TO THE EDITOR
Aerobic training improves thermal tolerance (Armstrong and Pandolf, 1988), which is postulated to result in part from improved evaporative cooling (Shibasaki et al., 2006; Taylor, 1986). Previous observations indicate that aerobic training either lowers the internal temperature at which sweating begins (sweating threshold) or increases the slopes of the internal temperature-sweat rate (Roberts et al., 1977) or exercise intensity-sweat rate relations (Yanagimoto et al., 2002). In contrast, deconditioning or detraining, associated with bedrest, increases the sweating threshold and decreases the slope of internal temperature-sweat rate relation (Lee et al., 2002). Importantly these deconditioning related responses can be prevented by exercising during bedrest (Shibasaki et al., 2003). These studies, although informative, do not provide mechanistic insight into whether altered sweating responses to aerobic training are mediated by a central sympathetic component or at the level of the eccrine gland.
Application of cholinergic agonists directly in the dermal space without repeated injections or electric current can isolate peripheral sweating responses (Crandall et al., 2003; Morgan et al., 2006; Schlereth et al., 2006). Cross-sectional studies (comparing aerobic trained vs. untrained) using electrical current drug delivery (iontophoresis) have observed increased sweating capacity and number of activated sweat glands with aerobic training (Buono and Sjoholm, 1988; Buono et al., 1992). However, these studies provided minimal insight into whether these adaptatory responses were mediated by changes in receptor responsiveness and sensitivity, or where simply a function of subject population selected. Accordingly, we tested the hypothesis that 8-weeks of aerobic training increases in vivo cholinergic sensitivity and responsiveness of eccrine sweat glands, without altering the number of exogenous acetylcholine-activated glands.
Eleven sedentary, young (age=26±1), healthy, non-obese (BMI<30kg/m2), normotensive (<140/90mmHg), non-smokers participated in this institutional (PSUCOM) approved longitudinal training study that adhered to Declaration of Helsinki guidelines. Each subject provided written informed consent and received a medical history and physical exam before participating in the study. Two intradermal microdialysis membranes were placed ~3cm apart in dorsal forearm skin in a similar location pre- and post-training which unlike previous studies allows for continuous monitoring of sweat glands at a prescribed agonist concentration (Morgan et al., 2006). This technique involved placing a small (200-μm outer diameter, 10-mm length) semipermeable membrane (20-kDa-cutoff) intradermally at a depthof approximately 0.3–1.0mm below the epidermis (Kellogg et al., 1999). Eight doses of acetylcholine (10−7 to 1M ACh) in a lactated Ringer’s vehicle were administered for 5 min at 2μl/min via a microdialysis infusion pump. Sweat rate was measured via capacitance hygrometry and the number of activated sweat glands was quantified using a starch-iodine technique during infusion of 1M ACh. Cholinergic dose-response relations were determined by logistic regression modeling. Endurance training consisted of running or cycling 4 times/week for 8 weeks. Subjects wore a heart rate monitor during all exercise sessions to ensure maintenance of target heart rates. Training began with exercising for 20 min at a work rate sufficient to achieve 80% of maximum heart rate. Exercise times increased to 60 min as training progressed, and high-intensity interval exercises were added twice/week during the second week to further stimulate training adaptations.
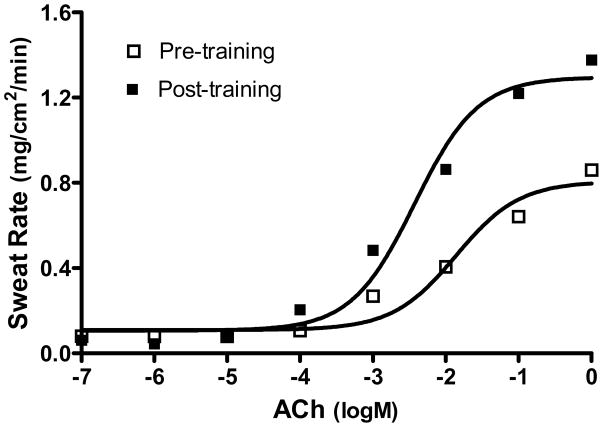
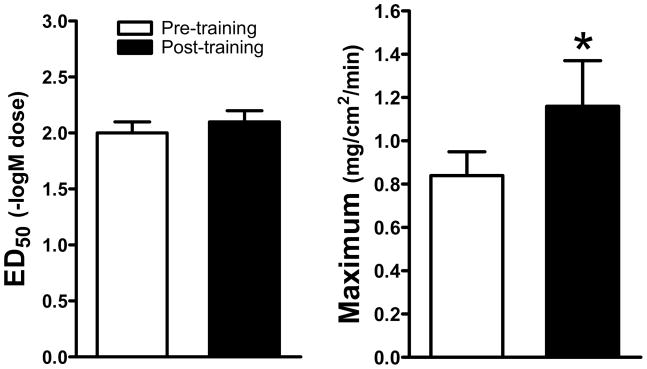
Peak oxygen uptake increased from 32.9 to 40.7ml•kg−1•min−1 or 19±2% (P<0.05) and resting heart rate decreased 9±2bpm (P<0.05) indicative of a training effect. The number of cholinergic-activated glands was unchanged by training (pre-training=40±6 and post-training=39±7 glands per 0.5cm2). This indicates that a similar number of glands were recruited both pre- and post-training and that this type and duration of training does not increase the number of exogenous cholinergic-activated glands. Dose-response relations were established with goodness of fit (R2) relations of 0.67±0.02 for pre-training and 0.72±0.02 for post-training, indicating that the logistic regression modeling adequately modeled the data (Figure 1). Training did not affect the ED50, but increased the maximal responses of the dose-response relation (Figure 2).
Figure 1.
Representative dose-response relations of acetylcholine (ACh) to sweat rate from pre- to post-training in one subject.
Figure 2.
Effect of aerobic training on in vivo cholinergic dose-response relations. ED50 is the effective dose causing 50% of the maximal response and is an indicator of sensitivity. Maximum is the maximal response of the cholinergic dose-response relation and is indicative of responsiveness. * denotes a significant difference from the previous time point (P<0.05).
These findings provide important insight into the adaptatory mechanism(s) of eccrine sweat glands to longitudinal aerobic training in humans. First, we did not observe a leftward shift in the ED50 of the cholinergic dose-response curve, strongly suggesting that exercise training does not alter eccrine sweat gland in vivo cholinergic sensitivity as previously suggested (Buono et al., 1992; Roberts et al., 1977). However, these reports suggesting an increase in cholinergic sensitivity used an iontophoresis drug delivery system to engage muscarinic receptors with pilocarpine or observed an increase in the slope of internal temperature-sweat rate relation. Pilocarpine iontophoresis, although beneficial for determining gross changes in sweating such as occur with cystic fibrosis (Quinton, 2007), has certain limitations (Hjortskov et al., 1995). These limitations include possible direct damage to the sweat gland that could result in erythemia and release of inflammatory mediators, and a poor ability to precisely adjust drug doses. Previous reports of increased slope of the internal temperature-sweat rate relation are also methodologically limited (Cheuvront et al., 2009) and likely gauge the sensitivity of the homeostatic gain rather than the sweat gland per se.
Second, the observed increases in in vivo cholinergic responsiveness in the eccrine sweat gland after aerobic training suggests that adaptations are due to changes in peripheral glandular function rather than via a central sympathetic component. Previously, isolated sweat glands from trained or acclimated individuals were reported to possess larger secretory coils that were more responsive to direct application of methylcholine (Sato and Sato, 1983). This finding of normal but enlarged ultrastructure is also observed in focal hyperhidrosis patients (Bovell et al., 2001). As adaptations to aerobic exercise training appear to also include larger, more responsive eccrine glands, the possibility exists that exercise training could provide a model for studying alterations in sweat gland structure and function associated with hyperhidrosis. Another dermatological application includes reduced sweating responses, such as occurs in skin graft patients, where sweat gland adaptations may be used to increase their sweating responses (Davis et al., 2009; Wingo et al., 2008). Combined with Crandall et al.’s (Crandall et al., 2003) cholinergic dose-response data, our findings allow the formation of a sweating adaptation model where bedrest attenuates, exercise during bedrest rescues, and exercise training accentuates in vivo cholinergic responsiveness.
Acknowledgments
This study was supported by National Institutes of Health Grants HL77670 and AG24420. Additional support was provided by NIH Grants M01 RR-10732 and C06 RR-016499.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Armstrong LE, Pandolf KB. Physical training, cardiorespiratory physical fitness and exercise-heat tolerance. In: Pandolf KB, Sawka MN, Gonzalez RR, editors. Human performance physiology and environmental medicine at terrestrial extremes. Carmel, IN: Cooper Publishing; 1988. pp. 199–226. [Google Scholar]
- Bovell DL, Clunes MT, Elder HY, Milsom J, Jenkinson DM. Ultrastructure of the hyperhidrotic eccrine sweat gland. Br J Dermatol. 2001;145:298–301. doi: 10.1046/j.1365-2133.2001.04351.x. [DOI] [PubMed] [Google Scholar]
- Buono MJ, Sjoholm NT. Effect of physical training on peripheral sweat production. J Appl Physiol. 1988;65:811–814. doi: 10.1152/jappl.1988.65.2.811. [DOI] [PubMed] [Google Scholar]
- Buono MJ, White CS, Connolly KP. Cholinergic sensitivity of the eccrine sweat gland in trained and untrained men. J Dermatol Sci. 1992;4:33–37. doi: 10.1016/0923-1811(92)90053-e. [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, Bearden SE, Kenefick RW, Ely BR, Degroot DW, Sawka MN, et al. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol. 2009;107:69–75. doi: 10.1152/japplphysiol.00250.2009. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Shibasaki M, Wilson TE, Cui J, Levine BD. Prolonged head-down tilt exposure reduces maximal cutaneous vasodilator and sweating capacity in humans. J Appl Physiol. 2003;94:2330–2336. doi: 10.1152/japplphysiol.00790.2002. [DOI] [PubMed] [Google Scholar]
- Davis SL, Shibasaki M, Low DA, Cui J, Keller DM, Wingo JE, et al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30:675–685. doi: 10.1097/BCR.0b013e3181abfd43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortskov N, Jepsen LT, Nielsen B, Juul A, Skakkebaek NE. Pilocarpine iontophoresis test: an index of physiological sweat secretion? Clin Physiol. 1995;15:409–414. doi: 10.1111/j.1475-097x.1995.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Lee SM, Williams WJ, Schneider SM. Role of skin blood flow and sweating rate in exercise thermoregulation after bed rest. J Appl Physiol. 2002;92:2026–2034. doi: 10.1152/japplphysiol.00105.2001. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Friedmann PS, Church MK, Clough GF. Cutaneous microdialysis as a novel means of continuously stimulating eccrine sweat glands in vivo. J Invest Dermatol. 2006;126:1220–1225. doi: 10.1038/sj.jid.5700197. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda) 2007;22:212–225. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol. 1977;43:133–137. doi: 10.1152/jappl.1977.43.1.133. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol. 1983;245:R203–208. doi: 10.1152/ajpregu.1983.245.2.R203. [DOI] [PubMed] [Google Scholar]
- Schlereth T, Dittmar JO, Seewald B, Birklein F. Peripheral amplification of sweating--a role for calcitonin gene-related peptide. J Physiol. 2006;576:823–832. doi: 10.1113/jphysiol.2006.116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol. 2006;100:1692–1701. doi: 10.1152/japplphysiol.01124.2005. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Levine BD, Crandall CG. Exercise throughout 6 degrees head-down tilt bed rest preserves thermoregulatory responses. J Appl Physiol. 2003;95:1817–1823. doi: 10.1152/japplphysiol.00188.2003. [DOI] [PubMed] [Google Scholar]
- Taylor NA. Eccrine sweat glands. Adaptations to physical training and heat acclimation. Sports Med. 1986;3:387–397. doi: 10.2165/00007256-198603060-00001. [DOI] [PubMed] [Google Scholar]
- Wingo JE, Low DA, Keller DM, Davis SL, Kowalske KJ, Purdue GF, et al. Heat acclimation of an adult female with a large surface area of grafted skin. J Burn Care Res. 2008;29:848–851. doi: 10.1097/BCR.0b013e3181848b5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimoto S, Aoki K, Horikawa N, Shibasaki M, Inoue Y, Nishiyasu T, et al. Sweating response in physically trained men to sustained handgrip exercise in mildly hyperthermic conditions. Acta Physiol Scand. 2002;174:31–39. doi: 10.1046/j.1365-201x.2002.00921.x. [DOI] [PubMed] [Google Scholar]