Abstract
The synthesis and evaluation of twenty dinitroanilines and related compounds against the obligate intracellular parasite Toxoplasma gondii is reported. Using in vitro cultures of parasites in human fibroblasts, we determined that most of these compounds selectively disrupted Toxoplasma microtubules, and several displayed sub-micromolar potency against the parasite. The most potent compound was N1,N1-dipropyl-2,6-dinitro-4-(trifuoromethyl)-1,3-benzenediamine (18b), which displayed an IC50 value of 36 nM against intracellular T. gondii. Based on these data and another recent report (Ma, C.; Tran, J.; Gu, F.; Ochoa, R.; Li, C.; Sept, D.; Werbovetz, K.; Morrissette, N. Antimicrob. Agents Chemother. 2010, 54, 1453), an antimitotic structure-activity relationship for dinitroanilines vs. Toxoplasma is presented.
Infection by the apicomplexan parasite Toxoplasma gondii can result in miscarriage, birth defects in infants, vision disturbances in immunocompetent hosts, and toxoplasmic encephalitis in the immunocompromised.1 Acute toxoplasmosis in otherwise healthy individuals is typically treated with the antifolate combination of pyrimethamine and sulfadiazine together with folinic acid.1 Pyrimethamine has potential side effects, including bone marrow suppression2 and teratogenicity.3, 4 Resistance to pyrimethamine in malaria chemotherapy can occur through mutations in the dihydrofolate reductase target protein,5 although no evidence for clinical resistance to pyrimethamine in Toxoplasma exists to date.6 Sulfadiazine use is also complicated by the occurrence of kidney stones,7 allergic reactions,8 and the development of resistance.6 Other alternatives to pyrimethamine-sulfadiazine treatment of toxoplasmosis include clindamycin, atovaquone, and spiramycin,1 but these drugs each possess their own limitations.9–11 An ideal drug against human toxoplasmosis would kill the different stages of T. gondii, be well distributed in the main sites of infection, including the brain and eyes, and lack the potential for teratogenicity.
Tubulin, which assembles into microtubules, is an essential protein for formation of the mitotic spindle. It continues to be a prime target for established and investigational anticancer agents12, 13 and is also believed to be the molecular target of anthelmintic benzimidazoles.14 Dinitroanilines are tubulin-binding herbicides which display microtubule selective toxicity for many different classes of protozoan parasites, including Leishmania, Trypanosoma, Plasmodium, and Toxoplasma. We have previously characterized the sensitivity of Toxoplasma to dinitroanilines and have found that IC50 values for commercially available dinitroanilines range from 45 nM to 6.7 µM.15–17 Toxoplasma parasites only replicate inside of host cells, and extracellular parasites do not have dynamic microtubules. Intracellular Toxoplasma parasites are sensitive to disruption by dinitroanilines: dinitroaniline-treated parasites lack all microtubules and cannot carry out microtubule-dependent functions including mitosis and cytokinesis.15, 18, 19
Following lysis of the original host cell, round, non-polar dinitroaniline-treated parasites are unable to invade new host cells and rapidly die. Thus, dinitroanilines could serve as excellent leads for the discovery of new drugs against toxoplasmosis since they disrupt protozoan parasite microtubules at nanomolar concentrations while showing little or no effect on host cell microtubules.16, 20–23 Computational methods have been used to identify a binding site for the dinitroanilines on parasite tubulin.16, 24 These studies predict that protofilament contacts in the microtubule lattice are disrupted when dinitroanilines selectively bind to protozoan α-tubulin beneath the H1-S2 loop. Analysis of vertebrate α-tubulin through computational methods indicates that oryzalin has non-specific, low affinity interactions with this protein and no consensus binding site, consistent with in vivo and in vitro observations that dinitroanilines do not bind to vertebrate tubulin or disrupt vertebrate microtubules.20, 25–28
Previous work in our laboratories has shown that structural modification of the commercial dinitroaniline oryzalin leads to increased potency against kinetoplastid parasites.23, 29, 30 However, a detailed structure-activity study of dinitroanilines against Toxoplasma has not been reported. We were therefore motivated to synthesize analogs of oryzalin (6i) and other dinitroaniline herbicides such as trifluralin (14a) and dinitramine (18a) for testing against Toxoplasma gondii. In the present manuscript, we describe the synthesis and biological activity of such derivatives.
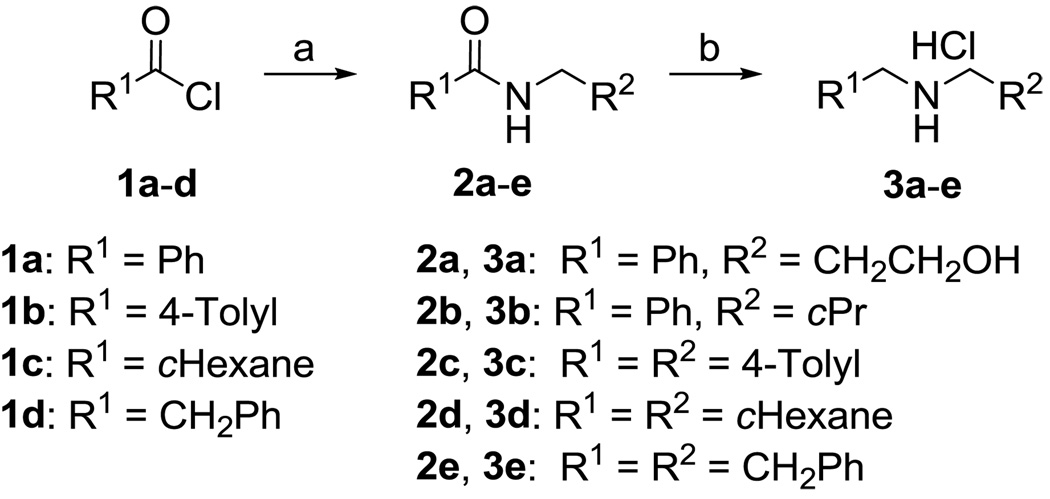
To synthesize several of the desired target compounds, unsymmetrical secondary amines were needed. The required secondary amine salts were synthesized using the method reported previously.29,31 Hence, the standard coupling of the acid chlorides 1a–d with amines gave the respective amides 2a–e, followed by reduction with borane-THF solution, treatment with methanol, protection with Boc, and deprotection using HCl in dioxane to afford the secondary amine salts 3a–e in good yields (see Scheme 1).
Scheme 1.
Reagents and conditions: a) RNH2, Et3N, CH2Cl2, 0 °C to rt, overnight; b) (i) BH3-THF (3 equiv), reflux, overnight; (ii) MeOH, reflux, 5h; (iii) Boc2O (1.5 equiv) in CH2Cl2, rt, overnight; (iv) 4 M HCl in 1,4-dioxane (1.3 equiv), CH2Cl2, rt, overnight.
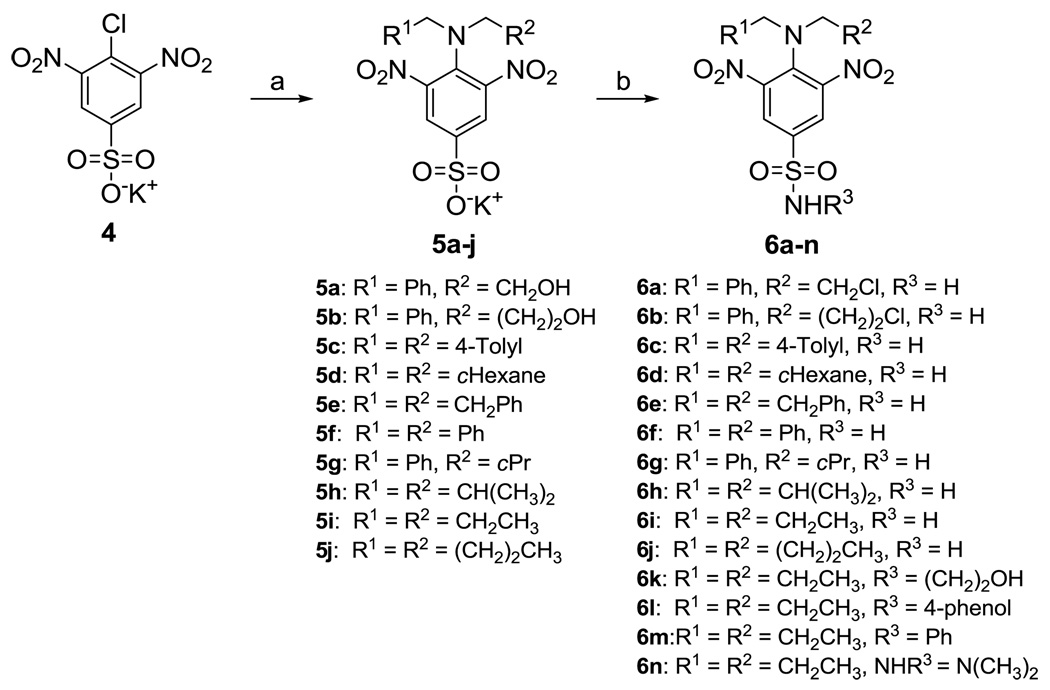
Dinitroaniline sulfonamide target compounds were prepared as shown in Scheme 2. The desired amines reacted with the potassium salt of 4-chloro-3,5-dinitrobenzenesulfonate 4 in the presence of triethylamine to give N4,N4-disubstituted-3,5-dinitrobenzenesulfonates 5a–e, 5f–h,31 and 5i–j30, 31 in good yields. These sulfonates contained traces of the secondary amine salts as determined from their 1H NMR spectra, but these salts did not interfere with further reactions. Sulfonyl chlorides were synthesized from benzenesulfonates 5a–j using PCl5 in dichloromethane. After brief work up, the resulting sulfonyl chlorides were treated with a methanolic solution of NH3 or the desired amines in dry THF to afford the target compounds 6a–h, 6i–j,29 6k–l, and 6m–n30 in good to excellent yields. Mono-N4-alkylated and free N4-amino sulfonamides were isolated as bi-products, especially for bulky N4,N4-disubstituted analogs such as 6a–g.
Scheme 2.
Reagents and conditions: a) Secondary amine, reflux, or secondary amine hydrochloride, Et3N, MeOH, reflux, 4 h; b) (i) PCl5, CH2Cl2, rt, 3 h; (ii) NH3 (7 N in MeOH), or amine, Et3N, THF, 0 °C to rt, 2h.
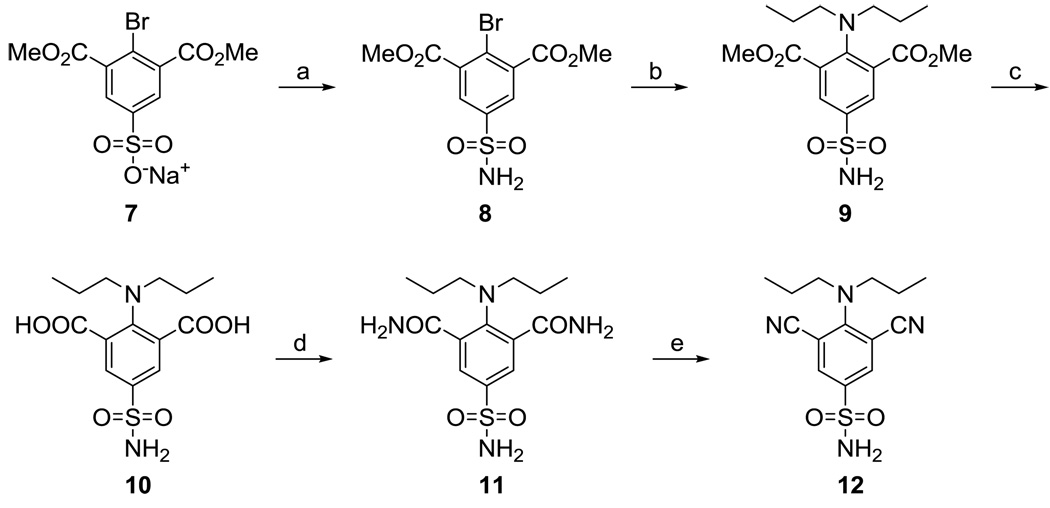
The synthesis of 12, where the nitro moieties present in 6i were replaced with nitrile groups, was accomplished according to Scheme 3. The sulfonyl chloride corresponding to sulfonate 732 was prepared in situ from 7 using PCl5/POCl3 in dry chlorobenzene; treatment of this sulfonyl chloride with methanolic NH3 afforded 8. An Ullmann reaction33 between sulfonamide 8 and dipropylamine in the presence of potassium carbonate and catalytic copper gave 9. Hydrolysis of this ester using aqueous NaOH in methanol afforded 10. Treatment of dicarboxylate 10 with hexachloroacetone and PPh3 in THF followed by the addition of NH3 in 1,4-dioxane provided bis-amide 11. Dehydration of 11 using PdCl2 in aqueous acetonitrile34 afforded the target compound 12 in excellent and reproducible yield.
Scheme 3.
Reagents and conditions: a) (i) PCl5/POCl3, chlorobenzene, reflux, 24; (ii) NH3 (0.5 M in 1,4-dioxane), 0 °C, 1 hr, rt, 1 h; b) dipropylamine, K2CO3, Cu, dioxane, reflux, 5h; c) 3N NaOH, MeOH, reflux, 2 days; d) (i) CCl3COCCl3, PPh3, THF, 0 °C, 1 h; (ii) NH3/dioxane, rt, 1 h; e) PdCl2, CH3CN/H2O, 24 h.
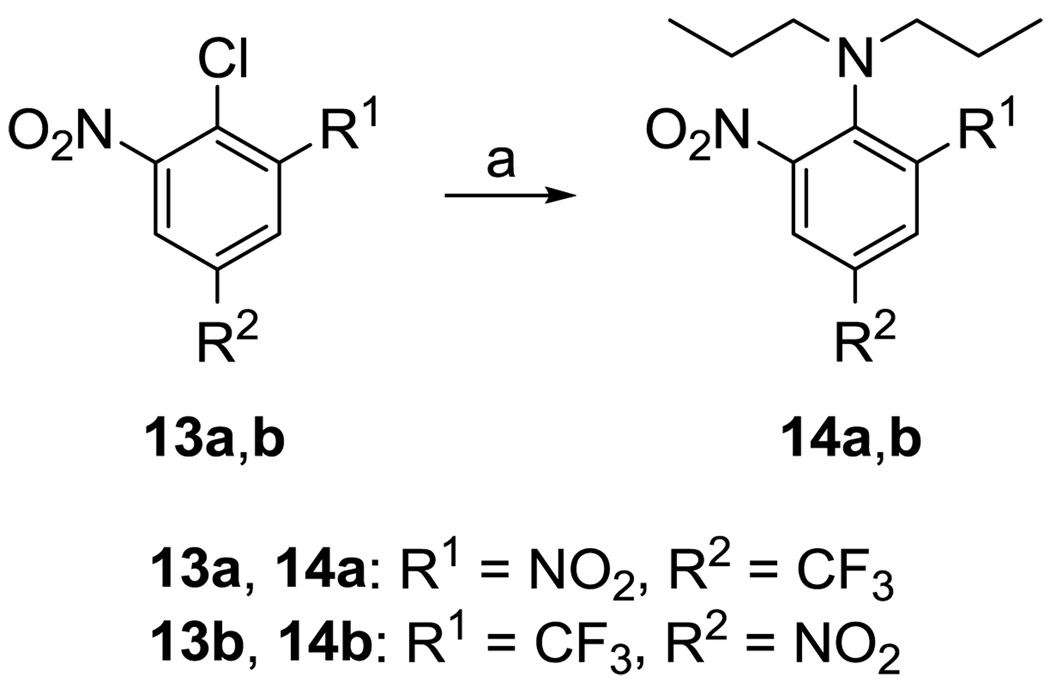
Trifluralin (14a) and its regioisomer 14b35 were prepared according to Scheme 4. Heating 13a and 13b to reflux in dipropylamine gave target compounds 14a and 14b, respectively, in excellent yield.
Scheme 4.
Reagents and conditions: a) Dipropylamine, reflux, overnight.
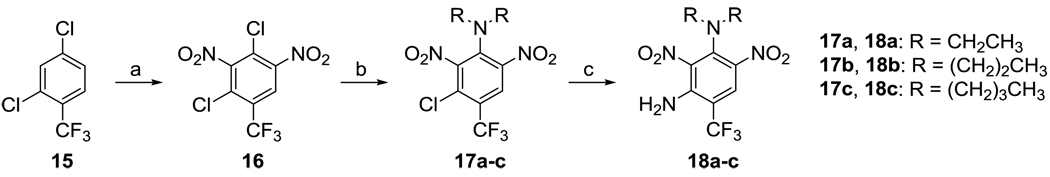
Dinitramine (18a) and its analogs were synthesized as shown in Scheme 5. 2,4-Dichloro-3,5-dinitrobenzotrifluoride (16) was prepared by nitration of 2,4-dichlorobenzotrifluoride (15) using fuming nitric acid in the presence of fuming sulfuric acid.36 Dinitrobenzotrifluoride 16 was treated with 2.2 equivalents of each of the desired secondary amines and heated in a sealed flask at 100 °C.36, 37 After a brief workup, the crude products 17a–c were dissolved in 3 equivalents of ammonia in dioxane and heated once again in a sealed flask at 100 °C, which gave the target compounds 18a–c in 60–80% yield for two steps. The use of excess amine and sealed flask conditions were critical for the success of the latter two reactions.
Scheme 5.
Reagents and conditions: a) Fuming sulfuric acid containing 30–33% SO3/fuming 90% nitric acid; b) secondary amine, absolute ethanol, 100 °C, 72 h; c) NH3 in 1,4-dioxane, 100°C, 40 h.
Structures for the proposed target compounds were confirmed with the aid of 1H and 13C NMR spectroscopy and mass spectrometry analysis, while the purity of the compounds was verified by elemental analysis. Synthetic procedures and spectral data for representative target compounds 6h, 12, and 18b are provided in the supplementary information.
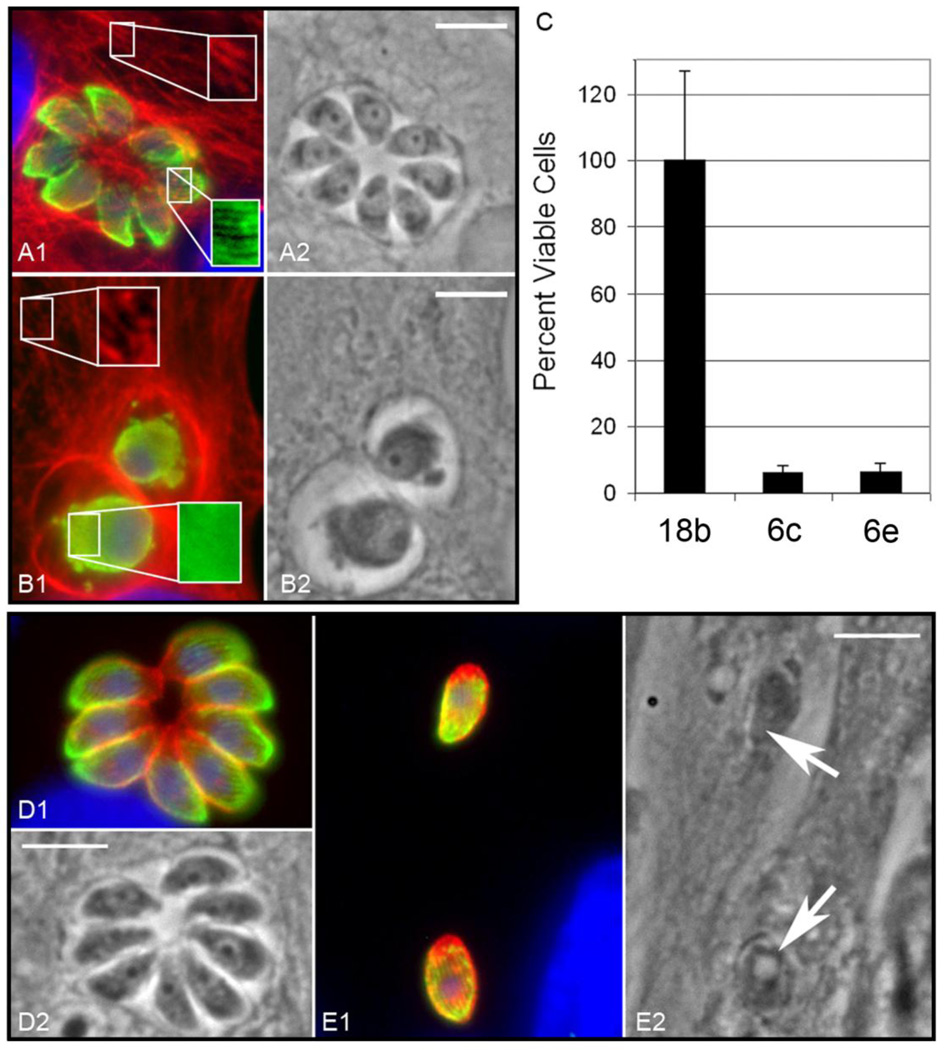
The growth inhibitory activity of compounds of interest against T. gondii in vitro was measured by a plaque assay,17 with the results shown in Table 1. The IC50 values for 6i, 14a, and 18a are consistent with those obtained previously in this assay.17 Toxoplasma microtubules were examined by an in vitro immunofluorescence assay (Figure 1). Figure 1A–B shows the effect of a selective antimitotic compound (18b), while Figure 1D–E illustrates the effect of a nonselective agent (6e). Experimental methods for both the plaque assay and the immunofluorescence assay are available in the supplementary information.
Table 1.
Activity of Target Compounds against Toxoplasma gondii In Vitro
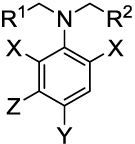 | ||||||
|---|---|---|---|---|---|---|
| IC50 (µM) vs. Toxoplasma gondiia |
||||||
| Compound | R1 | R2 | X | Y | Z | |
| 6a | Ph | CH2Cl | NO2 | SO2NH2 | H | 0.85 ± 0.03 |
| 6b | Ph | (CH2)2Cl | NO2 | SO2NH2 | H | 2.6 ± 0.1 |
| 6c | 4-Tolyl | 4-Tolyl | NO2 | SO2NH2 | H | Non-specific |
| 6d | cHexyl | cHexyl | NO2 | SO2NH2 | H | Non-specific |
| 6e | CH2Ph | CH2Ph | NO2 | SO2NH2 | H | Non-specific |
| 6f | Ph | Ph | NO2 | SO2NH2 | H | Non-specific |
| 6g | Ph | cPr | NO2 | SO2NH2 | H | 2.1 ± 0.1 |
| 6h | CH(CH3)2 | CH(CH3)2 | NO2 | SO2NH2 | H | 0.35 ± 0.02 |
| 6i | CH2CH3 | CH2CH3 | NO2 | SO2NH2 | H | 0.25 ± 0.01 |
| 6j | (CH2)2CH3 | (CH2)2CH3 | NO2 | SO2NH2 | H | 1.1 ± 0.2 |
| 6k | CH2CH3 | CH2CH3 | NO2 | SO2NH(CH2)2OH | H | 26 ± 2 |
| 6l | CH2CH3 | CH2CH3 | NO2 | SO2NH(4-phenol) | H | 2.6 ± 0.3 |
| 6m | CH2CH3 | CH2CH3 | NO2 | SO2NHPh | H | 6.7 ± 0.7 |
| 6n | CH2CH3 | CH2CH3 | NO2 | SO2N(CH3)2 | H | 3.4 ± 0.2 |
| 12 | CH2CH3 | CH2CH3 | CN | SO2NH2 | H | 4.8 ± 0.2 |
| 14a | CH2CH3 | CH2CH3 | NO2 | CF3 | H | 0.65 ± 0.06 |
| 14b | CH2CH3 | CH2CH3 | NO2, CF3 | NO2 | H | 7.8 ± 1.0 |
| 18a | CH3 | CH3 | NO2 | CF3 | NH2 | 0.070 ± 0.004 |
| 18b | CH2CH3 | CH2CH3 | NO2 | CF3 | NH2 | 0.036 ± 0.002 |
| 18c | (CH2)2CH3 | (CH2)2CH3 | NO2 | CF3 | NH2 | 0.51 ± 0.02 |
Mean ± standard deviation of three independent measurements, experimental methods are given in the supplementary information.
Figure 1.
(A–B) Compound 18b selectively disrupts microtubules in intracellular Toxoplasma gondii but not host vertebrate cells. (A1) Immunofluorescence of DMSO control treated parasite cultures illustrates that vehicle alone does not disrupt either vertebrate host cell (red) or Toxoplasma parasite (green) microtubules. Red: host cell microtubules retain a filamentous pattern, which can be seen more clearly in the boxed enlargement. Green: parasite subpellicular microtubules are present in a typical linear array. Blue: DAPI staining of host cell and parasite DNA. (A2) A phase contrast image of the same field; the scale bar represents 5 µm. (B1) Immunofluorescence of parasite cultures treated for 28 hours with 0.5 µM 18b in DMSO show that parasite but not host cells are disrupted. Red: host cell microtubules retain a filamentous pattern, which can be seen more clearly in the boxed enlargement. Green: parasite subpellicular microtubules are disrupted and free tubulin dimer staining is diffuse rather than organized into linear arrays. Blue: host cell and parasite DNA. (B2) A matched phase contrast image; the scale bar is 5 µm. (C) An MTS viability assay using the CellTiter reagent (Promega) illustrates that 6c and 6e are toxic to host cells at 30 µM, while 0.5 µM 18b (~10-fold greater than the antiparasitic IC50 value for this compound) does not have significant toxicity. (D–E) Compound 6e inhibits replication of intracellular Toxoplasma gondii but does not disrupt parasite microtubules, indicating that the compound interacts with target(s) other than tubulin. (D1) Immunofluorescence of a control culture: the rosette of eight daughter parasites indicates that the initial intracellular parasite has undergone 3 cycles of replication 28 hours after infection. Red: A parasite-specific surface antigen, P30/SAG1, labels the entire parasite surface. Green: parasite subpellicular microtubules are present in a typical linear array. Blue: host cell and parasite DNA. (D2) A matched phase contrast image; the scale bar is 5 µm. (E1) Immunofluorescence of a culture treated with 6e showing two single intracellular parasites which have not replicated 28 hours post infection. This culture was set up at the same time as the control culture, and parasite vacuoles should contain 4–16 parasites by 28 hours. Note the intact microtubules in the parasites. Fluorochromes are the same as in the control sample. (E2) A matched phase contrast image; the scale bar is 5 µm. Full experimental methods are given in the supplementary information.
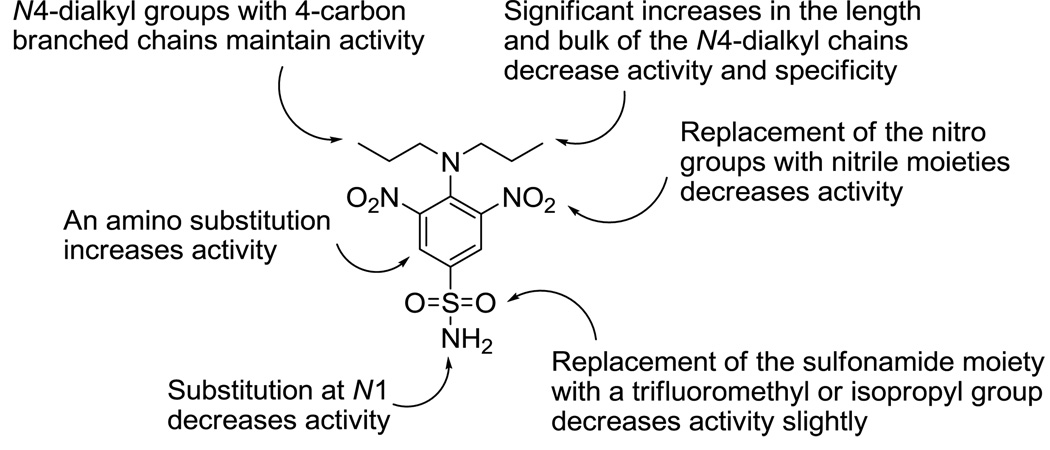
Among the compounds lacking a meta-amino group (6a–n, 12, 14a, and 14b), the dinitroaniline oryzalin (6i) was the most effective against Toxoplasma in this study, possessing an IC50 value of 0.25 µM. In other studies examining the efficacy of commercially available dinitroanilines, 6i was one of the most efficacious compounds to inhibit Toxoplasma growth as assessed by plaque assays.17 The other potent analogs of oryzalin are compounds 6h, 6a, and 6j, with IC50 values ranging from 0.35 µM to 1.1 µM. These results indicate that alkyl chains of 3 or 4 carbons in length at the aniline nitrogen of the dinitroanilines confer strong activity against intracellular T. gondii. Compound 6g, which possesses one four-carbon group and one benzyl group at the aniline nitrogen atom, is 8.5-fold less active than dipropyl derivative 6i and twofold less active than dibutyl congener 6j. Interestingly, compounds with two large substitutions at the aniline nitrogen (6c–f) do not appear to be tubulin specific inhibitors in Toxoplasma. These compounds inhibit parasite invasion, replication and growth at 10–25 µM without shortening the microtubules in the organism (Figure 1D–E, treatment with 30 µM 6e shown) and display host HFF cell toxicity at ~30 µM. We also used a tetrazolium dye assay (Figure 1C) to quantify viable host HFF cells after 17 hours of treatment with 30 µM of two of the non-specific compounds (6c and 6e) and with a parasite-specific compound (0.5 µM 18b). Compounds 6c and 6e cause host cell vacuolation and dramatically decrease the number of viable host cells. Although 6c and 6e inhibit parasite replication, parasite microtubules are not disrupted, suggesting that they have a distinct target. In contrast, the specific compound 18b does not compromise host cell viability at a concentration that selectively disrupts parasite microtubules.
Substitutions on the sulfonamide nitrogen decrease activity compared to 6i. Compounds with 4-hydroxyphenyl (6l), phenyl (6m), dimethyl (6n), or 2-hydroxyethyl (6k) substituents at the sulfonamide nitrogen are 10- to 100-fold less active than 6i. Interestingly, replacement of the nitro groups with nitrile moieties (12) results in a 19-fold drop in potency compared to 6i. This is in contrast to the structure-activity relationship observed in kinetoplastid parasites, where exchanging nitrile groups for the nitro moieties present in the potent antikinetoplastid compound 6m results in only a 1.5- to 2-fold loss of potency.32
Trifluralin (14a) is 2.6-fold less potent than 6i, indicating that the sulfonamide group conveys greater potency against Toxoplasma than the trifluoromethyl group. A 12-fold decrease in potency compared to 14a is observed for 14b, a compound where the nitro and trifluoromethyl groups of 14a are interchanged. The addition of the meta-amino group (compounds 18a–c), however, causes a dramatic increase in activity compared to 14a. Ma et al. recently reported that the meta-amino group present in 18a participates in two critical hydrogen bonds with the α-tubulin target, significantly increasing the activity of this compound compared to both 14a and 6i.17 Our present study confirms this result for 18a and also shows that the dipropylamino group found in 18b imparts twofold greater potency against Toxoplasma compared to the N,N-diethyl substitution present in 18a and 14-fold greater potency against this parasite compared to the N,N-di-n-butyl substitution occurring in 18c. The selective antimitotic action of 18b is illustrated in Figure 1A–B. Figure 1A1 shows well-defined microtubules present in both host cells and in Toxoplasma in the absence of compound treatment. In the presence of 0.5 µM 18b, host cell microtubules remain intact, while the diffuse staining of parasite tubulin reveals microtubule disruption in the parasites (Figure 1B1). Figure 1B also indicates that parasites exposed to 0.5 µM 18b are unable to replicate.
The results shown here, together with the data presented in Ma et al.17 concerning the evaluation of commercial dinitroanilines against T. gondii, permits us to develop an antimitotic structure-activity relationship for dinitroanilines vs. Toxoplasma. Isopropalin, a compound identical in structure to 6i and 14a except that it contains an isopropyl group para to the alkylamino position, reinforces the notion that sulfonamide-containing compounds are slightly superior to congeners with other substitutions at this position. As with compounds 6h and 6j, inclusion of a branched chain four carbon alkyl group in ethalfluralin leads to better activity than with benfluralin, which possesses an n-butyl group at the anilino nitrogen. The meta-amino derivatives 18a and 18b are 3.6- to 6.9-fold more potent than 6i against Toxoplasma in vitro, and studies with 18a–c suggest that the optimal chain length of the substitution on the aniline nitrogen is three carbons. These structure-activity data are summarized in Figure 2. Future efforts to optimize the anti-Toxoplasma potency and drug like properties of the dinitroanilines will focus on meta-amino containing derivatives 18a and 18b as lead compounds.
Figure 2.
Activity map for dinitroaniline analogs against Toxoplasma microtubules.
Supplementary Material
Acknowledgements
This work was funded by NIH Grants AI061021 (to K.A.W.) and AI067981 (to N.S.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Montoya J, Liesenfeld O. Lancet. 2004;363:1965. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Kongsaengdao S, Samintarapanya K, Oranratnachai K, Prapakarn W, Apichartpiyakul C. J. Int. Assoc. Physicians AIDS Care. 2008;7:11. doi: 10.1177/1545109707301244. [DOI] [PubMed] [Google Scholar]
- 3.Tsuda S, Kosaka Y, Matsusaka N, Sasaki Y. Mutat. Res. 1998;415:69. doi: 10.1016/s1383-5718(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 4.Misawa J, Kanda S, Kokue E, Hayama T, Teramoto S, Aoyama H, Kaneda M, Iwasaki T. Toxicol. Lett. 1982;10:51. doi: 10.1016/0378-4274(82)90266-1. [DOI] [PubMed] [Google Scholar]
- 5.Picot S, Olliaro P, Monbrison F, Bienvenu A, Price R, Ringwald P. Malaria J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meneceur P, Bouldouyre M, Aubert D, Villena I, Menotti J, Sauvage V, Garin J, Derouin F. Antimicrob. Agents Chemother. 2008;52:1269. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daudon M, Jungers P. Drugs. 2004;64:245. doi: 10.2165/00003495-200464030-00003. [DOI] [PubMed] [Google Scholar]
- 8.Dibbern D, Montanaro A. 2008;100:91. doi: 10.1016/S1081-1206(10)60415-2. [DOI] [PubMed] [Google Scholar]
- 9.Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. CMAJ. 2008;179:767. doi: 10.1503/cmaj.071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Megged O, Shalit I, Yaniv I, Stein J, Fisher S, Levy I. Pediatr. Transplantation. 2008;12:902. doi: 10.1111/j.1399-3046.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 11.Montoya J, Remington J. Clin. Infect. Dis. 2008;47:554. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 12.Morris P, Fornier M. Expert Rev. Anticancer Ther. 2009;9:175. doi: 10.1586/14737140.9.2.175. [DOI] [PubMed] [Google Scholar]
- 13.Kanthou C, Tozer G. Int. J. Exp. Pathol. 2009;90:284. doi: 10.1111/j.1365-2613.2009.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin R. Vet. J. 1997;154:11. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]
- 15.Stokkermans T, Schwartzman J, Keenan K, Morrissette N, Tilney L, Roos D. Exp. Parasitol. 1996;84:355. doi: 10.1006/expr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 16.Morrissette N, Mitra A, Sept D, Sibley L. Mol. Biol. Cell. 2004;15:1960. doi: 10.1091/mbc.E03-07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C, Tran J, Gu F, Ochoa R, Li C, Sept D, Werbovetz K, Morrissette N. Antimicrob. Agents Chemother. 2010;54:1453. doi: 10.1128/AAC.01150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrissette N, Sibley L. J. Cell Sci. 2002;115:1017. doi: 10.1242/jcs.115.5.1017. [DOI] [PubMed] [Google Scholar]
- 19.Shaw M, Compton H, Roos D, Tilney L. J. Cell Sci. 2000;113:1241. doi: 10.1242/jcs.113.7.1241. [DOI] [PubMed] [Google Scholar]
- 20.Chan M, Fong D. Science. 1990;249:924. doi: 10.1126/science.2392684. [DOI] [PubMed] [Google Scholar]
- 21.Arrowood M, Mead J, Xie L, You X. FEMS Microbiol. Lett. 1996;136:245. doi: 10.1111/j.1574-6968.1996.tb08056.x. [DOI] [PubMed] [Google Scholar]
- 22.Benbow J, Bernberg E, Korda A, Mead J. Antimicrob. Agents Chemother. 1998;42:339. doi: 10.1128/aac.42.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werbovetz K, Sackett D, Delfin D, Bhattacharya G, Salem M, Obrzut T, Rattendi D, Bacchi C. Mol. Pharmacol. 2003;64:1325. doi: 10.1124/mol.64.6.1325. [DOI] [PubMed] [Google Scholar]
- 24.Mitra A, Sept D. J. Med. Chem. 2006;49:5226. doi: 10.1021/jm060472+. [DOI] [PubMed] [Google Scholar]
- 25.Chan M, Triemer RE, Fong D. Differentiation. 1991;46:15. doi: 10.1111/j.1432-0436.1991.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 26.Chan M, Tzeng Y, Emge TJ, Ho C-T, Fong D. Antimicrob. Agents Chemother. 1993;37:1909. doi: 10.1128/aac.37.9.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugdahl JD, Morejohn LC. Plant Physiol. 1993;102:725. doi: 10.1104/pp.102.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morejohn L, Bureau T, Mole-Bajer J, Bajer A, Fosket D. Planta. 1987;172:252. doi: 10.1007/BF00394595. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya G, Salem M, Werbovetz K. Bioorg. Med. Chem. Lett. 2002;12:2395. doi: 10.1016/s0960-894x(02)00465-1. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya G, Herman J, Delfin D, Salem M, Barszcz T, Mollet M, Riccio G, Brun R, Werbovetz K. J. Med. Chem. 2004;47:1823. doi: 10.1021/jm0304461. [DOI] [PubMed] [Google Scholar]
- 31.George T, Johnsamuel J, Delfín D, Yakovich A, Mukherjee M, Phelps M, Dalton J, Sackett D, Kaiser M, Brun R, Werbovetz K. Bioorg. Med. Chem. 2006;14:5699. doi: 10.1016/j.bmc.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 32.George T, Endeshaw M, Morgan R, Mahasenan K, Delfín D, Mukherjee M, Yakovich A, Fotie J, Li C, Werbovetz K. Bioorg. Med. Chem. 2007;15:6071. doi: 10.1016/j.bmc.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beletskaya I, Cheprakov A. Coord. Chem. Rev. 2004;248:2337. [Google Scholar]
- 34.Maffioli S, Marzorati E, Marazzi A. Org. Lett. 2005;7:5237. doi: 10.1021/ol052100l. [DOI] [PubMed] [Google Scholar]
- 35.Soper Q. 3,442,639. U.S. Patent. 1969
- 36.Hunter D, Woods W, Stone J, LeFevre C. 3,903,078. U.S. Patent. 1975
- 37.Rasheed K, Warkentin J. J. Org. Chem. 1975;42:1265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.