Abstract
There are many distinct forms of dementia whose pharmacological and behavioral management differ. Differential diagnosis among the dementia variants currently relies upon a weighted combination of genetic and protein biomarkers, neuroanatomical integrity, and behavior. Diagnostic specificity is complicated by a high degree of overlap in the initial presenting symptoms across dementia subtypes. For this reason, reliable markers are of considerable diagnostic value. Communication disorders have proven to be among the strongest predictors for discriminating among dementia subtypes. As such, Speech-Language Pathologists may be poised to make an increasingly visible contribution to dementia diagnosis and its ongoing management. The value and durability of this potential contribution, however, demands an improved discipline-wide knowledge base about the unique features associated with different dementia variants. To this end we provide an overview of cognition, language, and clinical pathological features of four of the most common non-Alzheimer’s dementias: Frontotemporal Dementia, Vascular Dementia, Lewy Body Disease Dementia, and Parkinson’s Disease Dementia.
INTRODUCTION
The World Health Organization’s International Classification of Diseases (ICD-10) describes dementia as a spectrum of chronic progressive disorders that produce psychosocial or occupational impairment due to the compromise of higher cortical functioning within one or more of the following domains: memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgment (1992). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders-text revised (DSM-IV-TR) offers alternative diagnostic criteria for dementia, characterized as a significant decline in social and occupational functioning due to memory impairment with one or more of the following associated disturbances: aphasia, apraxia, agnosia, or dysexecutive disorder (2000).
Both the ICD-10 and DSM-IV-TR criteria have been criticized for lack specificity in discriminating among the many dementia variants whose pathologies, phenotypes, and clinical courses are distinct (Reisberg, 2006). Moreover, recent advances in pharmacological and behavioral management have created a compelling need for etiology-specific treatment and early diagnosis among the many dementia variants. Differential diagnosis is, however, complicated by a high degree of overlap in the initial presenting symptoms of the dementias. Specificity is further complicated by the lack of a definitive, non-surgically invasive biomarker that can confirm in vivo diagnosis. Current diagnostic protocols for dementia rely upon probabilistic weighting of a number of factors, including protein and genetic biomarkers, assays of metabolic functioning, neuroimaging, and behavior.
With respect to behavior, speech and language characteristics have proven to be among the most reliable behavioral markers for distinguishing among dementia variants (Cycyk & Wright, 2008; Garrard, Maloney, Hodges, & Patterson, 2005; Grossman, D’Esposito, et al., 1996; Neary, et al., 1998). For this reason, Speech-Language Pathology may be poised as a discipline to play an increasingly visible role in dementia diagnosis and management. This is especially true in light of the explosive projected growth of dementia in our aging population and the pragmatic necessity for promoting deinstitutionalized care for millions of affected patients (Hebert, Scherr, Bienias, Bennett, & Evans, 2004; Hebert & Brayne, 1995; Jorm, 1991; Zaccai, McCracken, & Brayne, 2005). One prerequisite for an informed contribution to dementia is an improved discipline-wide knowledge base regarding the unique characteristics of the various dementia subtypes. Clinical academic training in dementia is not yet universally mandatory for graduate education in Speech-Language Pathology in the United States (See American Speech-Language-Hearing Association Curriculum Guidelines, 2008), nor is such training mandated in the United Kingdom, Australia, or Canada. As a consequence, many practicing speech-language clinicians may lack competence in treating patients with dementia and counseling caregivers.
Our aim in this review is to acquaint the reader with four dementia subtypes that are likely to increasingly comprise their caseloads during the next two decades. We focus on cognition, language, and the clinical pathological features of the following non-Alzheimer’s disease dementias: Frontotemporal Dementia (FTD), Vascular Dementia (VaD), Lewy Body Disease Dementia (LBD), and Parkinson’s Disease Dementia (PDD). Although this is by no means an exhaustive list of non-Alzheimer’s dementias, these represent the four most common dementing conditions behind Alzheimer’s disease.
On the Relation between Aphasia and Dementia
Aphasia in the broadest sense of the word represents an acquired disorder of language comprehension, production, and/or symbolic knowledge (LaPointe, 2005). Given this broad definition, one might suspect that aphasia is evident across virtually all of the dementias, and indeed an inspection of formal diagnostic criteria across many of the dementias confirms this suspicion (American Psychiatric Association, 2000; McKhann, et al., 1984; Neary, et al., 1998; Wetterling, Kanitz, & Borgis, 1996). Despite the ubiquity of aphasia in dementia, however, its qualitative nature and severity vary substantially as functions of disease process and localization. Such heterogeneity has led to a longstanding theoretical debate reflected in the dichotomy between disorders of degraded storage vs. impaired linguistic access in dementia and stroke aphasia (Rapp & Caramazza, 1993; Warrington & Shallice, 1979).
Language disturbances in dementia have been historically attributed to the degradation of stored knowledge, whereas it is commonly assumed that language deficits in stroke aphasia reflect modality-specific impairment of access to intact conceptual knowledge (Adlam, Bozeat, Arnold, Watson, & Hodges, 2006; Beauregard, Chertkow, Gold, & Bergman, 2001; Rogers, Ivanoiu, Patterson, & Hodges, 2006; Salmon, Butters, & Chan, 1999). Although the storage-access dichotomy provides an intuitive framework, its assumptions have met with criticism (for discussion see Rapp & Caramazza, 1993). Counterevidence for a clean dementia-stroke dissociation is derived from work demonstrating that patients with various forms of stroke aphasia (e.g., optic aphasia, transcortical sensory aphasia, Broca’s aphasia) can experience non-verbal conceptual impairments that are consistent with degraded storage accounts (Chertkow, Bub, Deaudon, & Whitehead, 1997; Corbett, Jefferies, Ehsan, & Lambon Ralph, in press; Jefferies & Lambon Ralph, 2006; Jefferies, Patterson, & Lambon Ralph, 2008). An analogous body of research has demonstrated that patients with Alzheimer’s disease often show modality-specific advantages (e.g., pictures named better than words) that are strongly suggestive of impaired access (Bayles & Tomoeda, 1983; Bayles, Tomoeda, Kaszniak, & Trosset, 1991; Ober & Shenaut, 1999). Thus, it is distinctly possible for patients with stroke aphasia to satisfy criteria for storage impairment and for patients with dementia to behave consistently with access impairment. Variable performance in both populations has engendered perhaps the most controversy with respect to the nature of language and nonverbal conceptual impairment in Primary Progressive Aphasia.
Primary Progressive Aphasia and its Relation to Dementia
Mesulam (1982) proposed the term Primary Progressive Aphasia (PPA) to describe a slowly progressive language impairment that persists for a period of at least two years without dementia. Further diagnostic criteria for PPA include an insidious onset and gradual worsening of language (syntax, naming, word finding, word comprehension) in the absence of other specific causes of aphasia (e.g., stroke, malignancy). Patients with PPA may have co-morbid acalculia (i.e., inability to perform arithmetic) or ideomotor apraxia but must not manifest other amnestic, personality, or dysexecutive deficits that impair activities of daily living (Mesulam, 2001, 2003a). PPA has been described as a “language-based dementia” (Mesulam, 2003b) and as “slowly progressive aphasia without generalized dementia” (Mesulam, 1982).
Contemporary with Mesulam’s research on PPA, Warrington and colleagues independently characterized language disorders associated with primary semantic impairment (i.e., semantic dementia) (Warrington, 1975, 1981). Parallel research on PPA and semantic dementia continued until Snowden and colleagues argued that PPA and semantic dementia likely represent different stages of the same disease pathology (Snowden, Goulding, & Neary, 1989; Snowden & Neary, 1993). In many circles, semantic dementia is now used synonymously with fluent PPA (see Adlam, Patterson, et al., 2006; Amici, Gorno-Tempini, Ogar, Dronkers, & Miller, 2006). However, others have cautioned for a clear distinction between semantic dementia and semantic variant PPA (Mesulam, et al., 2009).
Perhaps the most compelling argument against interchangeable use of the terms PPA and semantic dementia lies in their differing levels of specificity. PPA represents a behavioral manifestation (i.e., a phenotype) of a number of possible disease etiologies, including frontotemporal dementia, Alzheimer’s disease, Parkinson’s Disease, and Creutzfeld-Jacob disease (Amici, et al., 2006; Grossman & Ash, 2004; Mesulam, 2007; Mesulam, et al., 2009). In contrast, semantic dementia is associated with a specific disease entity (i.e., frontotemporal lobar degeneration). Thus, it is exceedingly rare for Alzheimer’s disease to be described as semantic dementia. Yet it is not uncommon for Alzheimer’s disease to present as PPA. In fact, one recent postmortem confirmation study revealed that the distribution of disease pathology in one PPA cohort (N=38) consisted of almost 1/3 Alzheimer’s disease (32%) (Knibb, Xuereb, Patterson, & Hodges, 2006). Here we have attempted to avoid conflating phenotypic nomenclature (e.g., PPA) with clinicopathological disease classification. Instead, we focus upon clinicopathological groupings beginning with frontotemporal dementia.
FRONTOTEMPORAL DEMENTIA
FTD is the second most common dementia behind Alzheimer’s disease diagnosed in adults below the age of 65 years and the fourth most common dementia in industrialized nations (Heutink, 2000; Johnson, et al., 2005; Neary, Snowden, & Mann, 2005) Whereas age is an increasing risk factor for many other forms of dementia, this is not true of FTD, whose onset follows a roughly Gaussian (normal) distribution with a mean age of onset near 60 years and tapering frequency during earlier and later decades of life (Forman, et al., 2006; Johnson, et al., 2005; Ratnavalli, Brayne, Dawson, & Hodges, 2002). Symptoms of FTD typically appear about a decade earlier than AD, making age of onset a distinctive feature of the disease (Hodges, Davies, Xuereb, Kril, & Halliday, 2003; Neary, et al., 2005; Rascovsky, et al., 2005). Survival in FTD varies from between two to eight years post onset, although there is considerable variability in clinical course and rate of progression (Hodges, et al., 2003; Neary, et al., 2005).
FTD is characterized by progressive atrophy of regions of frontal and temporal cortex (Cairns, et al., 2007; Johnson, et al., 2005; Neary, et al., 2005). In early stage FTD, patients show relatively localized or circumscribed atrophy of these brain regions, but as FTD progresses, gyral atrophy is evident across both cerebral hemispheres (Chao, et al., 2007; Whitwell, Anderson, Scahill, Rossor, & Fox, 2004). While the etiology of FTD is idiopathic or unknown, a genetic component linked to chromosome-17 is apparent in 40% of familial cases (Miller, 2007; Neary, et al., 2005). In these cases, a mutation on an arm of Chromosome-17 affects a gene known as Progranulin (PGRN) whose disruption produces pathogenic encoding and aggregation of the proteins, tau and ubiquitin (Baker, et al., 2006). In healthy neurons, tau plays a critical role in cytoskeletal support through the maintenance of structural integrity (Avila, Lim, Moreno, Belmonte, & Cuello, 2002). However, abnormal aggregations of these proteins destroy neurons. Recent histopathlogical confirmation studies suggest that other neurodegenerative diseases such as corticobasal degeneration (CBD), Parkinsonism, progressive supranuclear palsy (PSP), motor neuron disease (MND) and amyotrophic lateral sclerosis (ALS) may all share a common protein pathology (i.e., TDP-43) (Bian & Grossman, 2007; Goldman, et al., 2004; Miller, 2007).
There are at least three distinct FTD phenotypes, each having a particular distribution of cortical damage. Figure 1 represents a schematic depiction of the following syndromes: a) Progressive Nonfluent Aphasia, b) Semantic Dementia, and c) Frontal variant FTD (Neary, 1998). Common to all of these FTD subtypes is insidious onset and gradual progression in the absence of an acute precipitating event (e.g., stroke). Supportive features include an onset before the age of 65, a positive family history for FTD in a first degree relative, and the presence of bulbar palsy (motor neuron disease) (Neary, et al., 1998).
Figure 1.
Cortical atrophy in three frontotemporal dementia variants
Progressive Nonfluent Aphasia
The core diagnostic feature of PNFA outlined by Neary et al. (1998) is nonfluent speech with one or more of the following: agrammatism, phonemic paraphasias, and anomia. Supportive diagnostic features include: stuttering, oral apraxia, impaired repetition, alexia, agraphia, early preservation of word meaning, late mutism. These impairments typically occur in the context of relatively preserved episodic memory, visuospatial functioning, and temporal orientation (McKinnon, et al., 2008; Peelle & Grossman, 2008; Rogers, et al., 2006).
PNFA is characterized by asymmetric damage to left posterior inferior frontal cortex, including the anterior insula, basal ganglia, left inferior and middle frontal gyri, dorsal, premotor and supplementary motor cortices (Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007). As left hemisphere perisylvian damage worsens, patients show increasing motor speech difficulties and impairment in phonological assembly (Croot, Patterson, & Hodges, 1998; Gorno-Tempini, et al., 2006; Nestor, et al., 2003). It is not uncommon for these profound difficulties in speech production to evolve to complete mutism (Gorno-Tempini, et al., 2006; Neary, et al., 1998). This classic progression toward mutism in PNFA has clear implications for guiding speech-language intervention. That is, given the typical progression toward a profound deficit in speech production, one logical means of facilitating communication is through augmentative communication devices (e.g., text- or picture-to-speech). Thus, one logical strategy for PNFA may involve training patients at early stages of the disease on communication devices that will later become essential.
The distribution of left hemisphere damage in PNFA corresponds to that commonly seen in nonfluent stroke aphasia (e.g., Broca’s Aphasia). Thus, one might expect to see behavioral parallels between PNFA and stroke aphasia, and to some extent this is the case. Like Broca’s aphasia, patients with PNFA tend to produce speech that is slow, effortful, and rife with phonemic paraphasias. Patients with PNFA have also shown disproportionate impairment for verbs relative to nouns (Hillis, Sangjin, & Ken, 2004; Hillis, Tuffiash, & Caramazza, 2002), reduced phrase length during production, and difficulties comprehending grammatically complex utterances (Ash, et al., 2006; Ash, et al., 2004; Grossman, Mickanin, Onishi, & Hughes, 1996; Peelle, Cooke, Moore, Vesely, & Grossman, 2007; Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997). One recent direct comparison between nonfluent stroke aphasia and PNFA, however, revealed that the extent of syntactic loss in production (i.e., agrammatism) was worse in the stroke aphasia group relative to PNFA (Patterson, Graham, Lambon Ralph, & Hodges, 2006). Although these differences may reflect a more posterior distribution of frontal lobe damage in PNFA, this speculative hypothesis awaits anatomical correlation.
Semantic Dementia
Elizabeth Warrington reported a groundbreaking case study of patients who experienced what she described as the “selective impairment of semantic memory” (Warrington, 1975). Snowden and colleagues (1989) later formally classified such patients as having a distinctive neurological disorder known as semantic dementia (SD). On structural imaging (e.g., MRI, CT), patients with SD tend to show circumscribed atrophy affecting anterior, lateral, and ventral temporal lobe structures with relative sparing of the medial temporal lobe (Neary, et al., 2005; Snowden, et al., 1989). A commonly held assumption, reflected in formal diagnostic criteria, holds that there is hemispheric asymmetry with the left hemisphere more prominently affected than the right (Galton, et al., 2001; Hodges, Patterson, Oxbury, & Funnell, 1992; Mummery, et al., 2000; Neary, et al., 1998; Snowden, et al., 1989). There is, however, a growing body of literature to suggest that homologous regions of the right anterior temporal lobe are also affected (for a computational model of the spread of atrophy see Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001).a Figure 2 shows coronal, axial, and sagittal T1-weighted MR images of a patient with early to moderate semantic dementia, illustrating this typical pattern of asymmetric left anterior temporal lobe atrophy on structural imaging.
Figure 2.
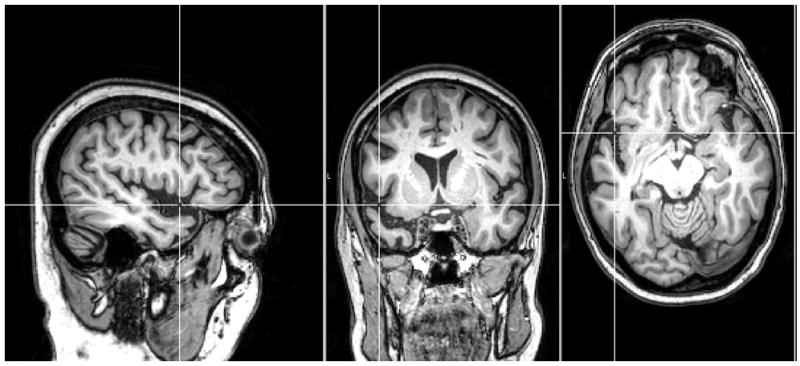
Structural magnetic resonance image of semantic dementia
Note: Structural image of a patient with early stage semantic dementia (<12 months post diagnosis). Asymmetric left hemisphere atrophy is evident in anterior temporal cortex as illustrated by the center of the white crosshairs.
Damage to the anterior and inferolateral temporal cortex in SD produces a distinctive language profile. Patients with SD produce fluent but empty speech that is rife with deictic expressions (e.g., I don t know… It s that thing) and semantic paraphasias (e.g., cat when the target word is Chihuahua) (Hodges, et al., 1992; Snowden, et al., 1989). These difficulties in narrative production are often amplified at the single word level and manifest as profound anomia (Lambon Ralph, et al., 2001; Lambon Ralph & Patterson, 2008; Neary, et al., 1998). Despite a severe naming impairment, however, patients with SD often produce over-learned phrases effortlessly. As a result, in cursory conversation it is often difficult to discern any hint of impairment. This was the first author’s impression upon testing his first SD patient. His combination of fluent speech, appropriate affect, and insight masked a conceptual impairment that soon became apparent through a combination of formal testing and observation of numerous functional errors in activities of daily living (e.g., adding salt to sweeten coffee, requesting (and succeeding) in having a young investigator eat an inedible plant from his herb garden) (for a description see patient BE reported in Reilly, Martin, & Grossman, 2005).
Loss of meaning is a core diagnostic feature of SD that affects both language production and comprehension. Other core features of SD include empty spontaneous speech, semantic paraphasias, and a perceptual disorder characterized by prosopagnosia (impaired face recognition) and/or associative visual agnosia (impaired recognition of object identity) (Neary, et al., 1998). For a diagnosis of SD, patients must also show relatively preserved perceptual matching abilities (e.g., pure tones, pictures of different people, nonsense objects), intact single word repetition, and an ability to read aloud orthographically regular words (Neary, et al., 1998).
The inability to read irregular words aloud (i.e., suface dyslexia) is ubiquitous in SD (Blazely, Coltheart, & Casey, 2005; Fushimi, et al., 2003; Jefferies, Lambon Ralph, Jones, Bateman, & Patterson, 2004; Wilson, et al., 2009). Surface dyslexia is a reading disorder that is characterized by regularization errors (e.g., yacht is written to dictation as yot or pronounced aloud as yaughched (Neary, et al., 1998; Woollams, Ralph, Plaut, & Patterson, 2007). One hypothesis regarding the high frequency of surface dyslexia in SD is that patients over-rely on reading via a direct grapheme-to-phoneme conversion process (Plaut, McClelland, Seidenberg, & Patterson, 1996). That is, patients exploit their preserved phonological and orthographic abilities to compensate for degraded semantic knowledge that would normally support reading or writing (Shallice, Warrington, & McCarthy, 1983, for a more comprehensive discussion of the relative merits and disadvantages of dual route models of reading and patient-based findings see Woolams et al, 2007).
Supportive features of SD include relative preservation of calculation, syntax, day-to-day memorizing (i.e., recent episodic memory), spatial skills, perception, and phonological processing. Additional impairments that are supportive of a diagnosis of SD include idiosyncratic word usage, press of speech, late stage loss of sympathy and empathy, narrowed preoccupations, and parsimony of interactions (e.g., seclusion, limited attempts to engage in conversation) (Neary, et al., 1998).
One of controversy in neuroscience over the past 20 years has been the nature of the impairment underlying SD and what it can tell us about the neural correlates of semantic memory (Caramazza & Mahon, 2003; Martin, 2007; Patterson, 2007; Patterson, Nestor, & Rogers, 2007; Reilly & Peelle, 2008). The crux of this debate centers upon whether deficits reflect a combination of agnosia and aphasia or a more central loss of conceptual knowledge. One clear prediction of a central semantic deficit is that patients should experience a pervasive multi-modal impairment (Patterson et al., 2006). For example, loss of the concept “DOG” should permeate all possible domains of DOGNESS, manifesting as difficulties in naming a picture of a dog, recognizing the distinctive sound of a dog’s bark, and identifying the odor of a dog. The Cambridge and Manchester research groups have elegantly (and consistently) demonstrated such a broad loss of domain-specific knowledge in SD (Bozeat, Lambon Ralph, Patterson, & Hodges, 2002; Patterson et al., 2006; Pulvermüller, et al., 2009; Rogers, Lambon Ralph, Hodges, & Patterson, 2004; Rogers & McClelland, 2004). Nonetheless, questions persist as to modality-specific advantages (e.g., reverse concreteness effects) and the role of the anterior temporal lobes in semantic memory (Simmons & Martin 2009; Simmons, Reddish, Bellgowan, & Martin, 2010).
A few studies have tracked the longitudinal progression of SD, and a picture of the deficits in late stages of the disease has begun to emerge. Typical of this progression, Jefferies et al. (2006) report a case study of patient MK who remained receptive to testing, pleasant, and without personality change even at a very advanced stage. For the patients we have observed, speech perception has remained remarkably intact, and we do not see the degree of speech struggle that is evident in PNFA (Kwok, Reilly, & Grossman, 2006; Reilly, Cross, Troiani, & Grossman, 2007). Nonetheless, patients do experience profound communicative impairment, and these difficulties often extend to fundamental interactions with the world.
Frontal Variant FTD / Behavioral Variant FTD
Frontal variant FTD (FvFTD) is behaviorally distinctive from PFNA and SD (Grossman, et al., 2007; Liu, et al., 2004; Rascovsky, et al., 2007). In FvFTD, cortical atrophy affects inferior, anterior regions of the frontal lobe (i.e., orbitofrontal cortex) (Liu, et al., 2004; Rascovsky, et al., 2007). Unlike PNFA and SD, individuals with FvFTD exhibit early changes in personality, organization, and attention (Neary, et al., 2005; Neary, et al., 1998). Core diagnostic criteria identified for FvFTD include a gradual decline in social conduct (i.e., inappropriate jokes, risk taking, hypersexuality), with loss of insight and early emotional blunting (Neary, et al., 1998). Supportive diagnostic features include a decline in personal hygiene, increased rigidity, hyperorality, diet changes, and the emergence of utilization behavior (i.e., compulsive use of implements such as combs or pencils that are in view of the patient).
Of the three primary FTD subtypes, FvFTD is perhaps the least likely to be referred for a speech-language clinical evaluation because social, behavioral, and emotional deficits predominate. Although it is true that aphasia is not a frank symptom of FvFTD, this disease variant does compromise language abilities. FvFTD produces impairments in executive functioning that are characterized by diminished attentional vigilance, poor inhibitory control (i.e., attending to salient stimuli while ignoring non-salient distracters), poor sequencing ability, and limitations in working memory (Rascovsky, et al., 2007). In the production of narrative discourse, the net effect of these deficits is reduced thematic cohesion and disjointed event organization (Ash, et al., 2006; Cooke, et al., 2003; Grossman, 2002).
VASCULAR DEMENTIA
Vascular Dementia (VaD), the second most frequent dementia subtype, describes a heterogeneous group of dementias associated with cerebrovascular damage (Fratiglioni, et al., 2000; Traykov, et al., 1999). VaD can evolve within the context of multiple cortical or subcortical strokes, cerebral hypoxemia, aneurysm, small vessel ischemic disease and genetic cerebrovascular disease. Since brain tissue requires diffuse perfusion, vascular compromise can potentially occur across many locations. Thus, the cumulative pattern of cerebrovascular damage in VaD dictates its particular phenotype. Phenotypic heterogeneity is further heightened by the fact almost half of all VaD patients have mixed vascular and Alzheimer’s disease pathologies (Rocca & Kokmen, 1999; Rockwood, 1997; Rockwood, Bowler, Erkinjuntti, Hachinski, & Wallin, 1999; Traykov, et al., 1999). Due to the multiplicity of potential causes of VaD, the ICD-10 has identified the following VaD variants: 1) VaD acute onset; 2) Multi-infarct dementia; 3) Subcortical vascular dementia; 4) Mixed subcortical and cortical vascular dementia; 5) Other VaD; 6) VaD unspecified (World Health Organization, 1992).
There currently exist five disparate sets of clinical criteria for VaD. These include the DSM-IV-TR (2000), the State of California Alzheimer’s Disease Diagnostic and Treatment Centers (Chui, et al., 1992), the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l Enseignement en Neurosciences (NINDS-AIREN) (Roman, et al., 1993), the Hachinski Ischemic Score (Hachinski, et al., 1975), and the ICD-10 (1992). Postmortem clinicopathological comparisons demonstrate poor agreement amongst these criteria (Wetterling, et al., 1996). It has been argued, however, that the DSM-IV-TR criteria for VaD may offer the best balance between specificity and sensitivity (Chui, et al., 2000; Gold, et al., 2002).
The DSM-IV-TR criteria for VaD includes social or occupational functional decline due to multiple cognitive deficits. Patients must have memory impairment and one or more of the following additional disorders: aphasia, agnosia, dysexecutive disorder. Patients must also show a combination of the following signs and symptoms in the absence of delirium: gait abnormalities, weakness of an extremity, pseuobulbar palsy, exaggeration of reflexes, extensor plantar response. Signs must also be present in the context of laboratory evidence (e.g., structural magnetic resonance imaging) demonstrating cerebrovascular disease. Importantly, symptoms must also be judged as etiologically-related to the cognitive disturbance.
Common across many reported investigations of VaD is psychomotor slowing, attentional deficits and frontal-executive dysfunction resulting in decreased self-regulation (Almkvist, Fratiglioni, Aguero-Torres, Viitanen, & Backman, 1999; Matsuda, Saito, & Sugishita, 1998). It is also common for patients with VaD to exhibit deficits in working memory, procedural memory (e.g., tying one’s shoe or using a bank machine) and episodic memory retrieval (Bowler, et al., 1997; Looi & Sachdev, 1999; Reed, Eberling, Mungas, Weiner, & Jagust, 2000). Patients with VaD are likely to show decreased phonemic fluency, diminished grammatical complexity in sentence production, and flattening of pitch and amplitude contours in spontaneous speech (i.e., dysprosodia) (Jones, Laukka, & Backman, 2006; Looi & Sachdev, 1999; Lukatela, Malloy, Jenkins, & Cohen, 1998; Tierney, et al., 2001). Progression of VaD is often characterized by a stepwise decline. That is, during the course of the disease, patients may experience periods of stability followed by punctuated declines in memory and other cognitive functions (Chui, et al., 2000).
LEWY BODY DEMENTIA
Lewy Body Dementia (LBD) is a relatively new dementia classification with about one-half the frequency of AD (Rahkonen, et al., 2003). Prevalence estimates for LBD vary from between 15% to 35% of all dementia cases (Geser, Wenning, Poewe, & McKeith, 2005; Zaccai, et al., 2005). If the upper range of LBD (35%) is correct, then LBD represents the second most common form of dementia behind AD, relegating VaD to the third most common form of dementia (Geser, et al., 2005; Heidebrink, 2002; Rahkonen, et al., 2003; Weisman & McKeith, 2007; Zaccai, et al., 2005). Age of onset in LBD is similar to AD, with a mean of 68 years (range 50–85 years) (Jellinger, Wenning, & Seppi, 2007; Korczyn & Reichmann, 2006; Neef & Walling, 2006). Reports on survival duration post onset of LBD vary, with a mean of approximately 6 years (range 1.8 to 9.5 years) (Walker, Allen, Shergill, Mullan, & Katona, 2000).
Standard diagnosis of LBD is based on presence of dementia as well as two of the following three core diagnostic features: 1) fluctuating cognition, 2) visual hallucinations, and 3) movement disorder (I. G. McKeith, 2006). It is estimated that 50–75% of individuals with LBD experience fluctuations in cognition that range from minutes to days (I. McKeith, et al., 2004) and 70% of individuals will develop akinetic-rigid syndrome (Geser, et al., 2005). Suggestive diagnostic features include rapid eye movement sleep disorder, neuroleptic sensitivity, and low dopaminergic uptake in the basal ganglia (Galvin, et al., 2008). Supportive features include syncope, postural instability with repeated falls, transient loss of consciousness, delusions, and multimodal hallucinations (i.e., auditory and tactile) (I. G. McKeith, 2006; Weisman & McKeith, 2007).
LBD is typically associated with cognitive complaints (e.g, amnestic impairment) rather than aphasia or other language disturbance early in the course of the disease. Doubleday et al (2002) report that only 5% of LBD patients present with primary language complaints, whereas memory impairment, or a combination of memory, visuospatial, and language impairment are more commonly reported (Doubleday, Snowden, Varma, & Neary, 2002). Specific cognitive features of LBD include severe memory impairment, inattention, visuospatial disturbance, prosopagnosia, color agnosia, constructional and ideomotor apraxia, and visual distractibility (Doubleday, et al., 2002; Galasko, Katzman, Salmon, & Hansen, 1996; Gibb, Luthert, Janota, & Lantos, 1989). Language disturbance in LBD is characterized by confabulation, incoherence, and perseveration during conversation, difficulty naming common objects, and a reduction in verbal fluency (Doubleday, et al., 2002; Galasko, et al., 1996; Gibb, et al., 1989). Progression of the disease is characterized by Parkinsonism, profound memory disturbance, severe neuroleptic reactions, and disproportionate visuospatial deficits (Ferman, et al., 2006; Hamilton, et al., 2008). Additionally, there is some evidence that severity of visuospatial deficits predicts rate of cognitive decline (Hamilton, et al., 2008). In late stages of LBD, global cognitive deficits lead to complete functional dependence.
PARKINSON’S DISEASE DEMENTIA
Parkinson’s Disease (PD) has a mean age of onset of 60 years, with only 10% of those affected being 45 years of age or younger (Korchounov, Schipper, Preobrazhenskaya, Kessler, & Yakhno, 2004; Rao, Hofmann, & Shakil, 2006). The risk of developing PD increases with advanced age, and males have a greater likelihood of contracting the disease (Nussbaum & Ellis, 2003). Estimates for idiopathic PD in the world’s ten most populated nations range from 4.1 to 6.6 million in 2005, with a projected expansion to 8.7 to 9.3 million by 2030 (Dorsey, et al., 2007). In PD, dopamine depletion compromises the integrity of the cortical-striatal motor loop, producing impairment in the relative timing and initiation of purposeful movement (Louis & Frucht, 2007; Rao, et al., 2006). Typically, when greater than 80% of the dopaminergic cells of the substantia nigra are depleted, individuals develop the cardinal motor symptoms of Parkinson’s disease (Louis & Frucht, 2007). These symptoms include an asymmetric onset with resting tremor, bradykinesia, rigidity, and postural instability (Jellinger, et al., 2007; von Campenhausen, et al., 2005).
Recent work has demonstrated that even in the absence of a frank dementia, PD is associated with a number of higher level cognitive and communication deficits (Caballol, Marti, & Tolosa, 2007; Grossman, et al., 2000; Henry & Crawford, 2004; Hochstadt, Nakano, Lieberman, & Friedman, 2006; Mahieux, et al., 1998; Riedel, et al., 2008). Moreover, the severity of cognitive impairment in PD is strongly correlated with severity of bradykinesia, indicating a relationship between generalized cognitive and motor slowing (Levy, et al., 2000; Sawamoto, Honda, Hanakawa, Fukuyama, & Shibasaki, 2002). With respect to communication, many hold that the overarching source of impairment is a dysexecutive disorder characterized by resource limitations that affect inhibitory control, speed of processing, vigilance, cognitive flexibility, and working memory (Grossman, et al., 2000; Grossman, Lee, Morris, Stern, & Hurtig, 2002; Hochstadt, et al., 2006). Resource-related deficits associated with prefrontal cortical dysfunction in PD produce ripple effects that are evident in tasks such as verbal fluency, sentence comprehension, and narrative production (Grossman, et al., 2002; Henry & Crawford, 2004; Hochstadt, et al., 2006). Interestingly, generative action naming fluency is disproportionately impaired in PD relative to semantic or phonemic fluency, and patients with PD are likely to show greater anomia for actions than objects (Cotelli, et al., 2007). Other communication-related deficits include difficulties with inference, metaphor comprehension, and perception of linguistic and emotional prosody (Monetta & Pell, 2007; Pell & Leonard, 2003, 2005).
As PD progresses, as many as 70 to 80% of individuals convert to PD with dementia (PDD) (Emre, et al., 2007). In most cases, this conversion happens within the first 10 years after onset and is insidious in nature. Upon conversion to PDD, survival duration is 5 years (mean 6.7 years), roughly similar to LBD (Emre, et al., 2007; Geser, et al., 2005). Predictors of survival duration in PDD and LBD include older age at onset, fluctuating cognition, severity of hallucinations, severity of akinesia, and degree of concurrent Alzheimer’s disease neurofibrillary plaque and tangle pathology (Jellinger, et al., 2007).
Core diagnostic criteria for PDD include an established diagnosis of PD and impairment in at least two of the following four domains: 1) attention; 2) executive functioning; 3) visuospatial processing; and 4) verbal free recall. For the diagnosis of probable PDD, individuals must also show at least one behavioral symptom (i.e., apathy, depression, hallucinations, delusions, or excessive daytime sleepiness) (Emre, et al., 2007).
Communication deficits include impaired concept formation, perseveration, and reduced verbal fluency across domains (Emre, et al., 2007). It has also been hypothesized that reduced action word (i.e., verb) fluency predicts conversion from PD to PDD (Piatt, Fields, Paolo, Koller, & Troster, 1999). As PDD progresses, the cognitive and language deficits become more pronounced.
PDD and LBD: Similarities
Histopathological investigations of both PDD and LBD reveal the presence of large, alpha-synuclein protein deposits termed Lewy Bodies in the brain (Nussbaum & Ellis, 2003; Singleton, et al., 2003; Theuns & Van Broeckhoven, 2008). Lewy bodies collect within the neuronal cytoplasm, ultimately destroying cells and resulting in neuronal dropout (Emre, et al., 2007; Singleton, et al., 2003). In PDD, Lewy body-induced cell death occurs within the substantia nigra, a midbrain structure that produces dopamine, the neurotransmitter critical for regulating communication between the basal ganglia and the motor cortex (Bear, Connors, & Paradiso, 2007). In LBD, Lewy Bodies are found in the substantia nigra, but are more numerous in the cerebral cortex (Byrne, Lennox, Lowe, & Godwin-Austen, 1989) In both PD and LBD, cell death is not restricted to a particular brain region. Thus, late stage PD may resemble LBD, and LBD often evolves into a Parkinsonian syndrome (Byrne, et al., 1989).
PDD and LBD have a similar set of genetic and environmental risk factors, and onset may involve an interaction of these components. In a minority of cases, PDD and LBD have been linked to several gene mutations including SNCA, PRKN, PINK1, DJ-1, and LRRK-2 (Pankratz & Foroud, 2007; Theuns & Van Broeckhoven, 2008). However, the large majority of patients with PDD or LBD have no family history of the disease and no evidence of specific gene mutations (Pankratz & Foroud, 2007). PDD and LBD share environmental factors such as exposure to pesticides, high soil concentrations of specific metals (i.e., manganese), neurotoxic drugs (i.e., synthetic heroin), and neuroleptic antipsychotic medications (Ascherio, et al., 2006; Gao, et al., 2007; Lai, Marion, Teschke, & Tsui, 2002).
Because of similar underlying neuropathology, there is considerable overlap between PDD and LBD with respect to clinical profiles (Ballard, et al., 2006; McKeith, 2000; Neef & Walling, 2006). While LBD is typically associated with greater memory loss and greater fluctuations in attention than PDD, no significant differences have been found between these two groups in deficits of executive function, constructional praxis, and visuospatial processing (Emre, et al., 2007). This has led some to suggest that PD and LBD are spectrum disorders of Lewy body disease (Jellinger & Attems, 2008). Due to the difficulty distinguishing between LBD and PDD, current practice instantiates an arbitrary “one-year rule”, whereby dementia preceding the onset of a movement disorder by one year is classified as LBD, whereas dementia that occurs within the context of an existing movement disorder is classified as PDD (Emre, et al., 2007).
CONCLUSION
One of the greatest public health challenges facing industrialized nations during the next three decades involves effectively coping with dementia. The psychosocial and economic necessities of promoting deinstitutionalized care for the many millions of people with dementia will soon become apparent in our rapidly aging society. As a clinical discipline, Speech-Language Pathology may be poised to contribute valuable information through careful observation and identification of cognitive-linguistic behaviors associated with different forms of dementia. In addition, it seems equally likely that differential diagnosis will rely on identification of motor speech disorders and swallowing impairments associated with each of the subtypes. In addition, knowledge of these clinical profiles may be useful in educating patients, families and caregivers regarding the cognitive-linguistic behavioral deficits expected from onset to progression of the disease. Thus, there is great potential for Speech-Language Pathology to emerge as a frontline discipline in dementia management. Nevertheless, it is clear that this contribution demands an improved discipline-wide knowledge base. The aim of this paper was to provide an overview of the cognitive, language and clinical pathological features of Non-Alzheimer’s dementias in order to begin to address this gap in knowledge.
Acknowledgments
This work was supported by NIH/NIDCD K23 DC010197 (to JR) and the American Speech-Language-Hearing Foundation (to AR).
Footnotes
The nature of hemispheric asymmetry in the progression of FTD remains controversial. Josephs and colleagues (2009) recently reported an imaging-behavioral correlation of the right temporal lobe variant of FTD, identifying two distinct right hemisphere FTD variants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jamie Reilly, Department of Speech, Language, and Hearing Sciences, University of Florida
Amy Rodriguez, Department of Speech, Language, and Hearing Sciences, University of Florida.
Martine Lamy, Department of Psychiatry, University of Cincinnati
Jean Neils-Strunjas, Department of Communication Sciences and Disorders, University of Cincinnati.
References
- Adlam A, Bozeat S, Arnold R, Watson PC, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2006;42:675–684. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- Adlam A, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta- Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Almkvist O, Fratiglioni L, Aguero-Torres H, Viitanen M, Backman L. Cognitive support at episodic encoding and retrieval: similar patterns of utilization in community-based samples of Alzheimer’s disease and vascular dementia patients. Journal of Clinical Experimental Neuropsychology. 1999;21(6):816–830. doi: 10.1076/jcen.21.6.816.862. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Speech-Language-Hearing Association. Standards for Accreditation of Graduate Education Programs in Audiology and Speech-Language Pathology 2008 [Google Scholar]
- Amici S, Gorno-Tempini ML, Ogar JM, Dronkers NF, Miller BL. An overview on Primary Progressive Aphasia and its variants. Behavioural Neurology. 2006;17(2):77–87. doi: 10.1155/2006/260734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, et al. Pesticide exposure and risk for Parkinson’s disease. Annals of Neurology. 2006;60(2):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Hauck R, Antani S, Katz J, Grossman M. Quantitative analysis of paraphasic errors in frontotemporal dementia. Neurology. 2004;62a:166–183. [Google Scholar]
- Avila J, Lim F, Moreno F, Belmonte C, Cuello AC. Tau function and dysfunction in neurons: its role in neurodegenerative disorders. Molecular Neurobiology. 2002;25(3):213–231. doi: 10.1385/MN:25:3:213. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Ballard C, Ziabreva I, Perry R, Larsen JP, O’Brien J, McKeith I, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–1934. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK. Confrontation naming impairment in dementia. Brain and Language. 1983;19(1):98–114. doi: 10.1016/0093-934x(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK, Kaszniak AW, Trosset MW. Alzheimer’s disease effects on semantic memory: Loss of structure or impaired processing? Journal of Cognitive Neuroscience. 1991;3(2):166–182. doi: 10.1162/jocn.1991.3.2.166. [DOI] [PubMed] [Google Scholar]
- Bear MF, Connors BW, Paradiso MA. Neuroscience: Exploring the brain. 3. Philadelphia, PA, US: Lippincott Williams & Wilkins Publishers; 2007. [Google Scholar]
- Beauregard M, Chertkow H, Gold D, Bergman S. The impact of semantic impairment on word stem completion in Alzheimer’s disease. Neuropsychologia. 2001;39(3):302–314. doi: 10.1016/s0028-3932(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Bian H, Grossman M. Frontotemporal lobar degeneration: recent progress in antemortem diagnosis. Acta Neuropathologica. 2007;114(1):23–29. doi: 10.1007/s00401-007-0235-4. [DOI] [PubMed] [Google Scholar]
- Blazely AM, Coltheart M, Casey BJ. Semantic impairment with and without surface dyslexia: Implications for models of reading. Cognitive Neuropsychology. 2005;22(6):695–717. doi: 10.1080/02643290442000257. [DOI] [PubMed] [Google Scholar]
- Bowler JV, Eliasziw M, Steenhuis R, Munoz DG, Fry R, Merskey H, et al. Comparative evolution of Alzheimer disease, vascular dementia, and mixed dementia. Archives of Neurology. 1997;54(6):697–703. doi: 10.1001/archneur.1997.00550180021007. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR. When objects lose their meaning: What happens to their use? Cognitive, Affective & Behavioral Neuroscience. 2002;2(3):236–251. doi: 10.3758/cabn.2.3.236. [DOI] [PubMed] [Google Scholar]
- Byrne EJ, Lennox G, Lowe J, Godwin-Austen RB. Diffuse Lewy body disease: clinical features in 15 cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52(6):709–717. doi: 10.1136/jnnp.52.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballol N, Marti MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Movement Disorders. 2007;22(S17):S358–S366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathologica. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ. The organization of conceptual knowledge: The evidence from category-specific semantic deficits. Trends in Cognitive Sciences. 2003;7(8):354–361. doi: 10.1016/s1364-6613(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Chao LL, Schuff N, Clevenger EM, Mueller SG, Rosen HJ, Gorno-Tempini ML, et al. Patterns of white matter atrophy in frontotemporal lobar degeneration. Archives of Neurology. 2007;64(11):1619–1624. doi: 10.1001/archneur.64.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Deaudon C, Whitehead V. On the status of object concepts in aphasia. Brain and Language. 1997;58(2):203–232. doi: 10.1006/brln.1997.1771. [DOI] [PubMed] [Google Scholar]
- Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, Tinklenberg J, et al. Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Archives of Neurology. 2000;57(2):191–196. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42(3 Pt 1):473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Cooke A, DeVita C, Gee J, Alsop D, Detre J, Chen W, et al. Neural basis for sentence comprehension deficits in frontotemporal dementia. Brain and Language. 2003;85(2):211–221. doi: 10.1016/s0093-934x(02)00562-x. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. Different Impairments of Semantic Cognition in Semantic Dementia and Semantic Aphasia: Evidence from the Nonverbal Domain. Brain. doi: 10.1093/brain/awp146. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Zanetti M, Aravalo A, Cappa SF, et al. Action and object naming in Parkinson’s disease without dementia. European Journal of Neurology. 2007;14(6):632–637. doi: 10.1111/j.1468-1331.2007.01797.x. [DOI] [PubMed] [Google Scholar]
- Croot K, Patterson K, Hodges JR. Single word production in nonfluent progressive aphasia. Brain and Language. 1998;61(2):226–273. doi: 10.1006/brln.1997.1852. [DOI] [PubMed] [Google Scholar]
- Cycyk LM, Wright HH. Frontotemporal dementia: Its definition, differential diagnosis, and management. Aphasiology. 2008;22(4):422–444. [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Doubleday EK, Snowden JS, Varma AR, Neary D. Qualitative performance characteristics differentiate dementia with Lewy bodies and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72(5):602–607. doi: 10.1136/jnnp.72.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Boeve BF, Graff-Radford NR, Lucas JA, Knopman DS, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clinical Neuropsychology. 2006;20(4):623–636. doi: 10.1080/13854040500376831. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal Dementia: Clinicopathological Correlations. Annals of Neurology. 2006;59(6):952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, et al. Incidence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S10–15. [PubMed] [Google Scholar]
- Fushimi T, Komori K, Ikeda M, Patterson K, Ijuin M, Tanabe H. Surface dyslexia in a Japanese patient with semantic dementia: evidence for similarity-based orthography-to-phonology translation. Neuropsychologia. 2003;41(12):1644–1658. doi: 10.1016/s0028-3932(03)00108-8. [DOI] [PubMed] [Google Scholar]
- Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain and Cognition. 1996;31(2):166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57(2):216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Boeve BF, Duda JE, Kaufer D, Leverenz JB, Lippa CF, et al. Current Issues in Lewy Body Dementia Diagnosis. Treatment and Research. 2008:1–14. [Google Scholar]
- Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, et al. Prospective study of dietary pattern and risk of Parkinson disease. American Journal of Clinical Nutrition. 2007;86(5):1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard P, Maloney LM, Hodges JR, Patterson K. The effects of very early Alzheimer’s disease on the characteristics of writing by a renowned author. Brain. 2005;128(Pt 2):250–260. doi: 10.1093/brain/awh341. [DOI] [PubMed] [Google Scholar]
- Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Movement Disorders. 2005;20(Suppl 12):11–20. doi: 10.1002/mds.20535. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Luthert PJ, Janota I, Lantos PL. Cortical Lewy body dementia: clinical features and classification. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52(2):185–192. doi: 10.1136/jnnp.52.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, et al. Clinicopathological Validation Study of Four Sets of Clinical Criteria for Vascular Dementia. American Journal of Psychiatry. 2002;159(1):82–87. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- Goldman JS, Farmer JM, Van Deerlin VM, Wilhelmsen KC, Miller BL, Grossman M. Frontotemporal dementia: genetics and genetic counseling dilemmas. Neurologist. 2004;10(5):227–234. doi: 10.1097/01.nrl.0000138735.48533.26. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Ogar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006;67(10):1849–1851. doi: 10.1212/01.wnl.0000237038.55627.5b. [DOI] [PubMed] [Google Scholar]
- Grossman M. Frontotemporal dementia: A review. Journal of the International Neuropsychological Society. 2002;8(4):566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, D’Esposito M, Hughes E, Onishi K, Biassou N, White-Devine T, et al. Language comprehension profiles in Alzheimer’s disease, multi-infarct dementia, and frontotemporal degeneration. Neurology. 1996;47(1):183–189. doi: 10.1212/wnl.47.1.183. [DOI] [PubMed] [Google Scholar]
- Grossman M, Kalmanson J, Bernhardt N, Morris J, Stern MB, Hurtig H. Cognitive resource limitations during sentence comprehension in Parkinson’s disease. Brain and Language. 2000;73(1):1–16. doi: 10.1006/brln.2000.2290. [DOI] [PubMed] [Google Scholar]
- Grossman M, Lee C, Morris J, Stern MB, Hurtig HI. Assessing resource demands during sentence processing in Parkinson’s disease. Brain and Language. 2002;80(3):603–616. doi: 10.1006/brln.2001.2630. [DOI] [PubMed] [Google Scholar]
- Grossman M, Libon DJ, Forman MS, Massimo L, Wood E, Moore P, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Archives of Neurology. 2007;64(11):1601–1609. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E. Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, et al. Cerebral blood flow in dementia. Archives of Neurology. 1975;32(9):632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- Halpern CH, Glosser G, Clark R, Gee J, Moore P, Dennis K, et al. Dissociation of numbers and objects in corticobasal degeneration and semantic dementia. Neurology. 2004;62:1163–1169. doi: 10.1212/01.wnl.0000118209.95423.96. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22(6):729–737. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2004;62(9):1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- Hebert R, Brayne C. Epidemiology of vascular dementia. Neuroepidemiology. 1995;14(5):240–257. doi: 10.1159/000109800. [DOI] [PubMed] [Google Scholar]
- Heidebrink JL. Is dementia with Lewy bodies the second most common cause of dementia? Journal of Geriatric Psychiatry and Neurology. 2002;15(4):182–187. doi: 10.1177/089198870201500402. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. Journal of the International Neuropsychological Society. 2004;10(4):608–622. doi: 10.1017/S1355617704104141. [DOI] [PubMed] [Google Scholar]
- Heutink P. Untangling tau-related dementia. Human Molecular Genetics. 2000;9(6):979–986. doi: 10.1093/hmg/9.6.979. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Sangjin O, Ken L. Deterioration of naming nouns versus verbs in Primary Progressive Aphasia. Annals of Neurology. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience. 2002;14(7):1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hochstadt J, Nakano H, Lieberman P, Friedman J. The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain and Language. 2006;97(3):243–257. doi: 10.1016/j.bandl.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61(3):349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic Dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129(8):2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA, Jones R, Bateman D, Patterson K. Surface dyslexia in semantic dementia: a comparison of the influence of consistency and regularity. Neurocase. 2004;10(4):290–299. doi: 10.1080/13554790490507623. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. The Natural History of Late-stage “Pure” Semantic Dementia. Neurocase. 2006;12(1):1–14. doi: 10.1080/13554790500428445. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46(2):649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathologica. 2008;114:427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Wenning GK, Seppi K. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegenerative Diseases. 2007;4(6):428–430. doi: 10.1159/000107703. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Archives of Neurology. 2005;62(6):925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Jones S, Laukka EJ, Backman L. Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia. Cortex. 2006;42(3):347–355. doi: 10.1016/s0010-9452(08)70361-7. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Cross-national comparisons of the occurrence of Alzheimer’s and vascular dementias. European Archives of Psychiatry and Clinical Neuroscience. 1991;240(4–5):218–222. doi: 10.1007/BF02189530. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73(18):1443–1450. doi: 10.1212/WNL.0b013e3181bf9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of Neurology. 2006;59(1):156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Schipper HI, Preobrazhenskaya IS, Kessler KR, Yakhno NN. Differences in age at onset and familial aggregation between clinical types of idiopathic Parkinson’s disease. Mov Disord. 2004;19(9):1059–1064. doi: 10.1002/mds.20061. [DOI] [PubMed] [Google Scholar]
- Korczyn AD, Reichmann H. Dementia with Lewy bodies. Journal of Neurological Sciences. 2006;248(1–2):3–8. doi: 10.1016/j.jns.2006.05.055. [DOI] [PubMed] [Google Scholar]
- Kwok S, Reilly J, Grossman M. Acoustic-phonetic processing in semantic dementia. Brain and Language. 2006;99:145–146. [Google Scholar]
- Lai BC, Marion SA, Teschke K, Tsui JK. Occupational and environmental risk factors for Parkinson’s disease. Parkinsonism Related Disorders. 2002;8(5):297–309. doi: 10.1016/s1353-8020(01)00054-2. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K. Generalization and differentiation in semantic memory: insights from semantic dementia. Annals of the New York Academy of Science. 2008;1124:61–76. doi: 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- LaPointe LL. Aphasia and related neurogenic language disorders. 3. New York, NY US: Thieme New York; 2005. [Google Scholar]
- Levy G, Tang MX, Cote LJ, Louis ED, Alfaro B, Mejia H, et al. Motor impairment in PD: relationship to incident dementia and age. Neurology. 2000;55(4):539–544. doi: 10.1212/wnl.55.4.539. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Sachdev PS. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology. 1999;53(4):670–678. doi: 10.1212/wnl.53.4.670. [DOI] [PubMed] [Google Scholar]
- Louis ED, Frucht SJ. Prevalence of essential tremor in patients with Parkinson’s disease vs. Parkinson-plus syndromes. Movement Disorders. 2007;22(10):1402–1407. doi: 10.1002/mds.21383. [DOI] [PubMed] [Google Scholar]
- Lukatela K, Malloy P, Jenkins M, Cohen R. The naming deficit in early Alzheimer’s and vascular dementia. Neuropsychology. 1998;12(4):565–572. doi: 10.1037//0894-4105.12.4.565. [DOI] [PubMed] [Google Scholar]
- Mahieux F, Fanelon G, Flahault A, Manifacier MJ, Michelet D, Boller Fo. Neuropsychological prediction of dementia in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(2):178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The Representation of Object Concepts in the Brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Matsuda O, Saito M, Sugishita M. Cognitive deficits of mild dementia: A comparison between dementia of the Alzheimer’s type and vascular dementia. Psychiatry Clin Neurosci. 1998;52(1):87–91. doi: 10.1111/j.1440-1819.1998.tb00978.x. [DOI] [PubMed] [Google Scholar]
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, et al. Dementia with Lewy bodies. Lancet Neurology. 2004;3(1):19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- McKeith IG. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurology Clinics. 2000;18(4):865–902. doi: 10.1016/s0733-8619(05)70230-9. [DOI] [PubMed] [Google Scholar]
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Journal of Alzheimer’s Disease and Associated Disorders. 2006;9(3 Suppl):417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Nica EI, Sengdy P, Kovacevic N, Moscovitch M, Freedman M, et al. Autobiographical memory and patterns of brain atrophy in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience. 2008;20(10):1839–1853. doi: 10.1162/jocn.2008.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Annals of Neurology. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Annals of Neurology. 2001;49(4):425–432. [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia--a language-based dementia. New England Journal of Medicine. 2003a;349(16):1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary Progressive Aphasia: A Language-Based Dementia. New England Journal of Medicine. 2003b;349(16):1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia: a 25-year retrospective. Alzheimer’s Disease and Associated Disorders. 2007;21(4):S8–S11. doi: 10.1097/WAD.0b013e31815bf7e1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132(9):2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL. Frontotemporal dementia and semantic dementia: anatomic variations on the same disease or distinctive entities? Alzheimer’s Disease and Associated Disorders. 2007;21(4):S19–22. doi: 10.1097/WAD.0b013e31815c0f7a. [DOI] [PubMed] [Google Scholar]
- Monetta L, Pell MD. Effects of verbal working memory deficits on metaphor comprehension in patients with Parkinson’s disease. Brain and Language. 2007;101(1):80–89. doi: 10.1016/j.bandl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of Semantic Dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurology. 2005;4(11):771–780. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neef D, Walling AD. Dementia with Lewy bodies: an emerging disease. American Family Physician. 2006;73(7):1223–1229. [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(Pt 11):2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. New England Journal of Medicine. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- Ober BA, Shenaut GK. Well-organized conceptual domains in Alzheimer’s disease. Journal of the International Neuropsychological Society. 1999;5(7):676–684. doi: 10.1017/s1355617799577102. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer’s Disease and Associated Disorders. 2007;21(4):S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Foroud T. Genetics of Parkinson disease. Genet Med. 2007;9(12):801–811. doi: 10.1097/gim.0b013e31815bf97c. [DOI] [PubMed] [Google Scholar]
- Patterson K. The reign of typicality in semantic memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362(1481):813–821. doi: 10.1098/rstb.2007.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Graham NL, Lambon Ralph MA, Hodges JR. Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology. 2006;20(9–11):1018–1034. [Google Scholar]
- Patterson K, Lambon Ralph MA, Jefferies E, Woollams A, Jones R, Hodges JR, et al. “Presemantic” cognition in semantic dementia: six deficits in search of an explanation. Journal of Cognitive Neuroscience. 2006;18(2):169–183. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Cooke A, Moore P, Vesely L, Grossman M. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. Journal of Neurolinguistics. 2007;20(6):482–494. doi: 10.1016/j.jneuroling.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Grossman M. Language processing in frontotemporal dementia: A brief review. Language and Linguistics Compass 2008 [Google Scholar]
- Pell MD, Leonard CL. Processing emotional tone from speech in Parkinson’s disease: A role for the basal ganglia. Cognitive, Affective & Behavioral Neuroscience. 2003;3(4):275–288. doi: 10.3758/cabn.3.4.275. [DOI] [PubMed] [Google Scholar]
- Pell MD, Leonard CL. Facial expression decoding in early Parkinson’s disease. Cognitive Brain Research. 2005;23(2):327–340. doi: 10.1016/j.cogbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson’s disease with and without dementia. Journal of Clinical Experimental Neuropsychology. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: computational principles in quasi-regular domains. Psychological Review. 1996;103(1):56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Cooper-Pye E, Dine C, Hauk O, Nestor PJ, Patterson K. The word processing deficit in semantic dementia: All categories are equal, but some categories are more equal than others. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21339. ahead of print. [DOI] [PubMed] [Google Scholar]
- Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R. Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74(6):720–724. doi: 10.1136/jnnp.74.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Hofmann LA, Shakil A. Parkinson’s disease: diagnosis and treatment. American Family Physician. 2006;74(12):2046–2054. [PubMed] [Google Scholar]
- Rapp B, Caramazza A. On the distinction between deficits of access and deficits of storage: A question of theory. Cognitive Neuropsychology. 1993;10(2):113–141. [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer’s Disease and Associated Disorders. 2007;21(4):S14–s18. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Lipton AM, Leverenz JB, DeCarli C, Jagust WJ, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65(3):397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Reed BR, Eberling JL, Mungas D, Weiner MW, Jagust WJ. Memory failure has different mechanisms in subcortical stroke and Alzheimer’s disease. Annals of Neurology. 2000;48(3):275–284. [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Cross K, Troiani V, Grossman M. Single word semantic judgments in Semantic Dementia: Do phonology and grammatical class count? Aphasiology. 2007;21(6/7/8):558–569. [Google Scholar]
- Reilly J, Martin N, Grossman M. Verbal learning in semantic dementia: Is repetition priming a useful strategy? Aphasiology. 2005;19:329–339. [Google Scholar]
- Reilly J, Peelle JE. Effects of semantic impairment on language processing in semantic dementia. Seminars in Speech and Language. 2008;29:32–43. doi: 10.1055/s-2008-1061623. [DOI] [PubMed] [Google Scholar]
- Reisberg B. Diagnostic criteria in dementia: a comparison of current criteria, research challenges, and implications for DSM-V. Journal of Geriatric Psychiatry and Neurology. 2006;19(3):137–146. doi: 10.1177/0891988706291083. [DOI] [PubMed] [Google Scholar]
- Riedel O, Klotsche J, Spottke A, Deuschl G, Forstl H, Henn F, et al. Cognitive impairment in 873 patients with idiopathic Parkinson’s disease: Results from the German Study on Epidemiology of Parkinson’s Disease with Dementia (GEPAD) Journal of Neurology. 2008;255:255–264. doi: 10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Kokmen E. Frequency and distribution of vascular dementia. Alzheimer’s Disease and Associated Disorders. 1999;13(Suppl 3):S9–14. [PubMed] [Google Scholar]
- Rockwood K. Lessons from mixed dementia. International Psychogeriatrics. 1997;9(3):245–249. doi: 10.1017/s1041610297004407. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Bowler J, Erkinjuntti T, Hachinski V, Wallin A. Subtypes of vascular dementia. Alzheimer’s Disease and Associated Disorders. 1999;13(Suppl 3):S59–65. [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer’s disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology. 2006;20(3):319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Hodges JR, Patterson K. Natural selection: The impact of semantic impairment on lexical and object decision. Cognitive Neuropsychology. 2004;21(2–4):331–352. doi: 10.1080/02643290342000366. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Semantic cognition: A parallel distributed processing approach. Cambridge, MA US: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N, Chan AS. The deterioration of semantic memory in Alzheimer’s disease. Canadian Journal of Experimental Psychology. 1999;53(1):108–116. doi: 10.1037/h0087303. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Hanakawa T, Fukuyama H, Shibasaki H. Cognitive slowing in Parkinson’s disease: a behavioral evaluation independent of motor slowing. Journal of Neuroscience. 2002;22(12):5198–5203. doi: 10.1523/JNEUROSCI.22-12-05198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Warrington EK, McCarthy RA. Reading without semantics. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1983;35A(1):111–138. [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. Journal of the International Neuropsychological Society. 2009;15(5):645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cerebral Cortex. 2010;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2:167–182. [Google Scholar]
- Snowden JS, Neary D. Progressive language dysfunction and lobar atrophy. Dementia. 1993;4(3–4):226–231. doi: 10.1159/000107327. [DOI] [PubMed] [Google Scholar]
- Theuns J, Van Broeckhoven C. alpha-Synuclein gene duplications in sporadic Parkinson disease. Neurology. 2008;70(1):7–9. doi: 10.1212/01.wnl.0000295508.10258.6b. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam MM. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11(4–5):297–331. [Google Scholar]
- Tierney MC, Black SE, Szalai JP, Snow WG, Fisher RH, Nadon G, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Archives of Neurology. 2001;58(10):1654–1659. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- Traykov L, Rigaud AS, Caputo L, Couderc R, Coste J, Michot JL, et al. Apolipoprotein E phenotypes in demented and cognitively impaired patients with and without cerebrovascular disease. European Journal of Neurology. 1999;6(4):415–421. doi: 10.1046/j.1468-1331.1999.640415.x. [DOI] [PubMed] [Google Scholar]
- von Campenhausen S, Bornschein B, Wick R, Bötzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson’s disease in Europe. European Neuropsychopharmacology. 2005;15(4):473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Walker Z, Allen RL, Shergill S, Mullan E, Katona CL. Three years survival in patients with a clinical diagnosis of dementia with Lewy bodies. International Journal of Geriatric Psychiatry. 2000;15(3):267–273. doi: 10.1002/(sici)1099-1166(200003)15:3<267::aid-gps107>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27(4):635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Concrete word dyslexia. British Journal of Psychology. 1981;72(2):175–196. doi: 10.1111/j.2044-8295.1981.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Semantic access dyslexia. Brain. 1979;102:42–63. doi: 10.1093/brain/102.1.43. [DOI] [PubMed] [Google Scholar]
- Weisman D, McKeith I. Dementia with Lewy bodies. Seminars in Neurology. 2007;27(1):42–47. doi: 10.1055/s-2006-956754. [DOI] [PubMed] [Google Scholar]
- Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN) Stroke. 1996;27(1):30–36. doi: 10.1161/01.str.27.1.30. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Anderson VM, Scahill RI, Rossor MN, Fox NC. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders. 2004;17(4):307–310. doi: 10.1159/000077160. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Brambati SM, Henry RG, Handwerker DA, Agosta F, Miller BL, et al. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132(Pt 1):71–86. doi: 10.1093/brain/awn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams AM, Ralph MA, Plaut DC, Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychological Review. 2007;114(2):316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases. Geneva, Switzerland: 1992. [Google Scholar]
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age and Ageing. 2005;34(6):561–566. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]



