCL, YSR, and TRF: comment on Lengua, Sadowksi, Friedrich, and Fischer (2001) J Consult Clin Psychol. 2001;69(4):699–702. [PubMed] [Google Scholar]
- Achenbach TM, Howell CT, Quay HC, Conners CK. National survey of problems and competencies among four- to sixteen-year-olds: parents' reports for normative and clinical samples. Monogr Soc Res Child Dev. 1991;56(3):1–131. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School Age Forms & Profiles. Burlington: University of Vermont Research Center for children, Youth & Families; 2001. [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev Psychopathol. 2002;14(2):333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethn Health. 2004;9(4):337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Bierman KL, Smoot DL, Aumiller K. Characteristics of aggressive-rejected, aggressive (nonrejected), and rejected (nonaggressive) boys. Child Dev. 1993;64(1):139–151. [PubMed] [Google Scholar]
- Birmaher B, Dahl RE, Perel J, Williamson DE, Nelson B, Stull S, Kaufman J, Waterman GS, Rao U, Nguyen N, Puig-Antich J, Ryan ND. Corticotropin-releasing hormone challenge in prepubertal major depression. Biol Psychiatry. 1996;39(4):267–277. doi: 10.1016/0006-3223(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Dahl R, Rabinovich H, Ambrosini P, Williamson DE, Novacenko H, Nelson B, Lo ES, Puig-Antich J. Dexamethasone suppression test in children with major depressive disorder. J Am Acad Child Adolesc Psychiatry. 1992;31(2):291–297. doi: 10.1097/00004583-199203000-00017. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R, Granger D. Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: further examination of the behavioral inhibition system/behavioral activation system scales for young children. Dev Psychobiol. 2004;45(3):113–124. doi: 10.1002/dev.20022. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. J Am Acad Child Adolesc Psychiatry. 2006;45(12):1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls' pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- Buss AH, Plomin R. Temperament: Early Developing Personality Traits. Hillsdale, New Jersey: Lawrence Erlbaum Associates, Inc; 1984. [Google Scholar]
- Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Liu PY, Veldhuis JD. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand Suppl. 2007;(433):90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Chong RY, Uhart M, McCaul ME, Johnson E, Wand GS. Whites have a more robust hypothalamic-pituitary-adrenal axis response to a psychological stressor than blacks. Psychoneuroendocrinology. 2008;33(2):246–254. doi: 10.1016/j.psyneuen.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol. 2001;13(4):783–804. [PubMed] [Google Scholar]
- Clingempeel WG, Colyar JJ, Brand E, Hetherington EM. Children's relationships with maternal grandparents: a longitudinal study of family structure and pubertal status effects. Child Dev. 1992;63(6):1404–1422. doi: 10.1111/j.1467-8624.1992.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Dahl R, Puig-Antich J, Ryan N, Nelson B, Novacenko H, Twomey J, Williamson D, Goetz R, Ambrosini PJ. Cortisol secretion in adolescents with major depressive disorder. Acta Psychiatr Scand. 1989;80(1):18–26. doi: 10.1111/j.1600-0447.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, Perel J. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biol Psychiatry. 1991;30(1):25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: individual differences in salivary cortisol response in relation to child temperament. Dev Psychobiol. 1999;35(3):188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- de Haan M, Gunnar MR, Tout K, Hart J, Stansbury K. Familiar and novel contexts yield different associations between cortisol and behavior among 2-year-old children. Dev Psychobiol. 1998;33(1):93–101. doi: 10.1002/(sici)1098-2302(199807)33:1<93::aid-dev8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Burgess ES, Susman EJ, von Eye A, DeBellis MD, Gold PW, Chrousos GP. Response to oCRH in depressed and nondepressed adolescents: does gender make a difference? J Am Acad Child Adolesc Psychiatry. 1996;35(6):764–773. doi: 10.1097/00004583-199606000-00016. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff-Germain G, Chrousos GP. Perceptions of puberty: Adolescent, parent, and health care personnel. Developmental Psychology. 1990;26(2):322–329. [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Fanurik D, Zeltzer LK, Roberts MC, Blount RL. The relationship between children's coping styles and psychological interventions for cold pressor pain. Pain. 1993;53(2):213–222. doi: 10.1016/0304-3959(93)90083-2. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 2006;59(1):24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Rogol AD, Knitter EF. Preliminary data on the dexamethasone suppression test in children with major depressive disorder. Am J Psychiatry. 1983;140(5):620–622. doi: 10.1176/ajp.140.5.620. [DOI] [PubMed] [Google Scholar]
- Gispen-de Wied CC, Jansen LM, Duyx JH, Thijssen JH, van Engeland H. Pituitary-adrenal function in adolescent psychiatric patients: impact of depressive symptoms. J Affect Disord. 2000;59(1):71–76. doi: 10.1016/s0165-0327(99)00116-0. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26(2):245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: a community-based longitudinal enquiry. Psychol Med. 2003;33(4):601–610. doi: 10.1017/s0033291702007286. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PM. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8–16 year olds. Biol Psychiatry. 2001;50(5):351–357. doi: 10.1016/s0006-3223(01)01120-9. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. J Abnorm Psychol. 1994;103(2):267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MM. Peer rejection, temperament, and cortisol activity in preschoolers. Dev Psychobiol. 2003;43(4):346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Dev Psychobiol. 1997;31(1):65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ha H, Kaplan S, Foley C. The dexamethasone suppression test in adolescent psychiatric patients. Am J Psychiatry. 1984;141(3):421–423. doi: 10.1176/ajp.141.3.421. [DOI] [PubMed] [Google Scholar]
- Harmon AG, Hibel LC, Rumyantseva O, Granger DA. Measuring salivary cortisol in studies of child development: watch out--what goes in may not come out of saliva collection devices. Dev Psychobiol. 2007;49(5):495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; 1975. [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58(6):1459–1473. [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13(3):451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Kenny FM, Gancayco GP, Heald FP, Hung W. Cortisol production rate in adolescent males in different stages of sexual maturation. J Clin Endocrinol Metab. 1966;26(11):1232–1236. doi: 10.1210/jcem-26-11-1232. [DOI] [PubMed] [Google Scholar]
- Kenny FM, Preeyasombat C, Migeon CJ. Cortisol production rate. II. Normal infants, children, and adults. Pediatrics. 1966;37(1):34–42. [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory Manual. North Tonawanda: New York: Multi-Health Systems, Inc; 1992. [Google Scholar]
- Kutcher S, Malkin D, Silverberg J, Marton P, Williamson P, Malkin A, Szalai J, Katic M. Nocturnal cortisol, thyroid stimulating hormone, and growth hormone secretory profiles in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1991;30(3):407–414. doi: 10.1097/00004583-199105000-00009. [DOI] [PubMed] [Google Scholar]
- La Greca AM. Peer Acceptance: The Correspondence between Children's Sociometric Scores and Teachers' Rating of Peer Interactions. Journal of Abnormal Child Psychology. 1981;9(2):167–178. doi: 10.1007/BF00919112. [DOI] [PubMed] [Google Scholar]
- Ledingham JE. Developmental patterns of aggressive and withdrawn behavior in childhood: a possible method for identifying preschizophrenics. J Abnorm Child Psychol. 1981;9(1):1–22. doi: 10.1007/BF00917854. [DOI] [PubMed] [Google Scholar]
- Ledingham JE, Younger AJ. The influence of the evaluator on assessments of children's social skills. In: Schneider BH, et al., editors. Social Competence in Developmental Perspective. Dordrecht: Kluwer; 1985. [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60(12):1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161(11):1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- Lupie SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13(3):653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Coplan JD, Goetz RR, Feder A, Greenwald S, Dahl RE, Ryan ND, Mann JJ, Weissman MM. Differentiating depressed adolescent 24 h cortisol secretion in light of their adult clinical outcome. Neuropsychopharmacology. 2003;28(7):1336–1343. doi: 10.1038/sj.npp.1300184. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Christ MA, Hanson KS. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. J Am Acad Child Adolesc Psychiatry. 1991;30(2):192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67(6):948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP. Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol Psychiatry. 1999;45(10):1293–1299. doi: 10.1016/s0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol Psychiatry. 1995;38(8):547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Pekarik EG, Prinz RJ, Liebert DE, Weintraub S, Neale JM. The Pupil Evaluation Inventory. A sociometric technique for assessing children's social behavior. J Abnorm Child Psychol. 1976;4(1):83–97. doi: 10.1007/BF00917607. [DOI] [PubMed] [Google Scholar]
- Petty LK, Asarnow JR, Carlson GA, Lesser L. The dexamethasone suppression test in depressed, dysthymic, and nondepressed children. Am J Psychiatry. 1985;142(5):631–633. doi: 10.1176/ajp.142.5.631. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Dahl R, Ryan N, Novacenko H, Goetz D, Goetz R, Twomey J, Klepper T. Cortisol secretion in prepubertal children with major depressive disorder. Episode and recovery. Arch Gen Psychiatry. 1989;46(9):801–809. doi: 10.1001/archpsyc.1989.01810090043008. [DOI] [PubMed] [Google Scholar]
- Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Giles DE, Rao R, Kaufman J, Nelson B. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biol Psychiatry. 1996;40(6):474–484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Rickels K, Garcia CR, Lipman RS, Derogatis LR, Fisher EL. The Hopkins Symptom Checklist. Assessing emotional distress in obstetric-gynecologic practice. Prim Care. 1976;3(4):751–764. [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogeness GA, Cepeda C, Macedo CA, Fischer C, Harris WR. Differences in heart rate and blood pressure in children with conduct disorder, major depression, and separation anxiety. Psychiatry Res. 1990;33(2):199–206. doi: 10.1016/0165-1781(90)90074-f. [DOI] [PubMed] [Google Scholar]
- Ronsaville DS, Municchi G, Laney C, Cizza G, Meyer SE, Haim A, Radke-Yarrow M, Chrousos G, Gold PW, Martinez PE. Maternal and environmental factors influence the hypothalamic-pituitary-adrenal axis response to corticotropin-releasing hormone infusion in offspring of mothers with or without mood disorders. Dev Psychopathol. 2006;18(1):173–194. doi: 10.1017/S095457940606010X. [DOI] [PubMed] [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30(5):483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: relationship to aggressive, hyperactive, and internalizing behaviors. J Am Acad Child Adolesc Psychiatry. 1994;33(8):1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Dev Psychobiol. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2003;42(9):1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: a prospective study. Child Dev. 2002;73(1):75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Sondeijker FE, Ferdinand RF, Oldehinkel AJ, Tiemeier H, Ormel J, Verhulst FC. HPA-axis activity as a predictor of future disruptive behaviors in young adolescents. Psychophysiology. 2008;45(3):398–404. doi: 10.1111/j.1469-8986.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Szanton SL, Gill JM, Allen JK. Allostatic load: a mechanism of socioeconomic health disparities? Biol Res Nurs. 2005;7(1):7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum SD, Capodanno AE. The dexamethasone suppression test in adolescent psychiatric inpatients. Am J Psychiatry. 1983;140(5):589–591. doi: 10.1176/ajp.140.5.589. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M. Children's adrenocortical responses to classroom activities and tests in elementary school. Psychosom Med. 1985;47(5):451–460. doi: 10.1097/00006842-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Tout K, de Haan M, Campbell EK, Gunnar MR. Social behavior correlates of cortisol activity in child care: gender differences and time-of-day effects. Child Dev. 1998;69(5):1247–1262. [PubMed] [Google Scholar]
- Tyrka AR, Wier LM, Anderson GM, Wilkinson CW, Price LH, Carpenter LL. Temperament and response to the Trier Social Stress Test. Acta Psychiatr Scand. 2007;115(5):395–402. doi: 10.1111/j.1600-0447.2006.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier LM, Price LH, Rikhye K, Ross NS, Anderson GM, Wilkinson CW, Carpenter LL. Cortisol and ACTH responses to the Dex/CRH test: influence of temperament. Horm Behav. 2008;53(4):518–525. doi: 10.1016/j.yhbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Horm Behav. 2008;54(2):253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Van Goozen SH, Matthys W, Cohen-Kettenis PT, Buitelaar JK, van Engeland H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol Psychiatry. 1998;43(7):531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Viru A, Laaneots L, Karelson K, Smirnova T, Viru M. Exercise-induced hormone responses in girls at different stages of sexual maturation. Eur J Appl Physiol Occup Physiol. 1998;77(5):401–408. doi: 10.1007/s004210050351. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13(3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. Third Edition. New York: The Psychological Corporation; 1991. [Google Scholar]
- Zobel A, Barkow K, Schulze-Rauschenbach S, Von Widdern O, Metten M, Pfeiffer U, Schnell S, Wagner M, Maier W. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic-pituitary-adrenocortical system in healthy volunteers. Acta Psychiatr Scand. 2004;109(5):392–399. doi: 10.1111/j.1600-0447.2004.00313.x. [DOI] [PubMed] [Google Scholar]