Abstract
Griscelli syndrome (GS), a rare autosomal recessive disorder characterized by partial albinism and immunological impairment and/or severe neurological impairment, results from mutations in the MYO5A (GS1), RAB27A (GS2), or MLPH (GS3) genes. We identified a Hispanic patient born of a consanguineous union who presented with immunodeficiency, partial albinism, hepatic dysfunction, hemophagocytosis, neurological impairment, nystagmus, and silvery hair indicative of Griscelli syndrome type 2 (GS2). We screened for point mutations, but only exons 2–6 of the patient’s DNA could be PCR-amplified. Whole genome analysis using the Illumina® 1M-Duo DNA Analysis BeadChip identified a homozygous deletion in the patient’s DNA. The exact breakpoints of the 47.5-kb deletion were identified as chr15q15-q21.1: g.53332432_53379990del (NCBI Build 37.1); the patient lacks the promoter and 5’UTR regions of RAB27A, thus confirming the diagnosis of GS2.
Keywords: GTPase, hypopigmentation, immunodeficiency, lytic granule, melanosome, SNP-array
Introduction
Griscelli syndrome (GS, MIM 214450]) is a rare autosomal recessive disorder caused by mutations in the MYO5A (GS1), RAB27A (GS2), or MLPH (GS3) genes [1]. All GS patients present with hypomelanosis of the skin due to abnormal melanosomal trafficking in melanocytes and silvery gray hair due to the presence of large clumps of pigment in hair shafts. In addition, GS1 patients present with severe central nervous system dysfunction, while GS2 patients develop hemophagocytic lymphohistiocytosis (HLH) with or without neurological involvement. HLH manifests as episodes of uncontrolled activation of T lymphocytes and macrophages and, without a bone marrow transplant, is typically fatal in early childhood for patients with GS2 [2–7]. For GS3 patients, the clinical phenotype is restricted to hypomelanosis of the skin; the same is true for GS1 patients with mutations in exon F of MYO5A [5].
Located on 15q21, RAB27A encodes the small GTPase, Rab27A, which has distinct functions in vesicular trafficking [8, 9]. Similar to other Rab proteins, Rab27A cycles between the guanosine triphosphate (GTP)-bound active state and the guanosine diphosphate (GDP)-bound inactive state [10]. Rab27A is subject to prenylation at two conserved cysteine residues within its C-terminus. This modification anchors Rab27A to membranes during vesicle transport [11, 12]. Previous studies have shown that Rab27A colocalizes with mature melanosomes, where it functions in concentrating melanosomes in the dendritic tips of normal melanocytes by forming a tripartite complex with melanophilin and Myosin 5A. Consequently, Rab27A defects result in perinuclear localization of melanosomes, and explain the albinism in patients with GS2 [13–15]. Rab27A also functions in lytic granule release from cytotoxic T lymphocytes [16, 17] as well as from natural killer cells [18, 19]; defects that underlie the immunological problems seen in GS2.
To date, mutations in RAB27A account for the largest portion of the around 50 reported GS patients [20, 21]. RAB27A mutations have been identified in numerous ethnicities including Turkish, Iranian, German, North African, and Brazilian patients; the identified genetic RAB27A alterations are largely frameshift or nonsense mutations [4, 20–27]. Previously, only two unique large scale deletions were identified in Palestinian and German patients [6, 25]. Here we report another unique 47.5-kb deletion on chromosome 15q15-q21.1 that removes the promoter region and exon 1 of RAB27A in a child from a consanguineous union of Hispanic individuals.
Materials and Methods
Patient
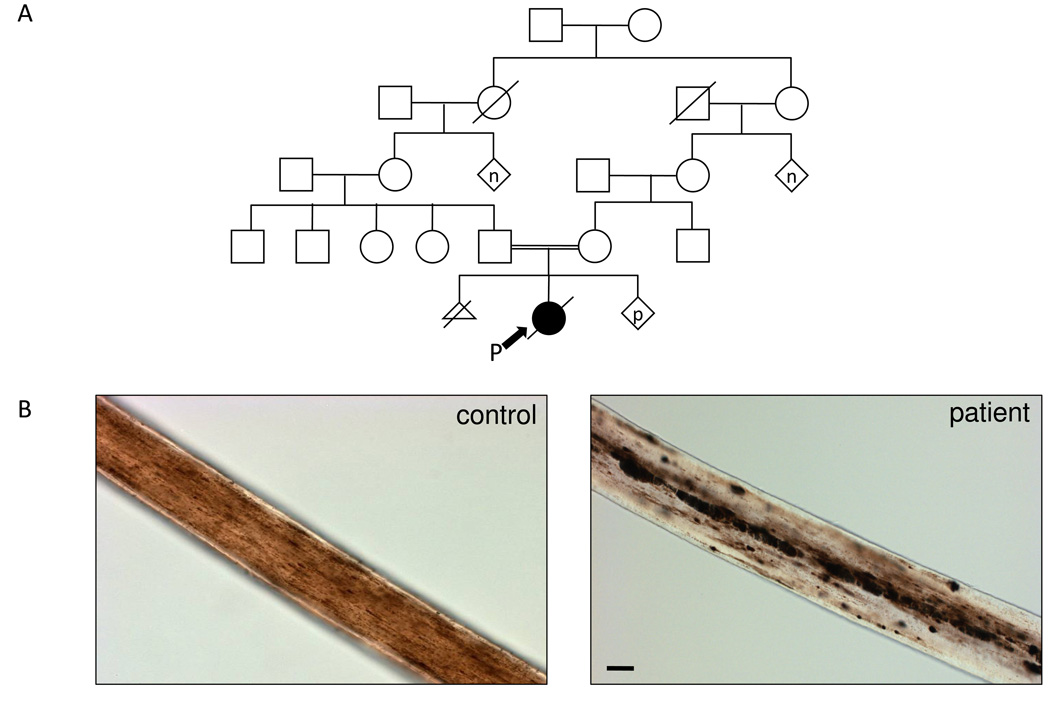
Written, informed consent by the patient’s parents was obtained for enrollment in a protocol for the diagnosis and treatment of patients with inborn errors of metabolism, approved by the National Human Genome Research Institute (NHGRI) Institutional Review Board. The female patient presented with hypopigmentation (lightly pigmented, much lighter than her mother), silvery hair, nystagmus, and a history remarkable for immunodeficiency, hepatic dysfunction, hemophagocytosis, and developmental delay. The patient was treated according to the HLH-2004 protocol [28]. Her parents are second cousins (Fig. 1A) and are of Hispanic descent. At 1.5 years of age, the girl had an episode of leptomeningeal encephalitis with hydrocephalus that was treated with a ventriculoperitoneal shunt. Three months later, she suffered from a spinal cord hemorrhage and disseminated intravascular coagulation. At 2.5 years, she failed to recover from a rapidly progressive respiratory infection, and became hypoxic and septic with Clostridium sordellii; fatal disseminated intravascular coagulation ensued. Post-mortem analyses identified erythrophagocytosis in the bone marrow, hepatomegaly, splenomegaly, thymic atrophy, and numerous central nervous system anomalies including severe brain atrophy with necrosis, cyst formation, hydrocephalus, and gliosis. The patient’s hair was obtained for visualization under light microscopy.
Fig. 1.
Clinical information of patient with Griscelli Type 2 Syndrome. (A) The pedigree of the proband’s (P) family shows consanguinity arising from a marriage of second cousins. (p = pregnancy; n = multiple siblings, number unknown) (B) The silvery hair of the patient with GS2 shows characteristic pigment clumping (right) compared to a hair of an unaffected dark haired person (left). Sizebar, 50 µm.
Molecular analysis
Given the clinical phenotype, the patient’s genomic DNA was screened for mutations in exons 1–6 of RAB27A (GenBank NM_004580) as previously described [6, 29]. It should be noted that the gene structure of RAB27A has been recently reviewed [27] and alternative transcripts indicated the presence of additional exons that contribute to the 5’untranslated region (UTR) in other RAB27A transcripts; however, amplification of these alternative exons were not attempted in this study.
After failure to amplify exon 1 using the patient’s DNA, we performed whole genome analysis using the Illumina® 1M-Duo DNA Analysis BeadChip (Illumina Inc., San Diego, CA), which contains informative markers approximately every 1.5-kb. The boundaries of the deletion were further narrowed by spanning the flanking regions with short PCR amplicons (~200–400bp) in areas without repetitive elements (Amplicon primer sets are available upon request). Subsequently, one final set of primers, 5’-CTCTGCCAACCAGTTTGAAA-3’ and 5’-GTAAGTCAAGAAACACCCTCCC-3’, was used to amplify across the breakpoints. The resultant amplicon was subjected to sequencing using BigDye® Terminator v3.1 chemistry and ABI 3130×l Genetic Analyzer (Applied Biosystems (ABI), Foster City, CA).
Results
The patient’s hypopigmentation (light tan), perinuclear accumulation of melanosomes within melanocytes, silvery hair, and nystagmus prompted analysis of her hair, which showed the characteristic clumping of pigmentation throughout the shaft (Fig. 1B) when compared to normal hair. This evidence and the patient’s extensive medical history suggested GS, so we screened the patient’s genomic DNA for mutations in RAB27A. Exons 2–6 amplified in both the patient and control DNA and showed no mutations in the patient; however, exon 1 only amplified in the control, not the patient.
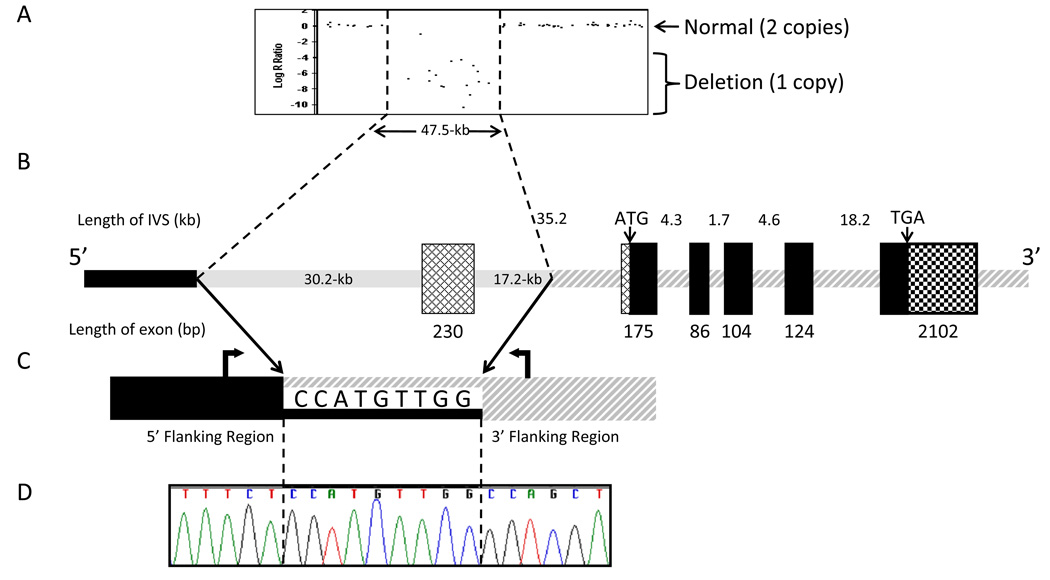
Subsequently, we utilized the Illumina® 1M-Duo DNA Analysis BeadChip to examine the entire genomic region surrounding RAB27A. This revealed 16 informative markers, spanning ~62.0-kb, that were homozygously deleted (Fig. 2A). The boundaries of the deletion were further assessed by placing small amplicons between the first diallelic single nucleotide polymorphism (SNP) and the first homozygously deleted SNP in each region encompassing the breakpoints, followed by a primer set that amplified across the deletion (Fig. 2B).
Fig. 2.
Experimental and schematic representations of homozygous deletion in Rab27A gene of patient with Griscelli Type 2 Syndrome.
(A) Illumina® 1M-Duo DNA Analysis BeadChip results illustrating a large scale deletion.
(B) Schematic basis of 47.5-kb deletion relative to the most abundant transcript of the Rab27A gene (GenBank NM_004580). The deletion (shaded gray line) removes the first exon, which is non-coding. The deletion does not contain any coding sequence. Cross-hatched boxes indicate 5’-UTR of the transcript and the checkered box indicates 3’-UTR of all transcripts. Black boxes indicate coding region of Rab27A.
(C) Two primers (block arrows) were used to amplify across the deletion that occurs within the 9-bp stretch shared by the two regions.
(D) Sequence analysis of the breakpoint region. Diagrams are not to scale.
With respect to the RAB27A gene on the antisense strand of chromosome 15, the 5’ area region containing the breakpoint was identified by the BeadChip to be between the 13.2-kb of sequence spanning from reference SNP rs11636687 to rs12901464 (dbSNP http://www.ncbi.nlm.nih.gov/projects/SNP/). The 3’ breakpoint region was narrowed to the 7.6-kb of sequence bound by rs8032859 and rs16976228. Informative amplicons were then spaced approximately 1–3 kb apart in unique regions of this highly repetitive region as annotated in the ensembl genome browser (www.ensembl.org). One of the four amplicons in the 5’ breakpoint region and two of five in the 3’ region failed to amplify. A final set of primers located at genomic positions 53380184–53380205 and 53332315–53332334 on chromosome 15q15-q21.1 (NCBI Build 37.1) amplified across the breakpoint to form a 343-bp amplicon. Sequencing of this amplicon (Fig. 2D) revealed that the deletion breakpoint occurred within the nine base pairs: CCATGTTGG (Fig. 2C, D). The final deletion of 47,548-bp was found to lie between genomic positions 53332432 and 53379990 on chromosome 15q15-q21.1 (NCBI Build 37.1).
RAB27A has four GenBank transcripts: NM_004580.3, NM_183234.1, NM_183235.1, and NM_183236.1. The large deletion in this patient removes the promoter region and exon 1 of the major RAB27A transcript NM_004580.3. At least part of the 5’UTR portions of the transcript are deleted in all of the four known transcripts. Although this mutation does not remove any coding regions of the gene in any of the transcripts, it is predicted to remove the elements required for the transcription of RAB27A. Cells and RNA were not available to verify the absence of the transcript and protein.
Discussion
We identified what is to our knowledge the first Hispanic patient with Griscelli Syndrome, type 2. The female infant presented with hypopigmentation (light tan, as described for other Griscelli cases [21, 30]), silvery hair (characteristic pigment clumps in the hair shaft), nystagmus, hemophagocytosis and severe neurological impairment. She carried a homozygous deletion (chr15q15-q21.1: g.53332432_53379990del (NCBI Build 37.1) that included the promoter and 5’ UTR regions of the RAB27A gene. A homozygous deletion was not surprising given the known consanguinity within the family.
The deletion breakpoints in this patient occurred within two Alu low copy repeat regions as annotated in the Ensembl genome browser and identified by Repeat Masker (www.repeatmasker.org). The Alu repeats span 294-bp at the 5’ and 1301-bp at the 3’ breakpoints relative to the RAB27A gene on the anti-sense strand of chromosome 15. Interestingly, these two repeats share 83% sequence identity across 289-bp of contiguous sequence. Alu repeats are genomic elements that can be transcribed by RNA polymerase III and subsequently inserted back into a different location in the genome by retroposition [31]. Recombination events between Alu elements, which likely occurred in this case, have been known to cause a wide range of diseases, including Duchenne’s muscular dystrophy, Fabry disease and Tay-Sachs disease [32–34].
In conclusion, we identified a novel 47.5-kb deletion on chromosome 15q15-q21.1 containing critical elements of the RAB27A gene. We determined that this deletion was likely the result of a recombination event on chromosome 15 that occurred between two Alu elements. Other studies of large scale RAB27A deletions fail to acknowledge the potential cause of the deletions in their patients [6, 25]; however, this novel deletion appears to have unique breakpoints unrelated to other known deletions in RAB27A.
Acknowledgments
We thank the patient’s family for participating in our protocol. We thank Jennifer Parkes for excellent laboratory assistance. This study was supported by the Intramural Research program of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu. Rev. Genomics Hum. Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am. J. Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 3.Pastural E, Barrat FJ, Dufourcq-Lagelouse R, Certain S, Sanal O, Jabado N, Seger R, Griscelli C, Fischer A, de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat. Genet. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- 4.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 5.Menasche G, Ho CH, Sanal O, Feldmann J, Tezcan I, Ersoy F, Houdusse A, Fischer A, de Saint Basile G. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1) J. Clin. Invest. 2003;112:450–456. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anikster Y, Huizing M, Anderson PD, Fitzpatrick DL, Klar A, Gross-Kieselstein E, Berkun Y, Shazberg G, Gahl WA, Hurvitz H. Evidence that Griscelli syndrome with neurological involvement is caused by mutations in RAB27A, not MYO5A. Am. J. Hum. Genet. 2002;71:407–414. doi: 10.1086/341606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein C, Philippe N, Le Deist F, Fraitag S, Prost C, Durandy A, Fischer A, Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J. Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Guo J, Miki T, Tachibana M, Gahl WA. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 1997;60:27–37. doi: 10.1006/bmme.1996.2559. [DOI] [PubMed] [Google Scholar]
- 9.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 10.Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000;301:1077–1087. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 11.Anant JS, Desnoyers L, Machius M, Demeler B, Hansen JC, Westover KD, Deisenhofer J, Seabra MC. Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry. 1998;37:12559–12568. doi: 10.1021/bi980881a. [DOI] [PubMed] [Google Scholar]
- 12.Larijani B, Hume AN, Tarafder AK, Seabra MC. Multiple factors contribute to inefficient prenylation of Rab27a in Rab prenylation diseases. J. Biol. Chem. 2003;278:46798–46804. doi: 10.1074/jbc.M307799200. [DOI] [PubMed] [Google Scholar]
- 13.Bahadoran P, Aberdam E, Mantoux F, Busca R, Bille K, Yalman N, de Saint-Basile G, Casaroli-Marano R, Ortonne JP, Ballotti R. Rab27a: A key to melanosome transport in human melanocytes. J. Cell Biol. 2001;152:843–850. doi: 10.1083/jcb.152.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA., 3rd Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- 16.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A, Latour S, de Saint Basile G. Genetic defects affecting lymphocyte cytotoxicity. Curr. Opin. Immunol. 2007;19:348–353. doi: 10.1016/j.coi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, Horiuchi H, Rosthoj S, Rutynowska O, Winiarski J, Stow JL, Nordenskjold M, Henter JI, Ljunggren HG, Bryceson YT. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13–4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117–4127. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- 19.Gazit R, Aker M, Elboim M, Achdout H, Katz G, Wolf DG, Katzav S, Mandelboim O. NK cytotoxicity mediated by CD16 but not by NKp30 is functional in Griscelli syndrome. Blood. 2007;109:4306–4312. doi: 10.1182/blood-2006-09-047159. [DOI] [PubMed] [Google Scholar]
- 20.Meeths M, Bryceson YT, Rudd E, Zheng C, Wood SM, Ramme K, Beutel K, Hasle H, Heilmann C, Hultenby K, Ljunggren HG, Fadeel B, Nordenskjold M, Henter JI. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr. Blood Cancer. 2009 doi: 10.1002/pbc.22357. [DOI] [PubMed] [Google Scholar]
- 21.Van Gele M, Dynoodt P, Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res. 2009;22:268–282. doi: 10.1111/j.1755-148X.2009.00558.x. [DOI] [PubMed] [Google Scholar]
- 22.Bizario JC, Feldmann J, Castro FA, Menasche G, Jacob CM, Cristofani L, Casella EB, Voltarelli JC, de Saint-Basile G, Espreafico EM. Griscelli syndrome: characterization of a new mutation and rescue of T-cytotoxic activity by retroviral transfer of RAB27A gene. J. Clin. Immunol. 2004;24:397–410. doi: 10.1023/B:JOCI.0000029119.83799.cb. [DOI] [PubMed] [Google Scholar]
- 23.Sanal O, Ersoy F, Tezcan I, Metin A, Yel L, Menasche G, Gurgey A, Berkel I, de Saint Basile G. Griscelli disease: genotype-phenotype correlation in an array of clinical heterogeneity. J. Clin. Immunol. 2002;22:237–243. doi: 10.1023/a:1016045026204. [DOI] [PubMed] [Google Scholar]
- 24.Schuster F, Stachel DK, Schmid I, Baumeister FA, Graubner UB, Weiss M, Haas RJ, Belohradsky BH. Griscelli syndrome: report of the first peripheral blood stem cell transplant and the role of mutations in the RAB27A gene as an indication for BMT. Bone Marrow Transplant. 2001;28:409–412. doi: 10.1038/sj.bmt.1703114. [DOI] [PubMed] [Google Scholar]
- 25.Zur Stadt U, Beutel K, Kolberg S, Schneppenheim R, Kabisch H, Janka G, Hennies HC. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum. Mutat. 2006;27:62–68. doi: 10.1002/humu.20274. [DOI] [PubMed] [Google Scholar]
- 26.Mamishi S, Modarressi MH, Pourakbari B, Tamizifar B, Mahjoub F, Fahimzad A, Alyasin S, Bemanian MH, Hamidiyeh AA, Fazlollahi MR, Ashrafi MR, Isaeian A, Khotaei G, Yeganeh M, Parvaneh N. Analysis of RAB27A gene in griscelli syndrome type 2: novel mutations including a deletion hotspot. J. Clin. Immunol. 2008;28:384–389. doi: 10.1007/s10875-008-9192-5. [DOI] [PubMed] [Google Scholar]
- 27.Meschede IP, Santos TO, Izidoro-Toledo TC, Gurgel-Gianetti J, Espreafico EM. Griscelli syndrome-type 2 in twin siblings: case report and update on RAB27A human mutations and gene structure. Braz. J. Med. Biol. Res. 2008;41:839–848. doi: 10.1590/s0100-879x2008001000002. [DOI] [PubMed] [Google Scholar]
- 28.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 29.Tolmachova T, Ramalho JS, Anant JS, Schultz RA, Huxley CM, Seabra MC. Cloning, mapping and characterization of the human RAB27A gene. Gene. 1999;239:109–116. doi: 10.1016/s0378-1119(99)00371-6. [DOI] [PubMed] [Google Scholar]
- 30.Westbroek W, Lambert J, De Schepper S, Kleta R, Van Den Bossche K, Seabra MC, Huizing M, Mommaas M, Naeyaert JM. Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-Myosin Va transcripts. Pigment Cell Res. 2004;17:498–505. doi: 10.1111/j.1600-0749.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 31.Rogers J. Retroposons defined Nature. 1983;301:460. doi: 10.1038/301460e0. [DOI] [PubMed] [Google Scholar]
- 32.Myerowitz R, Hogikyan ND. A deletion involving Alu sequences in the beta-hexosaminidase alpha-chain gene of French Canadians with Tay-Sachs disease. J. Biol. Chem. 1987;262:15396–15399. [PubMed] [Google Scholar]
- 33.Hu XY, Ray PN, Worton RG. Mechanisms of tandem duplication in the Duchenne muscular dystrophy gene include both homologous and nonhomologous intrachromosomal recombination. EMBO J. 1991;10:2471–2477. doi: 10.1002/j.1460-2075.1991.tb07786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornreich R, Bishop DF, Desnick RJ. Alpha-galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J. Biol. Chem. 1990;265:9319–9326. [PubMed] [Google Scholar]