Abstract
Azurin p28 (NSC745104) is a 28 amino acid peptide fragment that inhibits proliferation of human solid and hematological malignancies in vitro and in vivo by reducing proteasomal degradation of oncogene p53. The present study aimed at developing a novel and fast liquid chromatography/tandem mass spectrometry (LC/MS/MS) method for the bioanalysis of p28 in mouse serum, and determining Azurin p28 stability and pharmacokinetics in mice after full method validation. Both Azurin p28 and its internal standard MP-1 were separated and extracted from serum by using perchloric acid (7%, v/v) without time-consuming reconstitution. Chromatographic separation of Azurin p28 and MP-1 from the serum matrix was achieved using a C18 column with a gradient elution profile consisting of 5 mM ammonium acetate and acetonitrile, both containing formic acid. Mass analysis was conducted using positive ion electrospray ionization (ESI) and multiple reaction monitoring (MRM). It took 7.5 minutes to analyze one sample. The validated concentration range of the method extended from 100 to 10,000 ng/ml with accuracies of 85–115% and inter-day precision (CV) of <15%. Inter-day accuracy ranged from 96.4% to 103% and CV ranged from 4.61% to 6.90%. The average recovery of Azurin p28 from mouse serum at three concentrations (200, 1000, and 5,000 ng/ml) was determined to be 96.4%. Incubation of Azurin p28 at 37°C for 24 hours resulted in its degradation 55% in monkey serum, 41% in human serum, and 32–34% in mouse and dog serum. Intravenous administration of Azurin p28 to mice showed its t1/2β 0.23 hours, clearance 1.7 l/kg/hour, and volume of distribution at steady state 4.1 l/kg. In conclusion, the novel and fast bioanalytical method was proven to be useful for pharmacokinetic profiling of Azurin p28.
Keywords: Azurin p28, Pharmacokinetics, Cupredoxin bacterial redox protein, Liquid chromatography/tandem mass spectrometry
1. Introduction
Azurin p28 (p28; NSC745104) is a 28 amino acid peptide fragment (a.a. 50–77) of Azurin which is derived from a cupredoxin bacterial redox protein obtained from Pseudomonas aeruginosa [1]. This peptide forms a discrete helicial protein transport domain (PTD) and is responsible for the selective penetration into cancer cells. Azurin p28 inhibits the proliferation of human solid and hematological malignancies in vitro and in vivo in a dose-dependent manner probably through a p53-mediated mechanism [1–4]. The inhibitory effects of p28 at concentrations ranging from 5–100 μM on the growth of several cancer cell lines have been demonstrated [5–7]. Using the standard colorimetric MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) assay it has been shown that Azurin p28 induces cytostatic rather than immediate apopototic effects on cell lines [6,8]. Treatment of immunodeficient nude mice (inoculated subcutaneously with UI80-Mel-2 cells) with Azurin p28 at 8, 16 and 20 mg/kg (i.p., daily) for 4 week showed reduction in incidence of measurable tumors when compared to the control groups [unpublished observation]. The relatively low toxicity profile (> 200 mg/kg/day in CD1 mice) of Azurin p28 determined from a dose range finding study is attributed to the molecule’s selective penetration which makes it particularly attractive for use as a chemotherapeutic agent [8,9]. As of this writing, however, no bioanalytical method has been published for Azurin p28. The purpose of this study was to develop a novel and rapid liquid chromatography/tandem mass spectrometry (LC/MS/MS) method for quantitative determination of p28 in mouse serum, and utilize the method, after its full validation, to determine p28 stability in various species and pharmacokinetic parameters in mice in order to advance Azurin p28 further to preclinical toxicity and human clinical trials.
2. Experimental
2.1. Chemicals and materials
Azurin p28 (LSTAADMEGVVTDGMASGLDKDYLKPDD), a white to off-white crystal solid with a molecular weight of 2914 Daltons, was provided by the CDG Therapeutics, Inc (Chicago, IL). The identity, strength, quality and purity of Azurin p28 are documented in a certificate of analysis indicating its purity >95% by a HPLC method. MP-1, a 13 amino acid peptide (Ac-SYGJEHfRWGKPV-NH2, where J is abbreviation of L-Norleucine, and f, D-phenylalanine) was purchased from Mimotopes (Australia) at a purity of >95% (by HPLC) and used as the internal standard, HPLC grade acetonitrile, ammonium acetate, formic acid and perchloric acid were purchased from Fisher Scientific (Atlanta, GA. USA). Purified water was obtained from an in-house Millipore Milli-Q system (Bedford, MA, USA). Serum was obtained from Lampire Biologics (Pipersville, PA, USA).
2.2. Standard and sample preparation
2.2.1. Preparation of stock and working solutions
A stock solution of Azurin p28 (1 mg/ml) was prepared in 5 mM ammonium acetate. The stock solution was diluted with 5 mM ammonium acetate to make a series of seven working solutions over a concentration range of 1–100 μg/ml. A stock solution of MP-1 (1 mg/ml) was prepared in 5 mM ammonium acetate, and diluted with a 50/50 mixture of 5 mM ammonium acetate/acetonitrile to make a 10 μg/ml spiking solution.
2.2.2. Spiking and extraction from serum
For the preparation of the calibration standards, mouse serum (100 μl), was spiked with 10 μl the appropriate working solution of Azurin p28 to achieve concentrations in serum of 100, 200, 500, 1,000, 2,000, 5,000 and 10,000 ng/ml. After the addition of 10 μl of MP-1 spiking solution to each serum standard, serum proteins were precipitated by the addition of 100 μl perchloric acid (7% v/v). After centrifugation for 5 min at 9,000 × g, each supernatant was transferred to an autosampler vial and analyzed in positive ion mode by LC/MS/MS.
2.3. Instrumentation
The instrumentation was similar to that we used for a peptide molecular study [10]. Briefly, the LC/MS/MS system consisted of a Perkin-Elmer (Norwalk, CT) series 200 autosampler equipped with a Peltier cold tray set at 4°, and two Series 200 micro-flow pumps with an Aquasil C18, 100 × 2 mm 5 μm particle column (Thermo Scientific, Waltham, MA. USA) maintained at ambient temperature. Detection was performed using an Applied BioSystems 4000 QTRAP (Applied Biosystems, Foster City, CA) triple quadrupole mass spectrometer operated in the positive ion mode. Mass calibration, data acqusition and quantitation of Azurin p28 were performed using Applied Biosystem Analyst 1.4.1 software (Applied Biosystems, Foster City, CA) [11].
2.4. Chromatographic conditions
The mobile phase was delivered at a flow rate of 400 μl/min using a gradient elution profile consisting of 5 mM ammonium acetate with 0.5% formic acid (A) and acetonitrile with 0.5% formic acid (B). The starting mobile phase composition was 90% A/10% B and was increased linearly to 40% A/60% B over a 3.5 minute period after an initial 0.5 minute hold, then held at 40% A/60% B for 1 minute, and returned to 90%A/10% B (step gradient) and re-equilibrated for 2.5 minutes. The injection volume for the method was set at 10 μl.
2.5. Mass spectrometer conditions
The LC-MS-MS was equipped with an electrospray ion source operated at 450 °C and at a potential of 5 kV. The entrance potential was set at 10 V. High purity nitrogen was used as the curtain and collision gas with a CAD gas setting of 5 producing a pressure of 1.2 × 10−5 Torr. The analyte and internal standard were detected using multiple reaction monitoring (MRM) for the following transitions: Azurin p28 (m/z 972→1236 and 972→1272) and MP-1 (m/z 809→ 136). The measured intensities of the two Azurin p28 transitions were summed to achieve the necessary sensitivity. A dwell time of 150 ms was used for each ion transition. Collision energies were optimized for each transition and ranged from 40 eV for p28, to 95 eV for MP-1. The instrument was tuned and calibrated in the positive ion mode on each day of analysis using a 2 μM PPG solution (Applied Biosytems, Part number 4405231). The tuning criteria consisted of a Q1/Q3 resolutions of 0.7 amu FWHM with unit mass resolution and a peak intensity of ≥1E+07 at m/z 906.
2.6. Method validation
Method validation procedures were similar to those used by us [11–15], which consisted of analyzing two composite curves each comprised of three individual standard curves, where each single curve was prepared from a different lot of serum (n=6) and analyzed on separate days. The selectivity of the method was determined by measuring the level of interfering components in six individual sources of blank mouse serum following the US Food and Drug Administration’s guidance [18]. Accuracy and precision were determined from triplicate measurements per day of quality control samples at three different concentrations and from back calculated values of the seven calibration standards per curve. Recovery of Azurin p28 from serum was determined by comparing the response of Azurin p28 in serum after extraction to the response of the same concentration of Azurin p28 spiked into extracted blank serum.
To determine the integrity of Azurin p28 in extracted mouse serum samples during the time course of an analytical run, the stability of extracted samples was determined for a 18-hour time period during storage at 4°C in a Peltier autosampler tray. Calibration standards from a curve prepared on the first day of validation were stored in the autosampler and were re-injected on the second day of validation. The composite curve from the freshly prepared day 2 standards was used to quantitate the calibration standards from day 1.
The accuracy of diluting samples with an analyte concentration greater than the upper limit of quantitation (ULOQ: 10,000 ng/ml) was tested by spiking blank mouse serum at two concentrations (50,000 ng/ml and 20,000 ng/ml) above the ULOQ and diluting it with blank matrix to fall within the range of the curve. After dilution with blank plasma, samples were extracted and analyzed as described above. The ability to dilute samples into the range of the curve was based on percent of theoretical concentration after dilution.
Freeze-thaw stability of Azurin p28 in mouse serum was determined by measuring the amount of Azurin p28 in triplicate samples at two concentrations (100 ng/ml and 1,000 ng/ml) after three freeze-thaw cycles. The freeze thaw cycles consisted of first freezing the samples at approximately −80°C for 24 hours and then allowing them to thaw unassisted at room temperature. Once thawed, the samples were refrozen for 12 to 24 hours at −80 °C. This cycle was then repeated two more times and the samples assayed after the third cycle. Stability was measured in terms of measured Azurin p28 in mouse serum as compared to theoretical concentration.
2.7. Data analysis
Concentration calculations were performed using Analyst (Version 1.4.1). The amount of analyte in each serum sample (ng/ml) was back calculated using a calibration curve generated from a set of calibration standards. Linearity was assessed using the internal standard and up to 7 calibrators with Azurin p28 concentrations in the 100–10,000 ng/ml range. A quadratic fit with 1/× weighting was determined to be the best fit due to the wide concentration range investigated. The weighting factor was chosen based on goodness-of-fit-criteria including coefficient of determination (r2), the back-calculated concentration of individual calibrators, and minimizing intercept value [12, 13, 16]. Unless otherwise noted, all results were expressed as mean ± standard deviation (SD).
2.8. Stability in serum
Stability of Azurin p28 in mouse serum was investigated when stored refrigerated (4 °C) and at 37°C. Individual stock mixtures (5 ml) from mouse serum were spiked with Azurin p28 at 10 μg/ml and vortexed well. Immediately after vortexing, 0.1 ml portions of serum was put into microcentrifuge tubes and the tubes were placed at the designated incubation temperature (4 °C or 37 °C). At 0, 1, 2, 4, 8, 24, and 48 hours, a sample of serum were removed, fortified with internal standard, extracted and analyzed by LC/MS/MS. The stability of Azurin p28 was determined from the amount of the analyte measured in each stability study sample as compared with the amount of analyte measured in freshly prepared standards from the same serum species.
2.9. Pharmacokinetic study of Azurin p28 in mice
Male CD-1® ISG mice were procured from Charles River Laboratories (Raleigh, NC). The mice were approximately 7 weeks old and weighed between 32.0 ± 3 g on the day of dosing. The mice were group housed in polycarbonate cages lined with hardwood chip bedding. Animals were fed Teklad Certified Rodent Diet # 2016 (Harlan Teklad; Madision, WI) with tap water ad libitum. Cage size, animal care, and environmental conditions conformed to the guidelines of the U.S. Department of Agriculture (Animal Welfare Act; Public Law 99–198) and to those of the Guide for the Care and Use of Laboratory Animals.
The dose formulation of Azurin p28 was prepared in 0.9% sterile saline to contain 48 mg/ml of Azurin p28. Individual mice were dosed via a tail vein injection with 480 mg/kg of Azurin p28, in a dose volume of 10 ml/kg. At each of the following times, 4 mice were anesthetized with CO2/O2 and then bled from the retro-orbital sinus: 2, 5, 15, and 30 minutes and 1, 2, 4, 8, 12, and 24 hours after dosing. Each blood sample was collected into a 0.6 ml tube containing no anticoagulant, and maintained at room temperature for approximately 10 minutes, and then placed on ice. Individual blood samples were centrifuged to separate serum. Each serum sample was stored ≤−70°C until analyzed by LC/MS/MS for levels of Azurin p28.
Pharmacokinetic parameters were calculated from the serum concentration versus time data using WinNonlin® (Professional Version 4.1; Pharsight Corp; Mountain View, CA).
3. Results
3.1. Method validation
3.1.1. Selectivity and linearity range of the measurement
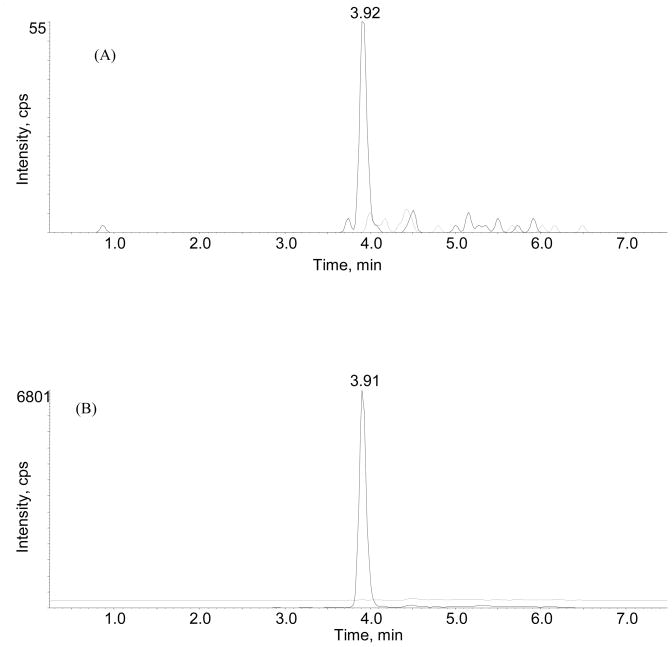
The selectivity of the method was determined by extracting and analyzing pooled drug-free mouse serum. Representative ion chromatograms of drug-free and spiked mouse serum containing 100 ng/ml of Azurin p28 and 1000 ng/ml of MP-1 are shown in Fig. 1A and B. The absence of peaks at the measured mass transitions and retention times of Azurin P28 (972.4→1272.3, 3.9 min) and internal standard (m/z 809.2→ 136.4, 3.9 min) in the drug-free trace provide evidence of no endogenous interferences in drug-free serum.
Fig. 1.
MRM chromatograms of the transition for (A) Azurin p28 m/z 972.4–1272.3 from mouse serum spiked with Azurin p28 for a final concentration of 100 ng/mL (solid line) and blank serum (dashed line), and (B) MP-1 (internal standard) m/z 809.1–136.4 spiked mouse serum (lower solid line) and blank serum (dashed line).
Linearity of the method was observed between Azurin p28 concentrations of 100–10,000 ng/ml as detected from a 100-μl sample size of serum. The resulting regression coefficients of the composite calibration curves were greater than 0.996 on each day of validation.
3.1.2. Intra- and inter-assay accuracy and precision
The intra- assay accuracy and precision of the method was determined from the results of the analysis of quality control samples and from the back calculated calibration standards prepared in triplicate at three concentrations on two separate days in mouse serum. The intra-assay accuracy of the method (n=3) ranged from 96.6% to 101% on the first day of validation and 95.7% to 109% on the second day of validation and the range of precision was 0.875% to 6.23% on day one and 2.83% to 6.33% on day two of the validation for mouse serum (Table 1). The inter-assay accuracy and precision of the method was determined from back calculated values (n=6) for the quality control samples and the calibration standards prepared in mouse serum and assayed in triplicate over two days. Inter-day accuracy ranged from 96.4% to 103% and inter-day precision ranged from 4.61% to 6.90% (Table 1). The accuracy of the back calculated values for the calibration standards (Table 2) ranged from 93 to 103% on day 1 and from 91.6 to 105% on day two. The precision of the method represented by %CV ranged from 1.38 to 9.82 on day 1 and from 2.30 to 10.6 on day two. Correlation coefficients for the composite curves were 0.9963 on day one and 0.9954 on day two.
Table 1.
Intra-day and inter-day accuracy and precision of Azurin p28 spiked into and extracted from mouse serum.
| Conditions | Concentrations | n | Mean± SD | %CV | Accuracy |
|---|---|---|---|---|---|
| Intra-day, Day 1 | 200 ng/mL | 3 | 201±12.5 | 6.23 | 101 |
| Intra-day, Day 2 | 200 ng/mL | 3 | 198±6.54 | 3.31 | 98.8 |
| Inter-day | 200 ng/mL | 6 | 199±9.19 | 4.61 | 99.7 |
| Intra-day, Day 1 | 1000 ng/mL | 3 | 970±37.5 | 3.87 | 97.0 |
| Intra-day, Day 2 | 1000 ng/mL | 3 | 957±60.6 | 6.33 | 95.7 |
| Inter-day | 1000 ng/mL | 6 | 964±45.6 | 4.73 | 96.4 |
| Intra-day, Day 1 | 5000 ng/mL | 3 | 4828±42.2 | 0.875 | 96.6 |
| Intra-day, Day 2 | 5000 ng/mL | 3 | 5449±154 | 2.83 | 109 |
| Inter-day | 5000 ng/mL | 6 | 5139±355 | 6.90 | 103 |
Table 2.
Intra-day accuracy and precision of calibration standards of mouse serum spiked with Azurin p28 and extracted according to the method.
| Nominal Concentration (ng/mL) | Day 1 |
Day 2 |
||
|---|---|---|---|---|
| Accuracy (%) | Precision (%CV) | Accuracy (%) | Precision (%CV) | |
| 100 | 100 | 1.35 | 104 | 3.70 |
| 200 | 102 | 5.30 | 104 | 7.23 |
| 500 | 93.0 | 9.82 | 91.6 | 2.30 |
| 1000 | 100 | 7.59 | 102 | 10.6 |
| 2000 | 103 | 8.18 | 92.0 | 4.51 |
| 5000 | 100 | 9.53 | 105 | 9.51 |
| 10000 | 99.4 | 11.1 | 99.1 | 2.82 |
To prepare for further pharmacokinetic and toxicokinetic studies with Azurin p28 in dogs and monkeys, and its clinical trials in humans, we examined the intra-day accuracy and precision of Azurin p28 spiked into dog, monkey and human serum at three concentrations (200, 1000, and 5000 ng/ml, n= 3). The results showed that %CV and accuracy of the analytical method for Azurin p28 in dog serum ranged from 2.63–12.9% and 93–100; in monkey serum, ranged from 1.63–7.35% and 93.1–102; in human serum, ranged from 0.81–11.2% and 88.4–102, respectively. Overall, the method is robust for determination of Azurin p28 in various species of sera.
3.1.3. Recovery from serum
The recovery Azurin p28 determined from mouse serum. Drug-free serum was spiked at three separate concentrations of Azurin p28 (200, 1000, and 5,000 ng/mL) and extracted as described above. An identical volume (100 μl) of extract produced from drug-free serum was spiked with Azurin p28 at the same concentrations listed above. The percent recovery was determined by comparing the peak areas of the spiked serum to those in the spiked serum extract. The average recovery of Azurin P28 from mouse, human, dog, and monkey serum at the three concentrations assayed was determined to be 96.4%, 108%, 90.2%, and 86.4%, respectively (Table 3). The results demonstrated the efficiency of the sample preparation with little variation.
Table 3.
Extraction recovery of Azurin p28 in mouse, human, dog and monkey serum (n=3)
| Concentrations (ng/mL) | 200 | 1000 | 5000 | Average |
|---|---|---|---|---|
| Mouse | 100 ± 2.38 | 100 ± 5.81 | 89.1± 7.94 | 96.4 ± 6.36 |
| Human | 89.4 ± 18.5 | 103 ± 14.8 | 94.1± 12.9 | 108 ± 8.37 |
| Dog | 92.5 ± 20.7 | 89.1±11.7 | 80.9 ± 14.5 | 90.2 ± 6.88 |
| Monkey | 81.0 ± 10.1 | 93.6 ± 6.39 | 89.2 ± 12.2 | 86.4 ± 4.91 |
3.1.4. Stability in mouse serum extracts
The stability of Azurin p28 in extracted mouse serum was determined by re-injecting a standard curve from the first day of validation after storage at 4°C for 18 hours in a Peltier autosampler tray. The results showed that after storage for 18 hours, the average response for concentrations ranging from 100–10000 ng/ml was 90.3% of day one (Table 4), suggesting that the established conditions for sample extraction, storage, and intermittent analysis are validated and suited for large scale sample analysis.
Table 4.
Stability of Azurin p28 in processed calibration standard extracts after 18 hours at 4°C.
| Nominal Concentration (ng/mL) | Day 1 | Day 2 | % of Day 1 |
|---|---|---|---|
| 100 | 101 | 98.8 | 97.8 |
| 200 | 216 | 191 | 88.4 |
| 500 | 497 | 436 | 87.7 |
| 1000 | 1080 | 962 | 89.1 |
| 2000 | 2230 | 2090 | 93.7 |
| 5000 | 5570 | 4960 | 89.0 |
| 10000 | 11200 | 9640 | 86.1 |
3.2. Stability in serum
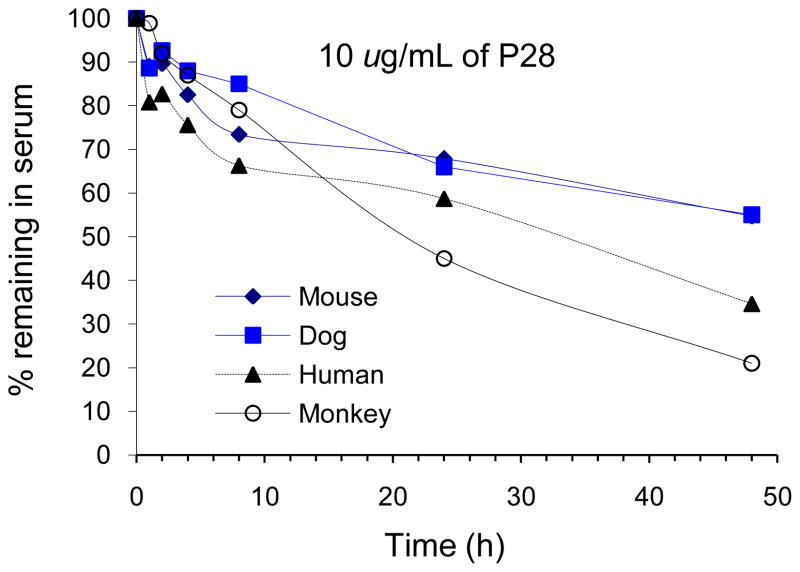
The stability of Azurin P28 in mouse serum (10 μg/ml) was determined at 4°C (refrigerated) and at 37°C at various times over a 48-hour period. The results of the serum stability study are presented in Fig. 2. At 4°C, Azurin p28 appeared to be stable in serum for at least 48 hours. At 37°C, Azurin p28 was most stable in mouse and dog serum followed by human serum and least stable in monkey serum. For example, after 24-h incubation at 37°C, the percent remaining of Azurin p28 in mouse, dog, human and monkey serum was 68, 67, 59, and 45%, respectively.
Fig. 2.
Stability profile of Azurin p28 (10 μg/ml) incubated at 37°C in various species of serum for up to 48 hours. The percentage of Azurin p28 remaining was calculated by integration of chromatographic peak area. Each point represents three tests.
3.3. Pharmacokinetics of Azurin p28 in mice
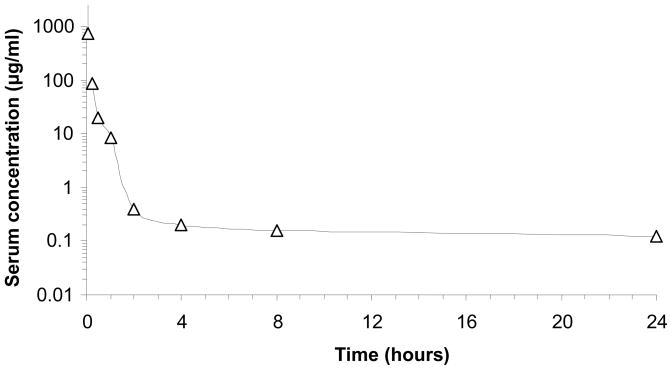
The serum concentration-time course of Azurin p28 in mice given a single i.v. bolus dose of 480 mg/kg is shown in Fig. 3. At 2 minutes after dosing, serum concentrations of Azurin p28 ranged from 1900 to 3200 μg/ml. Serum concentrations decreased rapidly during the first hour after dosing and ranged from 0.668 to 22.5 μg/ml at 1 hour. At 24 hours after dosing, Azurin p28 was measured at concentrations ranging from 0.103 to 0.149 μg/ml.
Fig. 3.
Serum concentration versus time plot for mice (n= 4 per time point) given an iv administration of 480 mg/kg of Azurin p28.
Pharmacokinetic parameters were calculated from the serum concentration versus time data using compartmental analysis. Data were fit to a three compartment model (Model 18). The estimated half-lives of Azurin p28 were 2 minutes, 0.23, and 34.1 hours. The estimated total body clearance (CL) and volume of distribution at steady state (Vdss) of Azurin p28 were 1727 ml/hr/kg and 4084 ml/kg, respectively. These results are summarized in (Table 5).
Table 5.
Pharmacokinetic parameters of Azurin p28 intravenously administered to mice (n= 4 per time point)
| Parameter | Dose (mg/kg) 480 |
|---|---|
| Cmax (μg/ml) | 2473 |
| Tmax (min) | 2 |
| T1/2α (min) | 2 |
| T1/2β (hours) | 0.23 |
| t1/2γ (hours) | 34.1 |
| AUC0-t (hr μg/ml)b | 278 |
| Clearance (ml/kg/hr)b | 1727 |
| Vdss (ml/kg) | 4084 |
Parameters were calculated from compartmental analysis of the data, except where indicated.
Calculated from non-compartmental analysis of the data
4. Discussion
In this work, we presented a novel and validated analytical method for the determination of Azurin p28 in serum of various species using the LC/MS/MS detection. The method allows for the direct and rapid extraction of Azurin p28 from serum using 7% of perchloric acid, and results in 96.4% of extraction recovery.
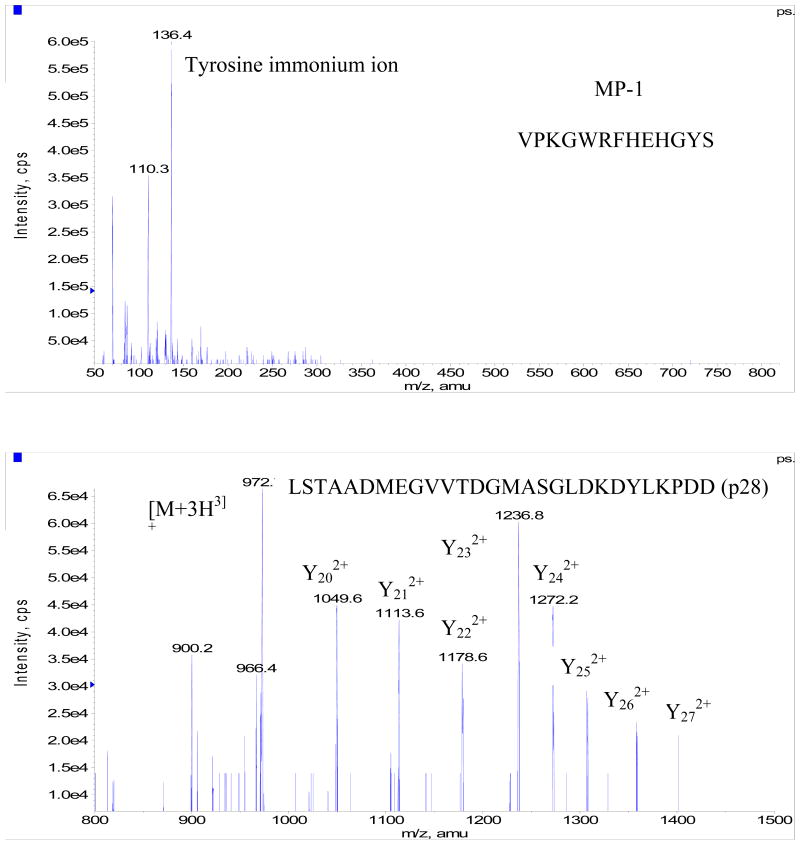
Development of the bioanalytical method consisted of separate infusions of 500 ng/ml solutions of Azurin p28 and MP-1 each dissolved in a 50:50 mixture of 5 mM ammonium acetate: acetonitrile solvent containing 0.5% formic acid (v/v). MP-1 was selected as an IS for this work as it has been well characterized and widely used in our laboratory as a peptide internal standard. The ion transitions for each compound were identified from product ion scans (Fig. 4) of the protonated molecular ion. Azurin p28 did not ionize very well with either electrospray or APCI however the strongest signal was obtained from electrospray and the predominate ion observed was [M+3H]3+ which corresponds to m/z of 972. The main fragment ions observed from the [M+3H]3+ pseudo-molecular of Azurin p28 were a doubly charged y24 (m/z 1272) and y23 ion (m/z 1236). In order to obtain the required sensitivity for Azurin p28 the sum of these two transitions were used. For the internal standard the predominate ion was the [M+2H]2+ at a observed m/z of 809 which produced a fragment ion at an observed m/z of 136 corresponding to the immonium ion of tyrosine. Chromatographic conditions for both analytes were optimized by evaluating a series of gradient profiles on various C18 HPLC columns in order to obtain good peak shape and response and to prevent ion suppression from co-elution of endogenous components in the extracted biological matrix. Sample extraction procedures were evaluated by spiking in known amounts of Azurin p28 and MP-1 into blank serum and evaluating reproducibility, recovery and applicability to sample dilution. Protein precipitation using a 7% perchloric acid solution in a ratio of 1:1 with serum was found to produce the best results.
Fig. 4.
Product ion scan of MP-1 (upper) and Azurin p28 (bottom). The upper panel shows m/z 136 as the immonium ion of tyrosine. The bottom panel shows the fragment ions used for quantification Y23 m/z 1236 and Y24 m/z 1272.
To assess accuracy (% deviation from theoretical value) and precision (% coefficient of variation), we determined the intra- and inter-day precision and overall accuracy by replicate analysis (n= 3, or 6) of each Azurin p28 concentration in serum over separate analytical batches. The results of the method validation showed that the predicted concentrations of Azurin p28 spiked in and extracted from mouse serum were within 10% of their theoretical values (Table 1–2). These results indicate that, consistent with our previous studies [10, 11, 14, 16], the method is reliable within the analytical range, and the use of the internal standard is effective for analytical reproducibility.
The in-vitro stability determination is one of the important secondary screening assays in drug development processes. It eliminates unstable candidate drugs and determines whether or not the in-vivo clearance is due to enzymatic degradation [17]. Of greater interest in this study is the relative stability of Azurin p28 in serum (Fig. 2). Using the same system, we reported that Phor21-βCG(ala), a lytic peptide conjugate, degraded fast when incubated with serum [10]. By comparison, Azurin p28 shows better drugability for further development. Together with the pilot pharmacokinetic study in mice showed in Fig. 3 and Table 5, the method has been proven to be validated and suited for analysis of large scale of samples from toxicokinetic and clinical trials.
In summary, a rapid LC/MS/MS method was developed and validated for the quantitation of Azurin p28 in various species of serum. Application of the extraction procedure yielded a recovery of greater than 96% of added Azurin p28. The assay showed good intra- and inter-assay accuracy and precision. The lower limit of quantitation of Azurin p28 in mouse serum was determined to be 100 ng/ml. The method was applied to the analysis of serum samples collected from mice given an intravenous administration of Azurin p28, and is currently used for analysis of pharmacokinetic and toxicokinetic samples obtained from monkeys and human beings to support investigational new drug application and clinical trials of the molecule.
Acknowledgments
This work was supported by NCI contract number N01-CM-52203
Footnotes
The study was partially presented at 2008 American Association of Cancer Research annual meeting, in April, 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taranta M, Bizzarri AR, Cannistraro S. Probing the interaction between p53 and the bacterial protein azurin by single molecule force spectroscopy. J Mol Recognit. 2008;21:63–70. doi: 10.1002/jmr.869. [DOI] [PubMed] [Google Scholar]
- 2.Punj V, Das Gupta TK, Chakrabarty AM. Bacterial cupredoxin azurin and its interactions with the tumor suppressor protein p53. Biochem Biophys Res Commun. 2003;312:109–114. doi: 10.1016/j.bbrc.2003.09.217. [DOI] [PubMed] [Google Scholar]
- 3.Yamada T, Fialho AM, Punj V, Bratescu L, Gupta TK, Chakrabarty AM. Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol. 2005;7:1418–1431. doi: 10.1111/j.1462-5822.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 4.Apiyo D, Wittung–Stafshede P. Unique complex between bacterial azurin and tumor-suppressor protein p53. Biochem Biophys Res Commun. 2005;332:965–968. doi: 10.1016/j.bbrc.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Hong CS, Yamada T, Hashimoto W, Fialho AM, Das Gupta TK, Chakrabarty AM. Disrupting the entry barrier and attacking brain tumors: the role of the Neisseria H.8 epitope and the Laz protein. Cell Cycle. 2006;5:1633–1641. doi: 10.4161/cc.5.15.2991. [DOI] [PubMed] [Google Scholar]
- 6.Mahfouz M, Hashimoto W, Das Gupta TK, Chakrabarty AM. Bacterial proteins and CpG-rich extrachromosomal DNA in potential cancer therapy. Plasmid. 2007;57:4–17. doi: 10.1016/j.plasmid.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhari A, Mahfouz M, Fialho AM, Yamada T, Granja AT, Zhu Y, Hashimoto W, Schlarb-Ridley B, Cho W, Das Gupta TK, Chakrabarty AM. Cupredoxin-cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochemistry. 2007;46:1799–1810. doi: 10.1021/bi061661x. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Goto M, Punj V, Zaborina O, Chen ML, Kimbara K, Majumdar D, Cunningham E, Das Gupta TK, Chakrabarty AM. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci U S A. 2002;99:14098–14103. doi: 10.1073/pnas.222539699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, Cunningham EA, Graves J, Yamada T, Constantinou AI, Christov K, White B, Li G, Majumdar D, Chakrabarty AM, Das Gupta TK. Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene. 2004;23:2367–2378. doi: 10.1038/sj.onc.1207376. [DOI] [PubMed] [Google Scholar]
- 10.Jia L, Noker PE, Piazza GA, Leuschner C, Hansel W, Gorman GS, Coward LU, Tomaszewski J. Pharmacokinetics and Pharmacodynamics of Phor21-βCG(ala), a lytic peptide conjugate. J Pharm Pharmacol. 2008;60:1441–48. doi: 10.1211/jpp.60.11.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coward LC, Kerstner-Wood, Noker P, Gorman GS, Pellecchia M, Reed JC, Jia L. Quantitative determination of Apogossypol, a pro-apoptotic analog of gossypol, in mouse plasma using LC/MS/MS. J Pharm Biomed Anal. 2006;42:581–586. doi: 10.1016/j.jpba.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Jia L, Yu L, Linnik DM, Jack RM. Stability and Pharmacokinetics of LJP 920, an octameric Gal (beta1–3) Gal conjugate for the inhibition of xenotransplantation rejection. J Pharm Pharmacol. 2001;53:999–1005. doi: 10.1211/0022357011776243. [DOI] [PubMed] [Google Scholar]
- 13.Wong H, Jia L, Camden J, Weitman S. Liquid chromatography-mass spectrometry assay of a thiodiazole derivative in mice: application to pharmacokinetic studies. J Chromatogr. 2001;765:55–62. doi: 10.1016/s0378-4347(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 14.Jia L, Tomaszewski JE, Noker PE, Gorman GS, Glaze E, Protopopova M. Simultaneous determination of pharmacokinetic properties of three anti-tubercular ethambutol analogs resulting from combinatorial lead optimization. J Pharm Biomed Anal. 2005;37:23–33. doi: 10.1016/j.jpba.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Song M, Wang L, Hang T, Wen A, Yang L, Jia L. Rapid and sensitive liquid chromatography-tandem mass spectrometry for rasagiline: assay development, validation and application to a human pharmacokinetic study. J Chromatogr. 2008;875:515–521. doi: 10.1016/j.jchromb.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Jia L, Coward LC, Kerstner-Wood, Gorman GS, Noker P, Kitada S, Pellecchia MP, Reed JC. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother Pharmacol. 2008;61:63–73. doi: 10.1007/s00280-007-0446-3. [DOI] [PubMed] [Google Scholar]
- 17.Jia L, Liu XD. The conduct of drug metabolism studies considered good practice (II): in vitro experiments. Curr Drug Metab. 2007;8:822–829. doi: 10.2174/138920007782798207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA. Guidance for Industry: Bioanalytical Method Validation. 2001 May; www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf.