Abstract
Glycogen synthase kinase-3 (GSK3 β) and casein kinase-1 alpha (CK-1α) are multifunctional kinases that play critical role in the regulation of a number of cellular processes. In spite of their importance, molecular imaging tools for non-invasive and real-time monitoring of their kinase activities have not been devised. Here, we report development of bioluminescent GSK3β and CK-1α reporter (BGCR) based on firefly luciferase complementation. Treatment of SW620 cells stably expressing the reporter with inhibitors of GSK3β (SB415286 and LiCl) or CK1α (CKI-7) resulted in dose and time dependent increase in BGCR activity which were validated using western blotting. No increase in bioluminescence was observed in case of S37A mutant (GSK3β inhibitors) or with S45A mutant (CKI-7) demonstrating the specificity of the reporter. Imaging of mice tumor xenograft generated with BGCR expressing SW620 cells following treatment with LiCl showed unique oscillations in GSK3β activity which were corroborated by phospho-GSK3β immunoblotting. Taken together, BGCR is novel molecular imaging tool that reveals unique insight into GSK3β and CK1α kinase activities and may provide powerful tool in experimental therapeutics for rapid optimization of dose and schedule of targeted therapies and for monitoring therapeutic response.
Keywords: Molecular imaging, GSK3 β, CK1α, split luciferase, reporter
Introduction
GSK3 is a ubiquitously expressing multifunctional serine/threonine kinase which was originally identified in mammals as a critical mediator in glycogen metabolism and insulin signaling. It is now known that GSK3 is a key regulator of diverse signaling pathways involved in the regulation of glycogen metabolism, protein synthesis, cell mobility, cell fate, proliferation and survival [1; 2]. Two GSK3 isoforms (GSK3α and GSK3β) are present in mammals which are encoded by two distinct genes located on different chromosomes. GSK3α and GSK3β are not functionally identical and redundant though they show a high degree of similarity (85%) [3]. The signaling pathway and protein function of GSK3β are much better investigated. GSK3β is active in resting cells and is inactivated during cellular responses. Due to its diverse cellular functions, the pathways in which GSK3β acts as a key regulator, when dysregulated, have been implicated in the development of a number of human diseases such as diabetes, some neurodegenerative diseases, bipolar disorder, cardiovascular disease, tumorigenesis and cancer progression [1; 2; 3; 4; 5]. GSK3β has preference for ‘primed’ substrates [6] and more than 40 proteins have been described as substrates of GSK3β, a number of them have been implicated in tumorigenesis like β-catenin, APC, AXIN, and p53.
Casein kinase-1 group (CK1) enzymes have been implicated in a variety of biological functions, including Wnt pathway regulation [7; 8], circadian rhythm [9], chromosome segregation [10; 11], spindle formation [12; 13; 14], nuclear import [15], cell cycle regulation [16] and apoptosis [17]. Deregulation of CK1 isoforms has been described in neurodegenerative diseases [18], Alzheimer [19], sleeping disorders [20] and in cancer [21]. GSK3β and CK1α have some common target protein like β-catenin and P53 [22] but do not share target sites.
β-catenin is phosphorylated at the N-terminus by CK1α at S45, and T41, S37 and S33 by GSK3β [23; 24] and marks it for ubiquitin-mediated degradation. Presence of ligand inactivates GSK3β and prevents it from phosphorylating β-catenin, thus stabilizing β-catenin in the cytoplasm. As β-catenin accumulates, it translocates into the nucleus where it binds to TCF/LEF family of transcription factors and turn on a number of target genes [25; 26]. In human malignancies such as cancers of colon, liver and skin, inappropriate activation of the Wnt/β-catenin signaling pathway has been shown to occur through gain-of-function mutations resulting in the loss of GSK3β and CK1α phosphorylation site in β-catenin.
Most of these studies utilize conventional modalities, such as western blotting, immunoprecipitation and other modalities which provide an excellent molecular snapshot image but fail to capture the dynamic variance in the signaling cascade. Here we report development of a genetically engineered chimeric reporter which monitors GSK3β and CK1α activity in real time non-invasively.
The bioluminescent GSK3β and CK-1α reporter (BGCR) is generated after a luciferase complementation based reporter [27]. BGCR is a sensitive, dynamic and quantitative sensor of both GSK3β and CK1α which responds in a dose and time dependent manner to the inhibition of these kinases. In a tumor xenograft mice model, BGCR reveals oscillatory changes in activity of GSK3β in response to LiCl. Such real time understanding of the kinase activity was not possible without the molecular imaging sensors for kinases. In summary this reporter represent a unique tool for sensitive real-time imaging of GSK3β and CK1α kinase activities and has the potential for use in enhancing our understanding of the biology of these key kinase regulators in living subjects and cells as well as for screening, characterization and pharmacodynamic analysis of potential regulators and drugs that target the GSK3β and CK1α.
Results
Construction of BGCR
To image GSK3β kinase activity, we constructed a recombinant chimeric reporter (Figure 1A) consisting of a 20 amino acid target peptide sequence (derived from β-catenin (aa 29-48, [26]) which is a substrate for GSK3β and CK1α [28]. The BGCR reporter also consisted of the FHA2 phospho-serine/threonine binding domain of Rad53 [29], flanked by N-Luc (residues 1 to 416 of firefly luciferase) and C-Luc (residues 398 to 550 of firefly luciferase)[30]. The functional basis (Figure 1B) of the reporter was that when kinase was active, phosphorylation of the substrate peptide would result in the binding of the phospho-serine/threonine residue to the FHA2 domain, thus preventing reconstitution of N-Luc with C-Luc due to stearic hindrance. Inhibition of kinase activity would result in loss of phosphorylation of the substrate peptide, thus releasing it from interaction with the FHA2 phospho-peptide binding domain. In this situation, N-Luc and C-Luc can associate and reconstitute luciferase activity, which can be detected by bioluminescence imaging.
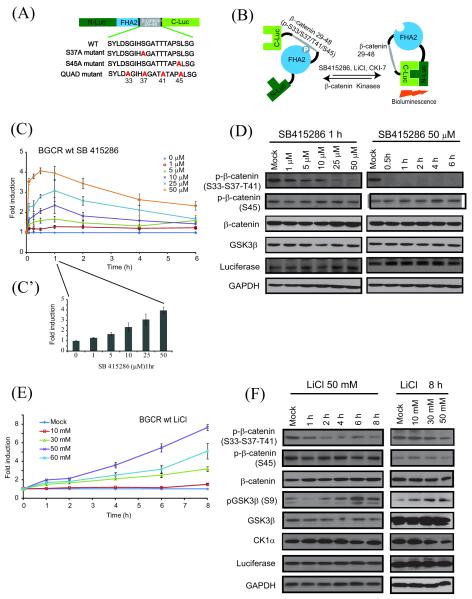
Figure 1.
Construction & validation of bioluminescent GSK3β and CK-1α reporter (BGCR) and time and dose dependent imaging of GSK3β activity. (A) The domain structure of the BGCR reporter. N-Luc and C-Luc are the amino- and carboxy- terminal domains of firefly luciferase that are fused to the appropriate ends of the reporter. The β-catenin peptide domain constitutes amino- terminal (aa 29-48) of CKIα and GSK3β substrate sequence with a flexible GGSGG linker on either side. At the amino- terminal of the β-catenin peptide domain the yeast FHA2 phospho-ser/thr binding domain (residues 420-582) was appended. Three different mutant versions of the BGCR WT reporter were developed. The S37A mutant molecule contained Ser to Ala substitution at a GSK3β phosphorylation site, S45A mutant where Ser to Ala substitution was done at the CK1α phosphorylation site and QUAD mutant where all four Ser/Thr within the substrate sequence were substituted to Ala. (B) The proposed mechanism of action for the BGCR reporter involves CKIα and GSK3β dependent phosphorylation of β-catenin target peptide which results in its interaction with the FHA2 domain. In this form the reporter has minimal bioluminescence activity. In the absence of kinase activity, association of the N-Luc and C-Luc domains restores bioluminescence activity. (C) Cells stably expressing the BGCR-WT reporter were treated with various doses (1 μM, 5 μM, 10 μM, 25 μM, and 50 μM) of SB415286 and BLI was measured serially from baseline to 6 h. The change in BLI activity over pretreatment (0 μM) levels was determined and plotted as fold induction. BLI measurements were done in triplicates. Error bars denote SEM. (C’) The bar diagram show dose dependent changes in reporter activity at 1 h with SB415286. (D) Western blot analysis of samples from Fig 1C using anti-phospho-(S33-S37-T41), phospho-(S45)-β-catenin, total β-catenin, GSK3β, reporter (luciferase) and GAPDH antibodies. (E) SW620 cells stably expressing reporter were treated in triplicates with various doses of LiCl (10 mM, 30 mM, 50 mM and 60 mM) and BLI was measured serially until 8 h. The change in BLI activity over vehicle control treated (mock) levels for each time point was calculated and plotted as fold change. All the BLI measurements were done in triplicates. Error bars denote SEM. (F) Western blot analysis of the cells stably expressing BGCR-WT reporter were treated with 50 mM LiCl for 1 h, 2 h, 4 h, 6 h and 8 h and various doses of LiCl (10 mM, 30 mM, and 50 mM) for 8 h using anti-phospho-(S33-S37-T41)-β-catenin, phospho-(S45)-β-catenin, total β-catenin, phospho-(S9)-GSK3β, total GSK3β, CK1α, luciferase and GAPDH antibodies.
Treatment with GSK3β inhibitors indicate high sensitivity of BGCR reporter
To investigate if BGCR senses inhibition of GSK3β and CK1α, stable cell lines expressing the reporter were constructed. SW620 colon carcinoma cells expressing the reporter were treated with the small molecular inhibitor SB415286 and LiCl, agents that have been shown to inhibit GSK3β [31; 32; 33; 34], as well as CKI-7, a CK1α inhibitor.
When SW620 cells stably expressing the WT reporter was treated with SB415286, a GSK3 inhibitor, a dose dependent increase in bioluminescence was observed. Quantitation of bioluminescence after treatment with 1-50 μM of SB415286 revealed a dose and time dependent increase in BGCR-WT activity (Figure 1C). The increase in bioluminescence was rapid which peaked between 30 min to 1 h (Figure 1C’). Influence of the SB415286 on phosphorylation of the substrate protein was confirmed by antibodies to phosphorylated S33-S37-T41 β-catenin. A dose dependent decrease in phospho-β-catenin and concomitant increase in total β-catenin was observed (Figure 1D). No significant change in levels of phosphorylated S45 β-catenin, total GSK3β and GAPDH was observed during the treatment. Additionally, expression of the BGCR-WT reporter, as detected by anti-luciferase antibody, did not change for the doses and time points tested. Therefore, the increase in BGCR-WT bioluminescence is based on changes in reporter conformation.
In response to increasing doses of LiCl (0-60 mM) BGCR-WT expressing SW620 cells had a 1.5-7.5 fold increase in bioluminescence activity over vehicle treated cells in a dose dependent manner (Figure 1E) over an eight hour period. To confirm if the observed changes in BGCR-WT activity correlated with changes in GSK3β kinase activity, representative samples were analyzed by western blot analysis. As shown in figure 1F, levels of phospho S33-S37-T41 β-catenin were found to decrease over time in response to 50 mM LiCl, without a significant change in phosphorylated S45 β-catenin and total β-catenin levels. To measure GSK3β activity we utilized an antibody that is specific for the inhibitory Ser-9 phosphorylation within GSK3β. As shown in figure 1c, a time dependent increase in signal was observed without a significant change in levels of total GSK3β or CK1α. In addition, expression levels of the reporter also remained unchanged indicating that changes observed in bioluminescence activity were due to changes in activity and not due to changes in expression levels.
Validation of BGCR reporter using CK1α inhibitor
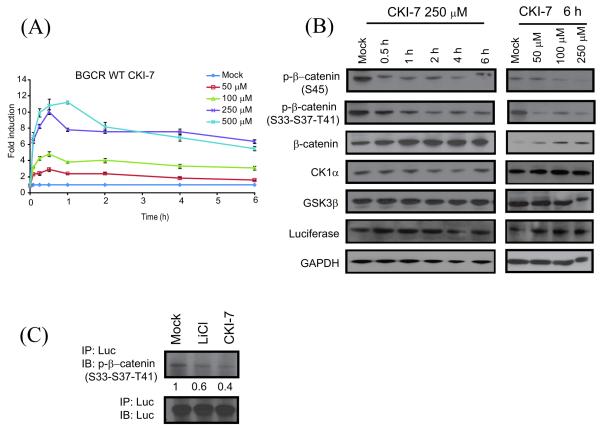
We designed the reporter such that the target peptide sequence present within the BGCR contains phosphorylation sites for GSK3β as well as CK1α. Therefore, the BGCR-WT reporter was also validated by treating with CKI-7, a small molecular weight inhibitor specific for CK1α. In response to treatment with CKI-7 a dose and time dependent increase in SW620 BGCR-WT bioluminescence activity was observed (Figure 2A). Western blot analysis of representative samples revealed a time dependent decrease in phosphorylation of β-catenin at S45 a known target of CK1α(Figure 2B). A decrease in phosphorylated S33-S37-S41 β-catenin was also observed after 2 h. No significant change in levels of total β-catenin, CK1α and GSK3β as well as the BGCR-WT reporter was observed. A similar decrease in phosphorylation of β-catenin at S45 was observed with increasing doses of CKI-7 without a significant change in BGCR, CK1α and GSK3β (Figure 2B). To determine whether the inhibitor mediated increase in BGCR-WT bioluminescence activity was due to decrease in phosphorylation of target peptide within the reporter, we immunoprecipitated the BGCR-WT with anti-luciferase antibody. Western blotting of these samples with a phospho(S33-S37-T41)-β-catenin antibody suggested a decrease in phosphorylation of target peptide within the reporter (Figure 2C).
Figure 2.
Measurement of CKIα activity in a dose and time dependent manner. (A) SW620 cells stably expressing the BGCR-WT reporter were treated with various doses (50 μM, 100 μM, 250 μM, and 500 μM) of the CK1α inhibitor CKI-7 and BLI was measured until 6 h. The change in BLI activity over DMSO control treated (mock) levels was determined and plotted as fold induction. BLI measurements were done in triplicates. Error bars denote SEM. (B) Western blot analysis of the cells stably expressing the BGCR-WT reporter were treated with 250 μM CKI-7 for 0.5 h, 1 h, 2 h, 4 h, and 6 h and various doses of CKI-7 (50 μM, 100 μM, and 250 μM) for 1 h using anti-phospho-(S33-S37-T41)-β-catenin, phospho-(S45)-β-catenin, total β-catenin, CK1α, total GSK3β, luciferase and GAPDH antibodies. (C) BGCR-WT reporter expressing cells were treated with 50 mM LiCl or 250 μM CKI-7 for 6 h. Luciferase specific antibody was used to immunoprecipitate the BGCR reporter molecule and western blot analysis was performed using phospho-(S33-S37-T41)-β-catenin antibody, as well as luciferase specific antibody as control.
Validation of specificity of the BGCR reporter using mutant reporters
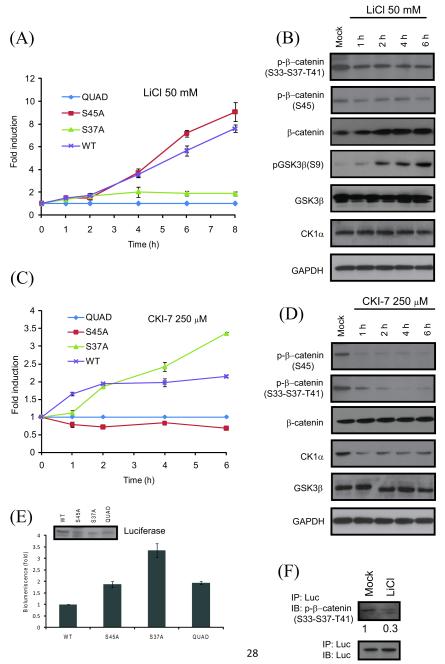
We constructed a series of mutant reporters (Figure 1A) to dissect the role of GSK3β and CK1α target sites in β-catenin derived peptide in BGCR. Based on reports that a single mutation was sufficient to abolish β-catenin phosphorylation by GSK3β [35], the residue corresponding to S37 of β-catenin was mutated to the neutral amino acid alanine within the reporter (BGCR S37A). Additionally, the CK1α target site corresponding to S45 within β-catenin was also independently mutated to Ala (BGCR S45A). Lastly, a control reporter was constructed wherein each of the relevant target phosphorylation sites (S33, S37, T41 and S45) were mutated to Ala (BGCR QUAD). Stable cell lines expressing each of these three reporters were generated in SW620 colon carcinoma cells. Treatment of wild-type BGCR and BGCR S45A expressing cells with LiCl (50 mM) resulted in a time dependent increase in bioluminescence activity (Figure 3A). In contrast, the BGCR S37A and the BGCR QUAD expressing cells did not exhibit a significant change in reporter activity (Figure 3A). Western blot analysis of the BGCR S45A cells demonstrated a time dependent increase in phospho(S9)-GSK3β levels (Figure 3B) and a corresponding decrease in levels of phospho(S33-S37-T41)-β-catenin but not in phospho(S45)-β-catenin or in CK1α.
Figure 3.
Confirmation of the BGCR reporter activity using mutant reporters. (A) SW620 cells stably expressing WT, S37A, S45A and QUAD mutant reporters were treated with 50mM LiCl and BLI was measured at 1h, 2h, 4h, 6h and 8h. The change in BLI activity over QUAD levels was determined and plotted as fold induction. BLI measurements were done in triplicates. Error bars denote SEM. (B) SW620 cells stably expressing S45A mutant were treated with 50 mM LiCl for 1 h, 2 h, 4 h and 6 h and western blot analysis was carried out using antibodies raised against phospho-(S33-S37-T41)-β-catenin, phospho-(S45)-β-catenin, total β-catenin, phospho-(S9)-GSK3β, total GSK3β, CK1α and GAPDH. (C) Cells stably expressing WT, S37A, S45A and QUAD mutant reporters were treated with the CK1α inhibitor CKI-7 (250 μM) and BLI was measured at 1 h, 2 h, 4 h, and 6 h. The change in BLI activity over QUAD levels was determined and plotted as fold induction. BLI measurements were done in triplicates. Error bars denote SEM. (D) SW620 cells stably expressing S45A mutant reporter were treated with 250 μM CKI-7 for 1 h, 2 h, 4 h and 6 h and western blot analysis was carried out using antibodies raised against phospho-(S33-S37-T41)-β-catenin, phospho-(S45)-β-catenin, total β-catenin, CK1α, total GSK3β, and GAPDH. (E) Basal bioluminescence was calculated for SW620 cells stably expressing WT, S37A, S45A and QUAD mutant reporters without any treatment. Expression of reporter in each stable cell line is shown with antibody against luciferase. (F) S45A mutant reporter expressing cells were treated with 50 mM LiCl for 6 h. Luciferase specific antibody was used to immunoprecipitate the BGCR reporter molecule and western blot analysis was performed using phospho-(S33-S37-T41)-β-catenin antibody, as well as luciferase specific antibody as control.
To investigate the specificity of the reporter for CK1α, the above four reporter expressing cells were treated with CKI-7 (250 μM). Bioluminescence activity measurements revealed a time dependent increase in BGCR wild-type and BGCR S37A expressing cells, while the BGCR S45A and the BGCR QUAD cells did not have a time dependent change in bioluminescence activity (Figure 3C). Western blot analysis of the CKI-7 treated cells revealed a time dependent decrease in phosphorylation of S45 β-catenin (Figure 3D), while no significant change in levels of total β-catenin, GSK3β or CK1α was observed. Similar level of reporter expression in stable cell lines was confirmed by western blotting (Figure 3E). Measurement of basal bioluminescence exhibited between 2-3 folds increase in S37A, S45A and QUAD mutant reporters over WT which was an expected result as replacement of target Ser/Thr with Ala in mutant reporters would mimic inhibition of phosphorylation (Figure 3E).
To determine whether the increase in BGCR S45A bioluminescence activity was due to decrease in phosphorylation of target peptide within the reporter, we immunoprecipitated the BGCR S45A with anti-luciferase antibody. Western blotting of these samples with a phospho(S33-S37-T41)-β-catenin antibody suggested a decrease in phosphorylation of target peptide within the reporter (Figure 3F).
Measurement of GSK3β kinase activity in response to Wnt3a
To monitor BGCR activity with a biological transducer of Wnt signaling, we treated SW620 BGCR WT cells with Wnt condition media obtained from Wnt3a secreting mouse fibroblast cell line (L-wnt3a cells; [36]). No Change in reporter activity was observed in SW620 BGCR. This corroborates existing literature that mutations of APC and/or Axin cause constitutive activation of Wnt-signaling and leads to desensitization of treatment with Wnt [37; 38]. Therefore, we created stable lines in HEK293 cells and characterized BGCR response to Wnt.
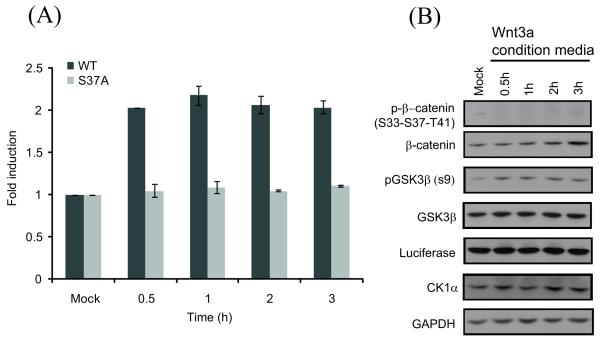
Upon treatment with Wnt3a conditioned media, more than a 2 fold induction in BGCR bioluminescence activity was observed with BGCR WT reporter cell line which remained active for 3 h (Figure 4A). HEK293 cells stably expressing S37A mutant reporter did not exhibit any induction of BGCR activity (Figure 4A). Cells treated similarly and harvested for western blotting indicated a decrease in phospho(S33-S37-T41)-β-catenin levels and increase in total β-catenin levels (Figure 4B). Further, an increase in inhibitory GSK3β phosphorylation at S9 was detected. Levels of total GSK3β and CK1α were not found to change with the treatments. Anti-luciferase blot confirms that a change in bioluminescence activity was due to conformational change and not due to change in reporter expression (Figure 4B).
Figure 4.
Measurement of Wnt modulated change in GSK3β activity. (A) HEK293 cells stably expressing the BGCR-WT and S37A mutant reporters were treated with the Wnt-conditioned media for 0.5 h, 1 h, 2 h, and 3 h and BLI activity was measured. The change in BLI activity over control levels was determined and plotted as fold induction. Error bars denote SEM. (B) HEK293 cells stably expressing the BGCR WT reporter were treated with the Wnt-conditioned media for 0.5 h, 1 h, 2 h, and 3 h and western blot analysis was carried out using antibodies to phospho-(S33-S37-T41)-β-catenin, total β-catenin, phospho-(S9)-GSK3β, total GSK3β, luciferase, CK1α and GAPDH.
Molecular imaging of GSK3β activity reveals oscillatory modulations in vivo
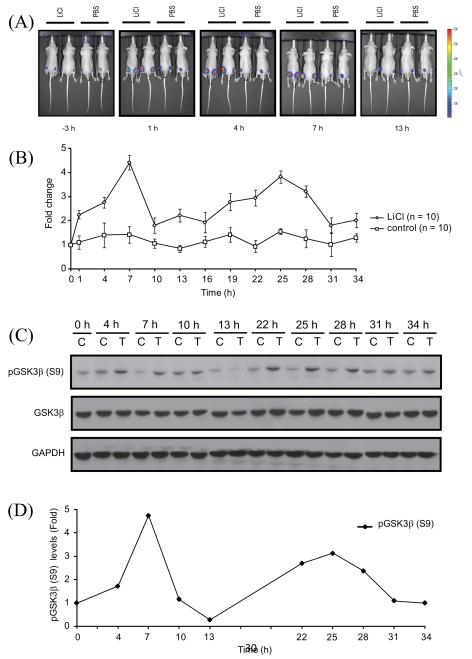
Having established the specificity and sensitivity of BGCR reporter in-vitro, we next investigated if BGCR activity can be used as surrogate marker for GSK3β and CK1α activities in-vivo. Tumor xenograft of SW620 BGCR WT cells were generated in athymic nude mice and once the tumor had grown to 40-60 mm3, animals were subjected to bioluminescence imaging to record the basal bioluminescence. Three hours later, animals were injected i.p. with a single dose of 400mg/kg body weight with LiCl. This dose was in accordance with a previous report to inhibit GSK3β activity [39]. Bioluminescence imaging of the animals was performed at 1 h following treatment and continued for 34 h with 3 h interval between imaging. Representative images at pretreatment and post treatment with either LiCl or vehicle control (saline) at 1 h, 4 h, 7 h and 13 h are shown in Fig. 5.
Figure 5.
Imaging of GSK3β activity in vivo. (A) CD-1 nude mice were subcutaneously implanted with SW620 cells stably expressing the BGCR-WT reporter. BLI activity was monitored in mice after 2 weeks when tumors reached about 40-60 mm3 size. BLI activity of pretreated and treated with vehicle control (PBS) and LiCl (400mg/kg) was monitored at 3 h interval till 34 h. Representative BLI images at −3 h, 1 h, 4 h, 7 h, and 13 h were shown. All window and level settings were consistent for all the acquisition. (B) ROI were drawn manually above the tumors and peak bioluminescence was measured until 34 h post treatment. BLI activity was expressed as fold induction as means ± SEM for each of the groups, based on pretreatment level. (C) CD-1 nude mice harboring xenograft of BGCR-WT expressing cells were treated with vehicle control PBS (control, C) or treated with 400mg/kg LiCl (treated, T) and tumors were harvested at times points mentioned and western blot analysis was done with pGSK3β(S9), total GSK3β and GAPDH antibodies. (D) Densitometric analysis of pGSK3β(S9) western blot for vehicle treated and LiCl treated tumor was done and shown as fold change from control levels at each time point.
Quantization of the bioluminescence averaged over 5 animals (10 tumors) revealed an increase in bioluminescence after 1 h (Figures 5A, B) which peaked at 7 h (greater than 4 folds) (Figure 5B). A second peak was then observed at 25 h (greater than 3 folds). Mice injected with saline (10 tumors) did not did not show a noticeable increase in bioluminescence during the 34 h period. These data indicate that GSK3β levels oscillate in vivo xenograft models. Since this reporter senses inhibition of GSK3β and phosphorylation at S9 has been reported in literature as an inhibitory post translational modification, we next evaluated correlation between phosphorylation at S9 in GSK3β and bioluminescence data. Towards this, tumors from SW620 BGCR WT xenografts bearing animals were harvested at the time points similar to bioluminescence imaging and subjected to western blotting using phospho(S9)-GSK3β antibody (Figure 5C). When the phospho(S9)-GSK3β reactivity was quantitated, a correlation between the levels of S9 phosphorylated GSK3β in tumor lysates and bioluminescence of BGCR activity was observed (Figure 5D). We attempted to measure CK1α activity in our tumor xenograft model but due to a lack of in-vivo compatible CK1α inhibitor, these experiments were not possible.
Discussion
Conventional biochemical assays for evaluation of signaling events only provide a “snapshot” view of the complex and dynamic activity of the pathway under investigation. It is increasingly becoming clear that signaling pathways are modulated at multiple levels, including positive and negative feedback loops. To obtain a true measure of the activity of a given signaling pathway, assays that take into consideration the temporal dynamics of the pathway as well as the impact of multiple regulatory molecules on the ultimate consequence of the signaling activity are required. Important role played by GSK3β and CK1α in multiple pathways including signal dependent phospho-regulation of β-catenin is one such example. The ability to non-invasively and in real-time evaluate the activity of these two key kinases would significantly enhance our understanding of their biology. To this end, we here describe the construction of four reporter molecules which can be used to evaluate the activation or inhibition of GSK3β and CK1α either independently or in combination.
The WT reporter contained the N-terminal phosphorylation domain of β-catenin that is responsive to phosphorylation by CK1α and GSK3β. The ability of the WT reporter to respond to treatment by SB415286, LiCl and CKI-7 in a time and dose dependent manner validated the specificity of the reporter for both kinases. In contrast the S37A mutant reporter wherein the key GSK3 target site was mutated to alanine only responded to CKI-7 but not to LiCl further demonstrating its specificity for sensing CK1α activity. Similarly, the S45A mutant wherein the CK1α target site was mutated to alanine failed to respond to CKI-7 but was responsive to LiCl, a modulator of GSK3β. In addition to having the expected specificity, these reporters demonstrated that bioluminescence activity could be used as a surrogate for each of the regulatory kinases in a dose and time dependent manner as demonstrated by the high degree of correlation observed between changes in photon counts and with changes in levels of target phospho-proteins.
Utilizing an elegant genetic approach and a plethora of distinct phospho-β-catenin antibodies, Liu et al., reported that for phosphorylation of β-catenin by GSK3 at the S33, S37 and T41, the S45 first has to be phosphorylated by CK1 [28]. This concept of priming kinase activity was later supported by a number of reports [1; 40; 41; 42; 43; 44; 45]. However, Wang et al., suggested that the S45 primer model for GSK3β is not universally applicable especially in human colon malignancies [46]. Interestingly, comparison of our data with BGCR activity and conventional western blotting using the phospho specific β-catenin antibodies suggest that loss of casein kinase phosphorylation site within the BGCR (S45A mutant) does impede its ability to sense inhibition by GSK3β inhibitors (Figure 3A). These results suggest that GSK3β mediated phosphorylation of β-catenin peptide in BGCR may not require priming by CK1α. However, monitoring the levels of phosphorylated β-catenin by western blotting suggest that a decrease in levels of phosphorylated S33-S37-T41-β-catenin is always observed with CKI-7 treatment (Figure 2B and 3D). Although, such observation suggests requirement of priming kinase activity for GSK3β, it does not rule out off target effects of CKI-7 and/or CKI-7 mediated changes in kinetics of phosphatases involved in dephosphorylation of β-catenin.
GSK3β is a unique kinase involved in regulating a number of distinct signaling pathways involved in processes ranging from embryogenesis to cell death [1]. Time lapse monitoring of inhibition of GSK3β activity following a single dose of LiCl showed an oscillatory modulation of kinase activity in tumor xenograft model (BGCR-WT). A peak in bioluminescence activity suggesting maximal inhibition was observed at 7 h after LiCl administration which correlated with an increase in levels of phospho(S9)-GSK3β (inactive form of GSK3 ). This was an expected result based on existing literature on LiCl mediated inhibition of GSK3β in experimental animals [47] and published plasma half life of LiCl in mice [48]. Continued imaging of these animals revealed the emergence of a second bioluminescence peak at approximately 25 h after the initial dose of LiCl which was consistent with an increase in the intra-tumoral levels of phospho(S9)-GSK3β as measured by immunoblotting (Figures 5C, D). A corresponding change in reporter activity was not observed in untreated animals harboring BGCR-WT expressing tumors, indicating that the second peak in bioluminescence activity was due to the initial LiCl treatment. The second peak in BGCR activity subsided at 31 h post-LiCl treatment, which was also confirmed by a corresponding decrease in intra-tumoral levels of phospho (S9)-GSK3β(Figures 5C, D). The oncological significance of such modulations is not clear yet and would require further investigations. However, similar oscillatory changes in GSK3β have been reported during circadian rhythms [49; 50; 51].
BGCR provides a unique tool for real-time monitoring of dynamic changes in kinase activities of GSK3β and CK1α noninvasively. A significant advantage of such assays is that the molecular events in question are interrogated in their appropriate cellular environment (unlike in vitro assays) and under physiological conditions. Additionally, the cellular assays described here have the potential to select against compounds that are non-specifically cytotoxic as the reporter is turned on when GSK3β or CK1α activity is inhibited (Figure 1A). This unique property of the reporter offers an opportunity for high throughput screening for novel small molecule inhibitors while reducing the number of non-specific hits. Further, these cell based assays also impart information on cell permeability, stability and solubility of the compound. In addition to its role in cancer, deregulated expression of GSK3β kinase is seen in innumerable human diseases such as, diabetes, schizophrenia, ADHD and Alzheimer disease [52; 53]. Therefore, use of BGCR in appropriate animal model will not only significantly enhance our understanding of the biology of cancer (and other diseases) but also allow investigation into efficacious therapeutic interventional modalities.
Materials and Methods
Construction of the reporter and generation of reporter expressing cell lines
The β-catenin substrate sequence (residues 29-47) harboring S45, T41, S37, S33 sites, flanked by linker (GGSGG) at each side was cloned into a pEF vector comprising split firefly luciferase and Rad53p FHA2 domain as described earlier [27] (Figure 1A). The primer sequences were as followed: BGCR wt forward primer CTAGAGGCGGTGGATCTTACCTGGACTCTGGTATTCACTCGGGTGCAACCACAACGGCGCCATCTTTATCGGGAAAGGGCGGTGGAC and BGCR wt reverse primer CCGGGTCCACCGCCCTTTCCCGATAAAGATGGCGCCGTTGTGGTTGCACCCGAGTGAATACCAGAGTCCAGGTAAGATCCACCGCCT. For generation of mutant reporters single primer mutagenesis protocol was used [54]. Primer sequences were as followed: S45A mutant GCAACCACAACGGCGCCAGCTTTATCGGGAAAGGGC, S37A mutant CCTGGACTCTGGTATTCACGCCGGCGCAACCACAACGGCGCC, and QUAD mutant CGGTGGATCTTACCTGGACGCTGGTATTCACGCGGGTGCAACCGCAACGGCGCCAGCTTTATCGGGAAAGGGC. All the clones were sequence verified. Colon cancer cell line SW620 and human embryonic kidney cells (HEK293) were obtained from ATCC and maintained in RPMI 1620 (Gibco-Invitrogen, Grand Island, NY) or DMEM respectively with 10% FBS. To generate stable cell lines expressing WT and mutant bioluminescent reporters, cells were transfected and selected in media containing 500 μg/ml G418 (Gibco-Invitrogen, Grand Island, NY).
Live cell imaging and western blotting
Reporter cell lines were plated in 12 well plates and were treated with various doses of GSK3β inhibitors SB415286 (Tocris Biosciences, Ellisville, MO), LiCl (Sigma Aldrich, St. Louis, MO) and CKIα inhibitor CKI-7 (Toronto Research Chemicals, North York, Ontario, Canada) for indicated period of time and bioluminescence was acquired on IVIS 200 imaging platform (Caliper Life Science, Hopkinton, MA) after adding 100 μg/ml D-Luciferin (Xenogen Corp, Alameda, CA). ROI values were calculated for each exposure and analyzed. All the BLI measurements were done in triplicates. Data were derived from a minimum of three independent experiments.
Western blotting was done using routine protocols. Protein lysate was made in RIPA buffer containing 50mM tris-HCL, 1% NP-40, 0.25% deoxycholate-sodium salt, 150 mM NaCl, 1 mM EDTA, 1X protease and phosphatase inhibitors (Roche, Indianapolis) and loaded on SDS-PAGE gels and probed against phospho-(S33-S37-T41)-β-catenin, phospho-(S45)-β-catenin, total β-catenin, phospho-(S9)-GSK3β, total GSK3β, GAPDH (Cell Signaling Technology, Baverly, MA), CKIα (Santa Cruz Biotechnology, MA) and luciferase (Abcam, Cambridge, MA). Immunoprecipitation (IP) was carried out with 400 μg total protein using antibodies raised against luciferase following routine protocol. Western blot intensity was measured using ImageJ v1.45 [55].
Tumor xenograft and in vivo bioluminescence imaging
All animal procedures were approved by the University of Michigan Committee for use and care of animals. Four to six weeks old athymic CD-1 male mice were procured from Charles River Laboratories (Wilmington, MA) and acclimatized for 4-5 days before use. The mice were injected with 2.5×106 cells stably expressing BGCR-WT reporter suspended in 50 μl serum free RPMI on each flank and let grow until palpable tumors formed. Mice were given i.p. injection of 400μg/100μl of D-luciferin (Xenogen Corp., Alameda, CA.). Animals were anesthetized with isofluran, and imaged 5 min after administration of D-luciferin on Xenogen IVIS Spectrum system (Caliper Life Science, Hopkinton, MA) for up to 30 minutes. Background photon flux was measured 4 h before drug administration. Mice were injected intraperitoneally with 400 mg/kg body weight of LiCl (50 μl of 200 mg/ml stock) or PBS and bioluminescence acquired after 1 h and every 3 h afterwards until 34 h.
Acknowledgements
We thank Dr Eric Fearon for critical comments on the work, Swathi Pasupulati for help in in-vitro bioluminescence data acquisition and Christin Hamilton in critical reading of the manuscript. This work was supported by the US National Institutes of Health research grants R01CA129623 (AR), R21CA131859 (AR), U24CA083099 (BDR) P50CA093990 (BDR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- [3].Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–62. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- [4].Manoukian AS, Woodgett JR. Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res. 2002;84:203–29. doi: 10.1016/s0065-230x(02)84007-6. [DOI] [PubMed] [Google Scholar]
- [5].Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–89. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001;8:593–6. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- [7].Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase 1 transduces Wnt signals. Nature. 1999;401:345–50. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- [8].Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–7. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Camacho F, Cilio M, Guo Y, Virshup DM, Patel K, Khorkova O, Styren S, Morse B, Yao Z, Keesler GA. Human casein kinase Idelta phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 2001;489:159–65. doi: 10.1016/s0014-5793(00)02434-0. [DOI] [PubMed] [Google Scholar]
- [10].Brockman JL, Gross SD, Sussman MR, Anderson RA. Cell cycle-dependent localization of casein kinase 1 to mitotic spindles. Proc Natl Acad Sci U S A. 1992;89:9454–8. doi: 10.1073/pnas.89.20.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Milne DM, Looby P, Meek DW. Catalytic activity of protein kinase CK1 delta (casein kinase Idelta) is essential for its normal subcellular localization. Exp Cell Res. 2001;263:43–54. doi: 10.1006/excr.2000.5100. [DOI] [PubMed] [Google Scholar]
- [12].Petronczki M, Matos J, Mori S, Gregan J, Bogdanova A, Schwickart M, Mechtler K, Shirahige K, Zachariae W, Nasmyth K. Monopolar attachment of sister kinetochores at meiosis 1 requires casein kinase 1. Cell. 2006;126:1049–64. doi: 10.1016/j.cell.2006.07.029. [DOI] [PubMed] [Google Scholar]
- [13].Behrend L, Milne DM, Stoter M, Deppert W, Campbell LE, Meek DW, Knippschild U. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene. 2000;19:5303–13. doi: 10.1038/sj.onc.1203939. [DOI] [PubMed] [Google Scholar]
- [14].Behrend L, Stoter M, Kurth M, Rutter G, Heukeshoven J, Deppert W, Knippschild U. Interaction of casein kinase 1 delta (CKldelta) with post-Golgi structures, microtubules and the spindle apparatus. Eur J Cell Biol. 2000;79:240–51. doi: 10.1078/s0171-9335(04)70027-8. [DOI] [PubMed] [Google Scholar]
- [15].Zhu J, Shibasaki F, Price R, Guillemot JC, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase 1 and MEK1. Cell. 1998;93:851–61. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- [16].Papoff G, Trivieri N, Crielesi R, Ruberti F, Marsilio S, Ruberti G. FADD-calmodulin interaction: A novel player in cell cycle regulation. Biochim Biophys Acta. doi: 10.1016/j.bbamcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- [17].Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases 1 and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–11. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- [18].Schwab C, DeMaggio AJ, Ghoshal N, Binder LI, Kuret J, McGeer PL. Casein kinase 1 delta is associated with pathological accumulation of tau in several neurodegenerative diseases. Neurobiol Aging. 2000;21:503–10. doi: 10.1016/s0197-4580(00)00110-x. [DOI] [PubMed] [Google Scholar]
- [19].Yasojima K, Kuret J, DeMaggio AJ, McGeer E, McGeer PL. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 2000;865:116–20. doi: 10.1016/s0006-8993(00)02200-9. [DOI] [PubMed] [Google Scholar]
- [20].Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, Fu YH. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- [21].Mishra SK, Yang Z, Mazumdar A, Talukder AH, Larose L, Kumar R. Metastatic tumor antigen 1 short form (MTAls) associates with casein kinase l-gamma2, an estrogen-responsive kinase. Oncogene. 2004;23:4422–9. doi: 10.1038/sj.onc.1207569. [DOI] [PubMed] [Google Scholar]
- [22].Venerando A, Marin O, Cozza G, Bustos VH, Sarno S, Pinna LA. Isoform specific phosphorylation of p53 by protein kinase CK1. Cell Mol Life Sci. 67:1105–18. doi: 10.1007/s00018-009-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Korinek V, Barker N, Morin PJ, vanWichen D, deWeger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC(−/−) colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- [24].Riggleman B, Schedl P, Wieschaus E. Spatial Expression of the Drosophila Segment Polarity Gene Armadillo Is Posttranscriptionally Regulated by Wingless. Cell. 1990;63:549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- [25].Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- [26].Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- [27].Zhang L, Lee KC, Bhojani MS, Khan AP, Shilman A, Holland EC, Ross BD, Rehemtulla A. Molecular imaging of Akt kinase activity. Nat Med. 2007;13:1114–9. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- [28].Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- [29].Durocher D, Jackson SP. The FHA domain. FEBS Lett. 2002;513:58–66. doi: 10.1016/s0014-5793(01)03294-x. [DOI] [PubMed] [Google Scholar]
- [30].Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101:12288–93. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- [32].MacAulay K, Hajduch E, Blair AS, Coghlan MP, Smith SA, Hundal HS. Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase. Eur J Biochem. 2003;270:3829–38. doi: 10.1046/j.1432-1033.2003.03777.x. [DOI] [PubMed] [Google Scholar]
- [33].Kao KR, Masui Y, Elinson RP. Lithium-induced respecification of pattern in Xenopus laevis embryos. Nature. 1986;322:371–3. doi: 10.1038/322371a0. [DOI] [PubMed] [Google Scholar]
- [34].Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102:17406–11. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- [36].Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- [37].Spink KE, Polakis P, Weis WI. Structural basis of the Axin-adenomatous polyposis coli interaction. Embo J. 2000;19:2270–9. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–81. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- [39].Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–8. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- [40].Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase 1 phosphorylates the Armadillo protein and induces its degradation in Drosophila. Embo J. 2002;21:1733–42. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matsubayashi H, Sese S, Lee JS, Shirakawa T, Iwatsubo T, Tomita T, Yanagawa S. Biochemical characterization of the Drosophila wingless signaling pathway based on RNA interference. Mol Cell Biol. 2004;24:2012–24. doi: 10.1128/MCB.24.5.2012-2024.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Polakis P. Casein kinase 1: a Wnt’er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- [44].van Es JH, Barker N, Clevers H. You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev. 2003;13:28–33. doi: 10.1016/s0959-437x(02)00012-6. [DOI] [PubMed] [Google Scholar]
- [45].Hagen T, Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem Biophys Res Commun. 2002;294:324–8. doi: 10.1016/S0006-291X(02)00485-0. [DOI] [PubMed] [Google Scholar]
- [46].Wang Z, Vogelstein B, Kinzler KW. Phosphorylation of beta-catenin at S33, S37, or T41 can occur in the absence of phosphorylation at T45 in colon cancer cells. Cancer Res. 2003;63:5234–5. [PubMed] [Google Scholar]
- [47].Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wood AJ, Goodwin GM, De Souza R, Green AR. The pharmacokinetic profile of lithium in rat and mouse; an important factor in psychopharmacological investigation of the drug. Neuropharmacology. 1986;25:1285–8. doi: 10.1016/0028-3908(86)90149-8. [DOI] [PubMed] [Google Scholar]
- [49].Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3 beta. Proc Natl Acad Sci U S A. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].litaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- [51].Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J Biol Chem. 2005;280:31714–21. doi: 10.1074/jbc.M506225200. [DOI] [PubMed] [Google Scholar]
- [52].Kannoji A, Phukan S, Sudher Babu V, Balaji VN. GSK3beta: a master switch and a promising target. Expert Opin Ther Targets. 2008;12:1443–55. doi: 10.1517/14728222.12.11.1443. [DOI] [PubMed] [Google Scholar]
- [53].Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–98. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen X, Liu W, Quinto L, Scala G. High efficiency of site-directed mutagenesis mediated by a single PCR product. Nucleic Acids Res. 1997;25:682–4. doi: 10.1093/nar/25.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]