Abstract
Rat small intestinal mucosa was examined for ability to produce mucins with human blood group A, B, and H activity. Blood group activity of the mucins was compared to antigenic activity of red blood cells in individual rats and the enzymatic basis for differences was investigated. Red cells in all the rats examined contained human blood group A and B antigens. All rats synthesized intestinal mucins having B and H antigenic activity but 57% failed to produce mucins with blood group A activity (A-); the remaining 43% (A+) produced A substance.
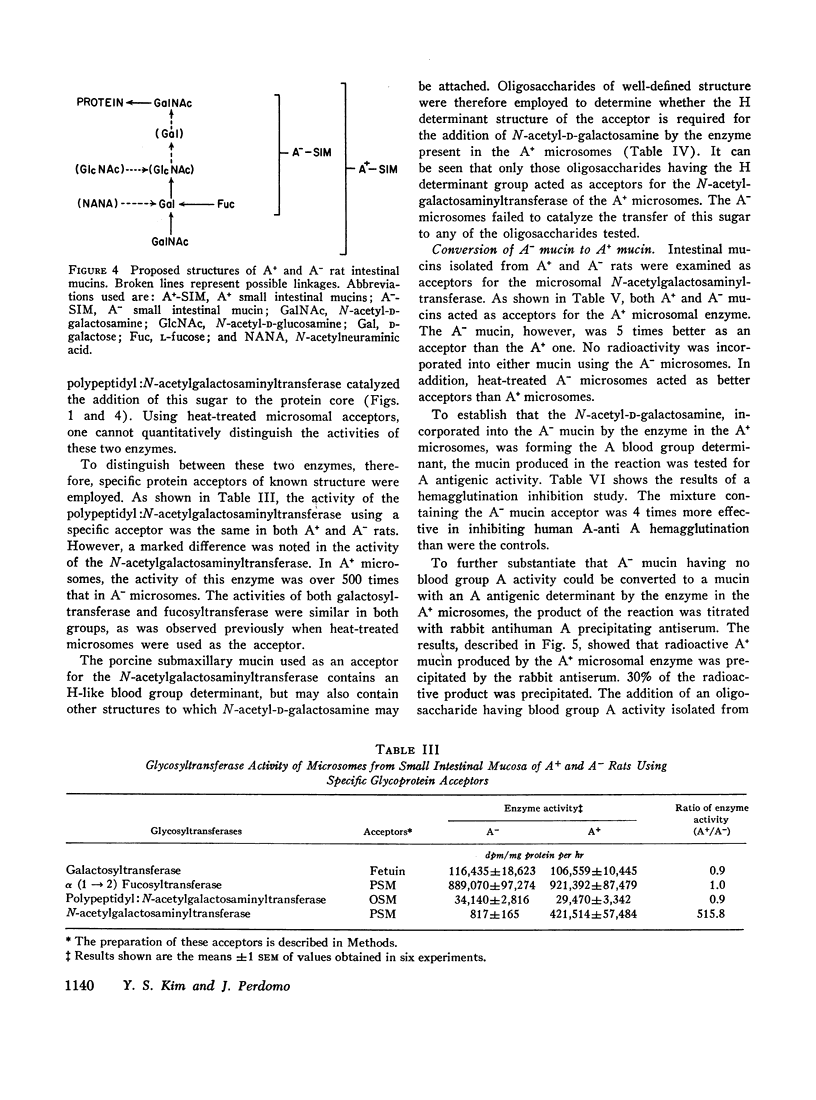
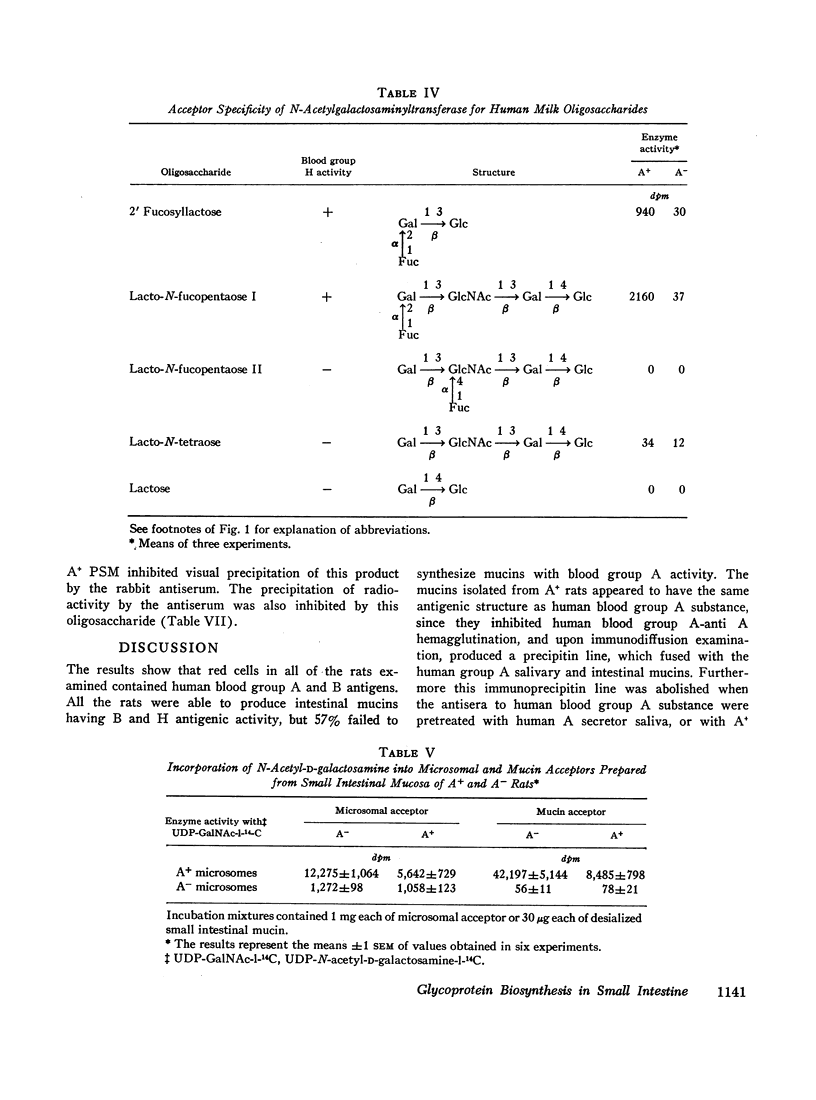
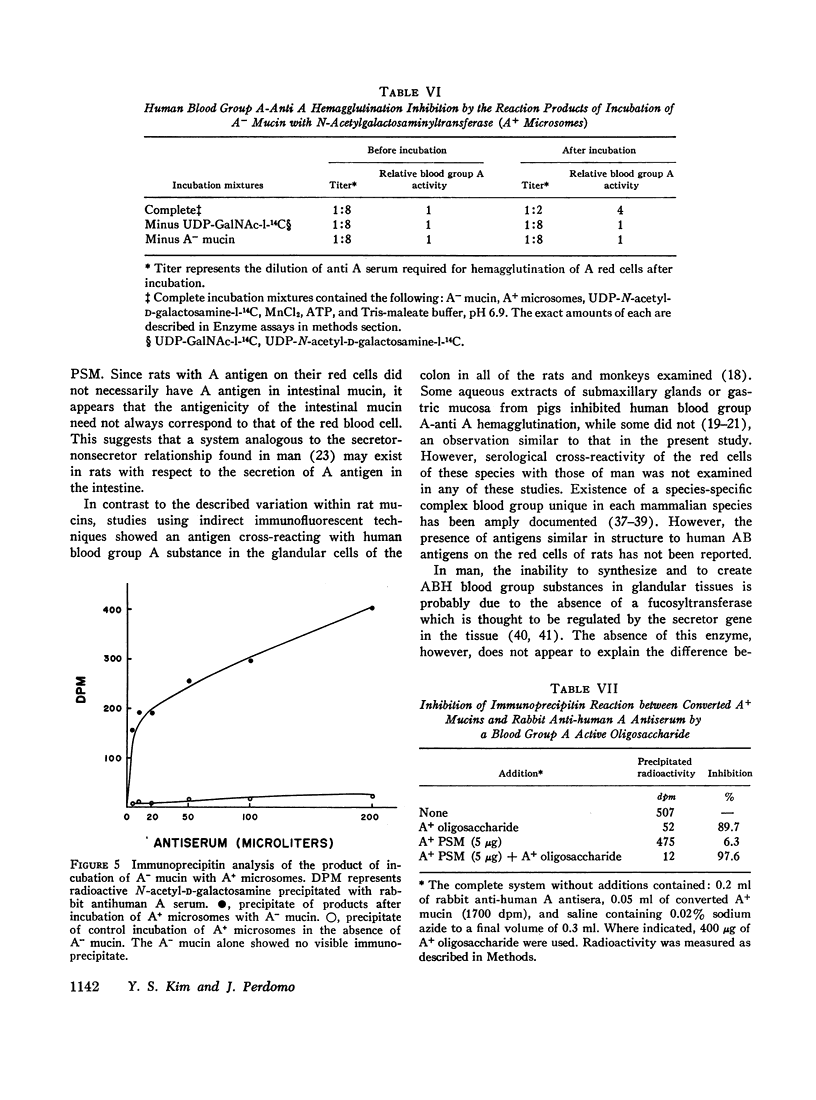
The activities of five glycosyltransferases including α(1→2) fucosyltransferase, the determinant of human secretor status, were measured in the intestine of A+ and A- rats. Four enzymes were the same in both groups, while the fifth, N-acetylgalactosaminyltransferase, was present only in A+ rats. The specificity of this latter enzyme, as found in the rat, appeared similar to that in humans, since it catalyzed addition of N-acetyl-D-galactosamine only to acceptors which had the H determinant structure. In the presence of the enzyme, A- mucin could be converted to A+ mucin; this was shown both by hemagglutination inhibition and immunoprecipitin studies of the products of incubation of A- mucin with UDP-N-acetyl-D-galactosamine and the enzyme.
These studies indicate that the difference between A+ and A- rats is due to the apparent absence of N-acetylgalactosaminyltransferase in the intestinal mucosa of A- rats. These rats may provide experimental models for studies on the effect of ABO and secretor status on susceptibility to ulceration and carcinogenesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIRD I., BENTALL H. H., MEHIGAN J. A., ROBERTS J. A. F. The blood groups in relation to peptic ulceration and carcinoma of colon, rectum, breast, and bronchus; an association between the ABO groups and peptic ulceration. Br Med J. 1954 Aug 7;2(4883):315–321. doi: 10.1136/bmj.2.4883.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIRD I., BENTALL H. H., ROBERTS J. A. F. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953 Apr 11;1(4814):799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRESEN E. Blood groups in pigs. Ann N Y Acad Sci. 1962 May 3;97:205–225. [PubMed] [Google Scholar]
- Bella A. M., Jr, Kim Y. S. An improved method of separating glucosaminitol from galactosaminitol and their amino sugars on an amino acid analyzer. J Chromatogr. 1970 Sep 16;51(2):314–315. doi: 10.1016/s0021-9673(01)96872-4. [DOI] [PubMed] [Google Scholar]
- Bella A., Jr, Kim Y. S. Biosynthesis of intestinal glycoprotein: a study of an (1 lead to 2) fucosyltransferase in rat small intestinal mucosa. Arch Biochem Biophys. 1971 Dec;147(2):753–761. doi: 10.1016/0003-9861(71)90435-8. [DOI] [PubMed] [Google Scholar]
- CARLSON D. M., SWANSON A. L., ROSEMAN S. PREPARATION OF CRYSTALLINE ALPHA-D-GALACTOSAMINE-1-PHOSPHORIC ACID AND ITS CONVERSION TO UDP-N-ACETYLGALACTOSAMINE. Biochemistry. 1964 Mar;3:402–405. doi: 10.1021/bi00891a016. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- DOLL R., DRANE H., NEWELL A. C. Secretion of blood group substances in duodenal, gastric and stomal ulcer, gastric carcinoma, and diabetes mellitus. Gut. 1961 Dec;2:352–359. doi: 10.1136/gut.2.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Salegui M., Plonska H. Preparation and properties of porcine submaxillary mucins. Arch Biochem Biophys. 1969 Jan;129(1):49–56. doi: 10.1016/0003-9861(69)90148-9. [DOI] [PubMed] [Google Scholar]
- Denborough M. A., Downing H. J., McCrea M. G. The ABO system in milk and saliva. Australas Ann Med. 1967 Nov;16(4):320–325. doi: 10.1111/imj.1967.16.4.320. [DOI] [PubMed] [Google Scholar]
- FRANKS D. Horse blood groups and hemolytic disease of the newborn foal. Ann N Y Acad Sci. 1962 May 3;97:235–250. doi: 10.1111/j.1749-6632.1962.tb34639.x. [DOI] [PubMed] [Google Scholar]
- GLYNN L. E., HOLBOROW E. J., JOHNSON G. D. The distribution of blood-group substances in human gastric and duodenal mucosa. Lancet. 1957 Nov 30;273(7005):1083–1088. doi: 10.1016/s0140-6736(57)90115-0. [DOI] [PubMed] [Google Scholar]
- Grollman E. F., Ginsburg V. Correlation between secretor status and the occurrence of 2'-fucosyllactose in human milk. Biochem Biophys Res Commun. 1967 Jul 10;28(1):50–53. doi: 10.1016/0006-291x(67)90404-4. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. I., JEANLOZ R. W. Isolation and characterization of glycolipids from erythrocytes of human blood A (plus) and B (plus). J Biol Chem. 1961 Nov;236:2827–2834. [PubMed] [Google Scholar]
- HOSKINS L. C., ZAMCHECK N. STUDIES ON GASTRIC MUCUS IN HEALTH AND DISEASE. II. EVIDENCE FOR A CORRELATION BETWEEN ABO BLOOD GROUP SPECIFICITY, ABH(O) SECRETOR STATUS, AND THE FUCOSE CONTENT OF THE GLYCOPROTEINS ELABORATED BY THE GASTRIC MUCOSA. Gastroenterology. 1965 Jun;48:758–767. [PubMed] [Google Scholar]
- Hagopian A., Bosmann H. B., Eylar E. H. Glycoprotein biosynthesis: the localization of polypeptidyl: N-acetylgalactosaminyl, collagen: glucosyl, and glycoprotein:galactosyl transferases in HeLa cell membrane fractions. Arch Biochem Biophys. 1968 Nov;128(2):387–396. doi: 10.1016/0003-9861(68)90045-3. [DOI] [PubMed] [Google Scholar]
- KOSCIELAK J., ZAKRZEWSKI K. Substance from erythrocytes of blood group A. Nature. 1960 Aug 6;187:516–517. doi: 10.1038/187516b0. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Bella A., Jr, Nordberg J. N-acetyl-D-galactosaminyltransferase in human serum and erythrocyte membranes. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1753–1756. doi: 10.1073/pnas.68.8.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Kobata A., Ginsburg V. Uridine diphosphate-N-acetyl-D-galactosamine: D-galactose alpha-3-N-acetyl-D-galactosaminyltransferase, a product of the gene that determines blood type A in man. J Biol Chem. 1970 Mar 25;245(6):1484–1490. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A. Immunochemical studies on blood groups. XLI. Proposed structures for the carbohydrate portions of blood group A, B, H, Lewis-a, and Lewis-b substances. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1470–1477. doi: 10.1073/pnas.61.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Rosenfield R. E. Immunochemical studies on blood groups. XXXV. The activity of fucose-containing oligosaccharides isolated from blood group A, B, and H substances by alkaline degradation. Biochemistry. 1966 May;5(5):1502–1507. doi: 10.1021/bi00869a008. [DOI] [PubMed] [Google Scholar]
- MARCUS D. M., KABAT E. A., SCHIFFMAN G. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXXI. DESTRUCTION OF BLOOD GROUP A ACTIVITY BY AN ENZYME FROM CLOSTRIDIUM TERTIUM WHICH DEACETYLATES N-ACETYLGALACTOSAMINE IN INTACT BLOOD GROUP SUBSTANCES. Biochemistry. 1964 Mar;3:437–443. doi: 10.1021/bi00891a023. [DOI] [PubMed] [Google Scholar]
- Marcus D. M. The ABO and Lewis blood-group system. Immunochemistry, genetics and relation to human disease. N Engl J Med. 1969 May 1;280(18):994–1006. doi: 10.1056/NEJM196905012801806. [DOI] [PubMed] [Google Scholar]
- PALM J. Current status of blood groups in rats. Ann N Y Acad Sci. 1962 May 3;97:57–68. doi: 10.1111/j.1749-6632.1962.tb34622.x. [DOI] [PubMed] [Google Scholar]
- Painter T. J., Watkins W. M., Morgan W. T. Serologically active fucose-containing oligosaccharides isolated from human blood-group A and B substances. Nature. 1965 May 8;206(984):594–597. doi: 10.1038/206594a0. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- SZULMAN A. E. The histological distribution of blood group substances A and B in man. J Exp Med. 1960 Jun 1;111:785–800. doi: 10.1084/jem.111.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Grollman E. F., Ginsburg V. An enzymatic basis for secretor status and blood group substance specificity in humans. Proc Natl Acad Sci U S A. 1968 Jan;59(1):224–230. doi: 10.1073/pnas.59.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Szulman A. E. Chemistry, distribution, and function of blood group substances. Annu Rev Med. 1966;17:307–322. doi: 10.1146/annurev.me.17.020166.001515. [DOI] [PubMed] [Google Scholar]
- Tuppy H., Staudenbauer W. L. Microsomal incorporation of N-acetyl-D-galactosamine into blood group substance. Nature. 1966 Apr 16;210(5033):316–317. doi: 10.1038/210316a0. [DOI] [PubMed] [Google Scholar]
- Watkins W. M. Blood-group substances. Science. 1966 Apr 8;152(3719):172–181. doi: 10.1126/science.152.3719.172. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Oudea P., Halpern B., Veyre C. Presence in colic glandular cells of various mammalian species of an antigen cross-reacting with human blood group A substance. Nature. 1966 Jan 8;209(5019):159–161. doi: 10.1038/209159a0. [DOI] [PubMed] [Google Scholar]