Abstract
To characterize the humoral response to the unglycosylated central region of the respiratory syncytial virus (RSV) attachment (G) protein, we generated glutathione S-transferase (GST)-RSV G subdomains (central core (CC), residues 151–190; proximal central core (PCC), 151–172, and distal central core (DCC), 173–190) to screen paired sera from RSV subtype A- or B-infected adults in hospitalized or outpatient settings. Following RSV infection, a ≥ 4-fold increase in homo- and heterosubtypic IgG response was noted in most subjects against the RSV G CC and PCC regions; in contrast, such titer increases against the RSV G DCC was only noted in a homosubtypic manner. Our results have implications for RSV G-based serological diagnostics and vaccine development.
Keywords: respiratory syncytial virus, attachment protein, humoral response
1. Introduction
Respiratory syncytial virus (RSV) is a significant cause of wintertime respiratory tract infections among patients of all ages [1,2]. Following RSV infection, one of the key targets of adaptive host immune response is the RSV attachment (G) protein that bears a genetically variable, extensively glycosylated ectodomain [3]. Previous serological studies of anti-RSV G antibody response have primarily utilized purified, full-length G protein, recombinant G protein fragments, or a series of overlapping G-derived peptides as screening antigens for immunoglobulin G (IgG) titer measurements in pediatric primary RSV infections[4–12]. RSV G-specific antibodies in adults, including G domain- and homo/heterosubtypic-specific immune responses, remain largely uncharacterized.
A structural hallmark of the RSV G protein is its central unglycosylated domain that is typically comprised of residues 151–190 [3]. Within these contiguous amino acids (aa), residues 151–172 (termed the proximal conserved core; PCC) bears overlapping epitopes for two partially neutralizing, RSV subtype-independent monoclonal antibodies (MAbs) K6 and L9 [13]. Within and carboxy-terminal to the PCC, residues 164–176 are universally conserved among all clinical isolates [3]. Cysteines at positions 173, 176, 182, and 186 are involved in two disulfide bonds that comprise a “loop” structure. Residues 182–186 comprise a CX3C chemokine (fractalkine) structural motif that contributes to the immunomodulatory functions of the RSV G protein[14]. Thus, humoral response against the unglycosylated region of RSV G may be critically important to RSV immunity with implications for vaccine development.
We have recently shown that the RSV G PCC subdomain is the target of a ≥ 4-fold increase in IgG response between acute- and convalescent-phase sera in ~40% of adults infected with subtype A RSV [13]; the ≥ 4-fold increase in IgG titers against RSV-encoded glycoproteins is the historical standard by which serological evidence of RSV infections has been determined[15,16]. To extend these results, we analyzed the serum reactogenicity against various bacterially derived RSV G subdomains using paired acute and convalescent sera from RSV A and B infected adult human subjects.
2. Materials and Methods
Mammalian cells and RSV isolates
HEp-2 cells and RSV A2 and B1 strains were obtained from the American Type Culture Collection (ATCC). HEp-2 cells were maintained in Modified Eagle’s Media (MEM) + 5% fetal calf serum + penicillin-streptomycin (Invitrogen).
DNA constructions and manipulations
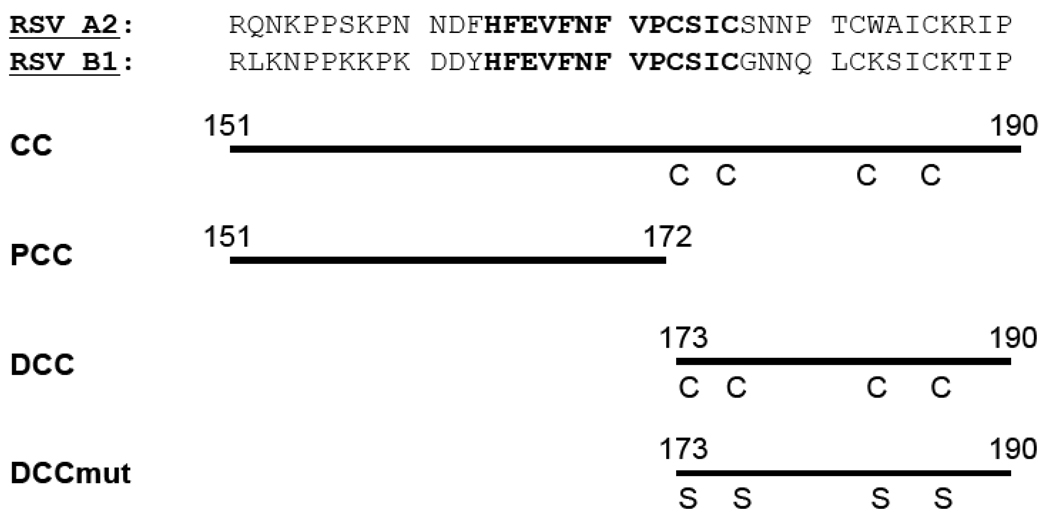
The RSV A2 and B1 strain G cDNAs (GI accession 138294 and 9629205, respectively) were codon-optimized and used for plasmid constructions. For glutathione S-transferase (GST) - RSV G 151–190 (numbers refer to the residue positions within the G protein), polymerase chain reaction (PCR) was used to amplify the cognate cDNA fragments from each of the codon-optimized G cDNAs. The resulting amplicons were ligated into the pGEX4T-1 BamHI site that generates the linker aa sequence …GS…between GST and the RSV G moieties. For plasmid constructions encoding shorter fragments of RSV G protein (residues 151–172, 173–190, or 173–190mut in which serines were substituted for each of the four cysteines at positions 173, 176, 182, and 186; Figure 1), complementary oligonucleotide pairs encoding the relevant RSV A2- or B1-derived G aa residues followed by a termination codon were phosphorylated, annealed, and ligated into the BamHI-EcoRI sites of pGEX4T-1. For plasmids encoding the GST-G 173–190 and 173–190mut, a slightly longer linker sequence (…GSGSG… with the underlined residues were derived from the BamHI site) was engineered between the GST and the RSV G moieties so as to reduce potential steric interactions. The integrity of the RSV G cDNA-derived sequences was verified by sequencing (Eurofins MWG Operon).
Figure 1.
The central unglycosylated region of the RSV G protein. Upper panel: Residues 151–190 of the RSV G protein from A2 and B1 strains. The invariantly conserved amino acids 164–176 are shown in bold. Lower panel: Schematic diagram of RSV G moieties that were bacterially expressed and purified as glutathione S-transferase fusion proteins. Labels to the left indicate the G conserved core (CC; residues 151–190), proximal conserved core (PCC; residues 151–172), distal conserved core (DCC; residues 173–190) subdomains as well as the DCCmut (residues 173–190 in which the four cysteines were altered to serines). Where relevant, the residue positions are indicated by numbers above the aligned amino acids and the positions of cysteine (C) or cysteine → serine substitutions (S) are also shown.
Bacterial manipulations and protein purification
Competent E. coli (DH5α strain) were transformed with each of the pGEX4T-1 derivatives and propagated in LB or 2XYT broth with carbenicillin. The recombinant GST-fusion proteins were expressed using 0.1–0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG; Invitrogen) at 37°C for 3–4 hrs and purified using glutathione-agarose beads (Sigma-Aldrich) according to the manufacturer’s instructions (Amersham).
For Coomassie staining of proteins, bacterially derived proteins (~0.5–1 µg) were diluted 1:1 (vol:vol) with 2X sodium dodecyl sulfate (SDS)-sample buffer containing β-mercaptoethanol, denatured at 95°C for 2–5 minutes, and then resolved on 12%/6% discontinuous SDS-polyacrylamide gel electrophoresis (PAGE). The resolved proteins were visualized by staining with Coomassie Blue R-250.
Collection and immunological analysis of human sera
Patient identifier-unlinked acute and convalescent paired sera were previously collected during an institutional review board-approved epidemiological study of RSV among elderly and hospitalized adults [2]. For this study, we identified a subset of archived, paired sera from RSV A or B-infected adults whose nasopharyingeal secretions were positive by reverse transcriptase-polymerase chain reaction (RT-PCR) for RSV and in whom there was a ≥ four-fold increase from acute to convalescent serum immunoglobulin G (IgG) titers (in reciprocal log2 dilutions) to purified, subtype-specific RSV G glycoprotein as measured in ELISAs. Such RSV diagnoses were made while the subjects were in hospitalized (subtype A-infected: n = 16; subtype B infected: n = 24) or outpatient (subtype A: n=16; subtype B: n=8) settings.
Patient demographics
For the non-hospitalized cohort, the median (range) age for subtype A and B infected outpatients were 79 (55–96) and 70 (59–83), respectively; the difference in the median age between the two groups was not statistically significant. The status of underlying medical conditions were as follows: no underlying cardiopulmonary diagnoses: n=10 and n=0 (subtype A- and B-infected, respectively); subjects with cardiac condition: n=1 and n=2; underlying pulmonary condition: n=4 and n=6; and nursing home resident: n=1 and n=0.
For the hospitalized cohort, the median (range) age for subtype A and B infected inpatients were 74 (55–96) and 78 (47–98), respectively; the difference in the median age between the two groups was not statistically different. Admission (ICD-9) diagnoses for the inpatient cohort were as follows: Subtype A: chronic obstructive pulmonary disease (COPD) exacerbation: 8; pneumonia: 6; myocardial infarction: 1; and respiratory arrest: 1; Subtype B: COPD exacerbation: 11; pneumonia: 4; asthma: 3; congestive heart failure: 2; and 1 each of myocardial infarction, respiratory arrest, and hypoglycemia.
Enzyme-linked immunosorbent assays (ELISAs)
Aliquots of each sera sample were tested in ELISAs that were performed essentially as previously described for reactogenicity against GST alone or each of the GST-G derivatives [17]. Typically, GST or each of the GST-RSV G derivatives was diluted in carbonate buffer pH 9.0 and plated at 100 ng/well onto 96 well ELISA plates (Nunc) followed by overnight incubation at 4°C. Following blocking of non-specific binding with PBS/0.5% Tween-20/1mM ethylenediamine tetra-acetic acid (EDTA)/0.5% gelatin, the plate-bound antigens were incubated with serial two-fold dilutions of human serum and then with alkaline phosphatase-conjugated goat anti-human secondary antibodies (Southern Biotech). Phosphatase substrate (p-nitrophenyl phosphate 104; Sigma-Aldrich) dissolved in diethanolamine buffer was then used to detect antigen-antibody complexes. The resulting colorimetric reactions were read at OD405nm using a 96-well enzyme linked immunosorbent assay (ELISA) plate reader (Molecular Devices). For each GST-G fusion protein, the OD 405nm generated by serum reactogenicity against GST alone was subtracted from that elicited by the GST-G protein. The resulting OD405nm [GST-G – GST alone] readings vs. serum dilutions were plotted using Excel 2003 (Microsoft) and used to calculate the end point serum titers (expressed as mean ± standard deviation reciprocal log2 dilutions) as previously described [17].
Graphical/statistical analyses
Statistical manipulations were performed using JMP version 8.0 (SAS, Cary, NC). For univariate analyses, means were compared with Wilcoxon rank-sum tests and proportions were compared using two-tailed Fisher’s exact tests.
3. Results
To examine the humoral response against the RSV G unglycosylated region, we first generated a series of GST fusion proteins, each bearing a portion of this domain from the G protein of the prototypical RSV A2 (subtype A) and B1 (subtype B) strains. For each of the two RSV subtype-derived G proteins, we generated four GST-G derivatives: 1) those bearing the entire conserved core (CC; residues 151–190); 2) those bearing the proximal central core (PCC; residues 151–172); 3) those bearing the distal conserved core (DCC; residues 173–190, including the four cysteines); and 4) those bearing cysteine → serine substitutions at each of the four cysteines within the DCC (DCCmut; Figure 1). Each of the GST-G fusion proteins were affinity purified to >95% homogeneity (data not shown).
Using these recombinant proteins as screening reagents, we assayed the serum reactogenicity to various portions of the RSV G central unglycosylated region in ELISAs using paired acute and convalescent sera from RSV-infected adults. Subjects were classified by the infection-associated RSV subtype A or B (N = 32 for each group) and were further categorized into one of two subgroups (hospitalized/inpatient vs. outpatient) based on the initial location of patient screening. In all paired sera, there was a ≥ four-fold increase in the anti-RSV Ga or Gb IgG titers from acute to convalescent sera.
Among RSV A-infected adults, we observed a relatively high degree of acute and convalescent serum reactogenicity against the entire RSV A2-encoded CC domain (mean ± SD log2 reciprocal dilution: 10.2 ± 2.3 → 13.8 ± 2.0) and to the PCC (8.8 ± 1.7 → 10.8 ± 2.0) and DCC subdomains (10.3 ± 1.9 → 14.0 ± 2.8; Table 1). In all three cases, such homosubtypic-specific titer increase was statistically significant (p < 0.05) and the proportion of paired sera that bore ≥ four-fold increase in IgG titers against the A2-encoded RSV G subdomains was ≥ 50% (Table 1). In parallel, we also observed statistically significant titer increases against the RSV B1-derived PCC and CC subdomains (Table 1). However, there was no significant change between the acute and convalescent IgG response against GST-RSV B1 G DCC subdomain (10.0 ± 2.4 → 10.7 ± 2.8; p = 0.28) and the proportion of subjects with a ≥ four-fold titer increase between paired sera was only 16% (n = 5). In all sera, we noted no detectable serum reactogenicity against GST-A2 or –B1 DCCmut in which the four RSV A2 G-encoded cysteines were substituted by serines (data not shown).
Table 1.
RSV subtype A-infected adults: acute and convalescent serum reactogenicity against GST-G derivatives.
| RSV Subtype A infected (N=32) |
GST-RSV A2 G | GST-RSV B1 G | ||||
|---|---|---|---|---|---|---|
| PCC | DCC | CC | PCC | DCC | CC | |
| Acute | 8.8±1.7 | 10.3±1.9 | 10.2±2.3 | 10.6±2.2 | 10.0±2.4 | 12.1±1.8 |
| Convalescent | 10.8±2.0 | 14.0±2.8 | 13.8±2.0 | 12.0±1.9 | 10.7±2.8 | 14.8±1.3 |
| p value | <0.001 | <0.001 | <0.001 | 0.013 | 0.28 | <0.001 |
| Number (%) with ≥ 4-fold titer increase |
16 (50%) |
23 (72%) |
20 (63%) |
12 (38%) |
5 (16%) |
18 (56%) |
| Change in convalescent to acute titers |
2.0±1.8 | 3.7±2.5 | 3.6±2.8 | 1.3±1.5 | 0.8±1.5 | 2.7±2.3 |
Results are expressed as mean ± SD reciprocal log2 titers.
Where relevant, p values were calculated using the Wilcoxon sign-rank test.
We also performed similar analyses with paired sera from RSV B-infected adults (Table 2). Against RSV subtype B-derived G residues, we observed robust acute and convalescent homosubtypic serum reactogenicity against the RSV B1 CC, PCC and the DCC; in all three cases, such increase was statistically significant (p < 0.05) and the proportion of paired sera that bore ≥ four-fold increase in IgG titers against the B1-derived subdomains was ≥ 56% (Table 2). Against RSV A2- derived residues, we observed heterosubtypic titer increases of 10.0 ± 1.8 → 12.2 ± 2.3 and 8.3 ± 1.7 → 10.2 ± 2.8 for the A2 CC and PCC, respectively (Table 2). However, we noted minimal change in paired serum reactogenicity against the A2-derived DCC and only one (3%) of subjects demonstrated a ≥ four-fold titer increase between paired sera (Table 2). As in the case of subtype A-infected adults, we noted no detectable serum reactogenicity against GST-A2 or –B1 DCCmut (data not shown).
Table 2.
RSV subtype B-infected adults: acute and convalescent serum reactogenicity against GST-G derivatives.
| RSV Subtype B infected (N=32) |
GST-RSV A2 G | GST-RSV B1 G | ||||
|---|---|---|---|---|---|---|
| PCC | DCC | CC | PCC | DCC | CC | |
| Acute | 8.3±1.7 | 10.6±1.9 | 10.0±1.8 | 10.5±2.2 | 9.0±1.2 | 11.2±2.0 |
| Convalescent | 10.2±2.8 | 10.5±1.8 | 12.2±2.3 | 12.4±2.3 | 11.5±2.7 | 14.5±2.3 |
| p value | 0.006 | 0.69 | <0.001 | 0.002 | <0.001 | <0.001 |
| Number (%) with ≥ 4-fold titer increase |
16 (50%) |
1 (3%) |
19 (59%) |
19 (59%) |
18 (56%) |
27 (84%) |
| Change in convalescent to acute titers |
1.9±2.7 | −0.6±0.9 | 2.2±2.0 | 1.9±2.8 | 2.5±2.4 | 3.3±2.4 |
Results are expressed as mean ± SD reciprocal log2 titers.
Where relevant, p values were calculated using the Wilcoxon sign-rank test.
Taken together, these data suggest that for RSV infected adults, there is a statistically significant acute to convalescent titer increase against the homo- and heterosubtypic RSV G CC and PCC. However, such titer increases specific to the RSV G DCC are homosubtype-specific. The serine substitutions of the four DCC-embedded cysteines abolishes the serum recognition of the RSV G DCC subdomain.
We performed a subgroup analysis to determine the correlation between the clinical setting in which the patients were identified (inpatient vs. outpatient) and the titers and the proportion of patient sera that reacted against the RSV G-derived residues. For RSV subtype A-infected adults, there were no statistically significant differences between the mean acute titers of outpatient vs. hospitalized groups for any of the A2- or B1-derived G subdomains (Table 3). However, in convalescent sera, the mean titers were greater and in a statistically significant manner (p < 0.05) in the inpatient population than in the outpatient cohort for IgG response against the A2 PCC and DCC subdomains but not against the A2 CC and all three B1-derived subdomains (Table 3). For RSV B-infected adults, the acute titers were higher in the outpatient group (n = 8) as compared to the hospitalized cohort (n = 24) against both RSV A2- and B1-derived PCC residues (Table 4). For convalescent serological response among subtype B-infected patients, the inpatient group bore higher titers than the outpatient cohort with respect to the A2 and B1 CC domains (Table 4); such increase was statistically significant for anti-B1 PCC response (p < 0.05) and the anti-A2 PCC response followed a similar trend (p = 0.054). These data suggest that: 1) the serum reactogenicity to the RSV G PCC may correlate to severity of disease at the time of diagnosis for RSV subtype B infection among adults; and 2) convalescent titers against portions of the RSV G central region are higher in hospitalized than in outpatient subjects.
Table 3.
RSV subtype A infected adults: subgroup analysis of anti-GST G serological reactogenicity among inpatient vs. outpatient subjects.
| RSV Subtype A infected (N=32) |
GST-RSV A2 G | GST-RSV B1 G | |||||
|---|---|---|---|---|---|---|---|
| PCC | DCC | CC | PCC | DCC | CC | ||
| Acute | Outpatient | 8.7±1.6 | 10.5±1.8 | 9.9±2.5 | 10.7±2.2 | 10.7±2.5 | 12.7±1.2 |
| Inpatient | 8.9±1.9 | 10.5±2.1 | 10.5±2.1 | 10.6±2.2 | 9.2±2.1 | 11.5±2.1 | |
| p value | 0.86 | 0.40 | 0.32 | 0.94 | 0.07 | 0.06 | |
| Convalescent | Outpatient | 9.9±1.6 | 13.0±2.9 | 13.2±2.1 | 11.7±1.8 | 11.5±2.8 | 14.7±1.5 |
| Inpatient | 11.7±2.0 | 15.0±2.2 | 14.5±1.8 | 12.3±2.1 | 10.0±2.6 | 15.0±1.2 | |
| p value | 0.0056 | 0.046 | 0.08 | 0.39 | 0.21 | 0.48 | |
| Number (%) with ≥ 4-fold titer increase |
Outpatient (n=16) |
4 (25%) |
10 (63%) |
10 (63%) |
4 (25%) |
2 (13%) |
6 (38%) |
| Inpatient (n=16) |
12 (75%) |
13 (81%) |
12 (75%) |
8 (50%) |
3 (19%) |
12 (75%) |
|
Results are expressed as mean ± SD reciprocal log2 titers.
Where relevant, p values were calculated using the Wilcoxon sign-rank test.
Table 4.
RSV subtype B infected adults: subgroup analysis of anti-GST G serological reactogenicity among inpatient vs. outpatient subjects.
| RSV Subtype B infected (N=32) |
GST-RSV A2 G | GST-RSV B1 G | |||||
|---|---|---|---|---|---|---|---|
| PCC | DCC | CC | PCC | DCC | CC | ||
| Acute | Outpatient | 9.3±1.5 | 10.1±2.0 | 9.5±2.1 | 12.5±2.6 | 9.6±2.3 | 10.6±2.3 |
| Inpatient | 8.0±1.7 | 10.8±1.8 | 10.2±1.7 | 9.8±1.7 | 8.7±2.2 | 11.3±1.9 | |
| p value | 0.054 | 0.29 | 0.37 | 0.011 | 0.23 | 0.25 | |
| Convalescent | Outpatient | 9.2±1.0 | 9.7±1.5 | 10.8±1.6 | 11.8±2.1 | 10.1±2.4 | 12.3±2.8 |
| Inpatient | 10.6±0.6 | 10.8±1.8 | 12.7±2.2 | 12.6±2.4 | 12.0±2.7 | 15.2±1.5 | |
| p value | 0.31 | 0.11 | 0.013 | 0.59 | 0.084 | 0.0026 | |
| Number (%) with ≥ 4-fold titer increase |
Outpatient (n=8) |
1 (13%) |
1 (13%) |
3 (38%) |
1 (13%) |
1 (13%) |
5 (63%) |
| Inpatient (n=24) |
15 (63%) |
0 (0%) |
16 (67%) |
18 (75%) |
17 (71%) |
22 (92%) |
|
Results are expressed as mean ± SD reciprocal log2 titers.
Where relevant, p values were calculated using the Wilcoxon sign-rank test.
4. Discussion
To study the humoral response against the central unglycosylated region of the RSV G protein, we screened sera from RSV-infected adults for reactogenicity against recombinant GST-RSV G fusion proteins. Our approach utilized rational partitioning of the RSV G unglycosylated central residues into the PCC, DCC, and the entire CC region; in contrast, previous reports have utilized overlapping peptides or bacterially derived hypervariable G domains with or without the unglycosylated core as screening reagents to determine RSV G domain-specific serological responses [4–11].
For each of the six RSV G derivatives (PCC, DCC, and CC encoded by RSV A2 and B1 strains), we observed robust IgG titers in acute sera and in a homo- and heterosubtypic-specific manner. This is not unexpected since adults in this study would have experienced a number of RSV subtype A and B infections during their lifetimes [2]. In convalescent sera, we noted statistically significant titer increases against the homosubtypic and heterosubtypic RSV G PCC and CC domains. The subtype-independent reactogenicity against the PCC and CC regions may primarily be due to the canonical aa sequence HFEVFNFVPCSIC (residues 164–176; Figure 1) that overlaps most of the PCC residues and is embedded within the CC [3,13]. Our observations are also consistent with the fact that anti-RSV G MAbs with cognate epitopes that overlap residues 164–176 typically recognize RSV G in a subtype-independent manner [13,18].
Interestingly, convalescent sera from subtype A-infected adults responded weakly to B1-derived DCC and subtype B-infected adults responded minimally to A2-derived DCC, i.e. there is homosubtype-specific serum reactogenicity against the RSV G DCC subdomain. It has previously been reported that peptides bearing RSV A2 or B1 strain-derived G residues 158–189 were recognized in a subtype-specific manner in paired sera from pediatric patients with primary RSV infection [7,8]. Our results with adult convalescent sera confirm and extend these results by defining the potential residues that may be responsible for such subtype-specific targets of immune response to those within the DCC.
Between the RSV A2- and B1-derived DCC, we note the following six residue differences: A2: CSICSNNPTCWAICKRIP vs. B1: CSICGNNQLCKSICKTIP, in which differences at positions 177, 180, 181, 183, 184, and 188 are underlined and in bold. Based on comparative analysis of reference RSV G sequences, the RSV A2 and B1 DCC are likely to be representative of subtype A and B-specific residues 173–190 of the RSV G protein ([19–23]Murata, et al., unpublished observations). The existence of subtype-specific differences within the DCC subdomain may be evolutionarily conserved since bovine RSV (BRSV) G residues 180, 182, and 184 of strain 391-2 (subtype A) (CSTCEGNLACLSLCHIET) differ from those of BOV-X (subtype B) (CSTCEGNPACSPLCQIGL; differences underlined and in bold) [7]. We conclude that ≥ 1 of the six residue differences between subtype A and B viral strains confers homosubtype specificity of acute to convalescent serum anti-RSV G DCC reactogenicity increases in adult RSV infections. Furthermore, our analysis of corresponding BRSV DCC residues implies the existence of similar DCC homosubtype-specific humoral response in BRSV infections.
Our results are relevant to the structural and functional aspects of the RSV G DCC region, which is not essential for RSV replication in vitro or in vivo but elicits robust immunomodulatory effects, e.g. CX3C motif-mediated cellular trafficking [14,24]. Our results suggest that the presence of cysteines is required for the antigenic/structural integrity of DCC-embedded epitopes since the substitution of all four cysteine residues with serine rendered the resulting DCCmut moiety non-reactogenic. Particularly notable is our observation that for both RSV and BRSV, the subtype-specific residue differences at residues 183 and 184, i.e. between C182 and C186, involve the CX3C motif; for BRSV G protein, mutations of residues 183 and 184 are predicted to have major structural consequences of the antigenic surface of the G “loop” region [25,26]. Taken together, these observations raise the possibility of viral subtype-specific, evolutionarily conserved immunomodulatory effects of the RSV DCC subdomain, including the CX3C homology region.
Our data also have implications for clinical course and severity of disease among RSV-infected adults. For subtype A-infected adults, there were greater and statistically significant increases in A2 PCC- and DCC-specific convalescent titers among hospitalized patients as compared to those of subjects diagnosed with RSV as outpatients; a similar but non-statistically significant (p = 0.08) trend was also observed for A2 CC domain (Table 3). Among subtype B-infected adults, outpatients bore higher anti-B1 PCC titers than did inpatients at the time of diagnosis (i.e. acute sera) and hospitalized adults bore higher anti- A2 and –B1 CC convalescent titers than did outpatients. Thus for RSV-infected adults, hospitalization may be correlated with greater burden of disease, i.e. elevated viral loads, and subsequent IgG increases in convalescent sera titers against RSV G CC and its subdomains [27]. For RSV subtype B-infected adults, elevated acute titers against the RSV G PCC may correlate with severity of illness at diagnosis and may potentially be utilized as serological correlates of disease severity. We acknowledge the limitation that the number of non-hospitalized subtype-B infected adults was relatively small (n=8) and thus the clinical utility of such serological correlates requires additional studies.
Lastly, our results have implications for immunological function of RSV G and potential epitopes for host adaptive humoral response and vaccine development. The RSV G CC domain contains immunologically important structural and functional motifs, including: 1) aa 184–198, thought to be involved in CD4+ T cell-mediated eosinophila; 2) canonically conserved residues + G “loop” that collectively have been associated with broad anti-inflammatory effects; and 3) the presence of a human HLA DP4-restricted CD4+ T-cell epitope (minimum epitope residues defined as either 163–171 or 162–175) [28–31]. Adult and pediatric subjects who were either naturally infected with RSV or received live attenuated RSV vaccine candidate generated IgG response that inhibited RSV G-mediated leukocyte chemotaxis and RSV G-CXC3 receptor interactions [14]. To enhance such RSV G-specific immune response, particularly against potential subtype-specific immunomodulatory effects, it may be necessary to engineer the RSV G “loop” region as DCC or the entire CC domain from both subtype A and B viruses for potential vaccine epitopes. As an alternative immunogen, the RSV G PCC from either subtype A or B virus strain may be sufficient to elicit viral subtype-independent antibody production and protective efficacy. Such possibilities require empirical testing in animal immunogenicity studies.
Acknowledgments
This work was supported by Public Health Service grants from the National Institutes of Allergy and Infectious Diseases (R21 AI076781 to YM and R01 AI045969 to EEW). We thank Patricia Hennessey, RN, for compiling the clinical demographic data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Melero JA, Garcia-Barreno B, Martinez I, Pringle CR, Cane PA. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol. 1997;78(Pt 10):2411–2418. doi: 10.1099/0022-1317-78-10-2411. (Pt 10) [DOI] [PubMed] [Google Scholar]
- 4.Cane PA. Analysis of linear epitopes recognised by the primary human antibody response to a variable region of the attachment (G) protein of respiratory syncytial virus. J Med Virol. 1997;51(4):297–304. doi: 10.1002/(sici)1096-9071(199704)51:4<297::aid-jmv7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Cane PA, Thomas HM, Simpson AF, Evans JE, Hart CA, Pringle CR. Analysis of the human serological immune response to a variable region of the attachment (G) protein of respiratory syncytial virus during primary infection. J Med Virol. 1996;48(3):253–261. doi: 10.1002/(SICI)1096-9071(199603)48:3<253::AID-JMV7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Jones LP, Zheng HQ, Karron RA, Peret TC, Tsou C, Anderson LJ. Multiplex assay for detection of strain-specific antibodies against the two variable regions of the G protein of respiratory syncytial virus. Clin Diagn Lab Immunol. 2002;9(3):633–638. doi: 10.1128/CDLI.9.3.633-638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langedijk JP, Brandenburg AH, Middel WG, Osterhaus A, Meloen RH, van Oirschot JT. A subtype-specific peptide-based enzyme immunoassay for detection of antibodies to the G protein of human respiratory syncytial virus is more sensitive than routine serological tests. J Clin Microbiol. 1997;35(7):1656–1660. doi: 10.1128/jcm.35.7.1656-1660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langedijk JP, Middel WG, Schaaper WM, Meloen RH, Kramps JA, Brandenburg AH, et al. Type-specific serologic diagnosis of respiratory syncytial virus infection, based on a synthetic peptide of the attachment protein G. J Immunol Methods. 1996;193(2):157–166. doi: 10.1016/0022-1759(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 9.Norrby E, Mufson MA, Alexander H, Houghten RA, Lerner RA. Site-directed serology with synthetic peptides representing the large glycoprotein G of respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987;84(18):6572–6576. doi: 10.1073/pnas.84.18.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akerlind-Stopner B, Utter G, Norrby E, Mufson MA. Evaluation of subgroup-specific peptides of the G protein of respiratory syncytial virus for characterization of the immune response. J Med Virol. 1995;47(2):120–125. doi: 10.1002/jmv.1890470203. [DOI] [PubMed] [Google Scholar]
- 11.Palomo C, Cane PA, Melero JA. Evaluation of the antibody specificities of human convalescent-phase sera against the attachment (G) protein of human respiratory syncytial virus: influence of strain variation and carbohydrate side chains. J Med Virol. 2000;60(4):468–474. [PubMed] [Google Scholar]
- 12.Scott PD, Ochola R, Sande C, Ngama M, Okiro EA, Medley GF, et al. Comparison of strain-specific antibody responses during primary and secondary infections with respiratory syncytial virus. J Med Virol. 2007;79(12):1943–1950. doi: 10.1002/jmv.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata Y, Lightfoote PM, Falsey AR, Walsh EE. Identification of and human serum reactogenicity to neutralizing epitopes within the central unglycosylated region of the respiratory syncytial virus attachment protein. Clin Vaccine Immunol. 2010;17(4):695–697. doi: 10.1128/CVI.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harcourt JL, Karron RA, Tripp RA. Anti-G protein antibody responses to respiratory syncytial virus infection or vaccination are associated with inhibition of G protein CX3C-CX3CR1 binding and leukocyte chemotaxis. J Infect Dis. 2004;190(11):1936–1940. doi: 10.1086/425516. [DOI] [PubMed] [Google Scholar]
- 15.Gardner PS, Elderkin FM, Wall AH. Serological Study of Respiratory Syncytial Virus Infections in Infancy and Childhood. Br Med J. 1964;2(5424):1570–1573. doi: 10.1136/bmj.2.5424.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsey AR, Walsh EE. Humoral immunity to respiratory syncytial virus infection in the elderly. J Med Virol. 1992;36(1):39–43. doi: 10.1002/jmv.1890360108. [DOI] [PubMed] [Google Scholar]
- 17.Walsh EE, Falsey AR. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73(2):295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 18.Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol. 2009;183(10):6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- 19.Cane PA, Matthews DA, Pringle CR. Analysis of relatedness of subgroup A respiratory syncytial viruses isolated worldwide. Virus Res. 1992;25(1–2):15–22. doi: 10.1016/0168-1702(92)90096-r. [DOI] [PubMed] [Google Scholar]
- 20.Cane PA, Matthews DA, Pringle CR. Analysis of respiratory syncytial virus strain variation in successive epidemics in one city. J Clin Microbiol. 1994;32(1):1–4. doi: 10.1128/jcm.32.1.1-4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia O, Martin M, Dopazo J, Arbiza J, Frabasile S, Russi J, et al. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J Virol. 1994;68(9):5448–5459. doi: 10.1128/jvi.68.9.5448-5459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlateva KT, Lemey P, Moes E, Vandamme AM, Van Ranst M. Genetic variability and molecular evolution of the human respiratory syncytial virus subgroup B attachment G protein. J Virol. 2005;79(14):9157–9167. doi: 10.1128/JVI.79.14.9157-9167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang ZY, Du LN, Chen X, Zhao Y, Liu EM, Yang XQ, et al. Genetic variability of respiratory syncytial viruses (RSV) prevalent in Southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J Clin Microbiol. 2010;48(4):1201–1207. doi: 10.1128/JCM.02258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2(8):732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 25.Doreleijers JF, Langedijk JP, Hard K, Boelens R, Rullmann JA, Schaaper WM, et al. Solution structure of the immunodominant region of protein G of bovine respiratory syncytial virus. Biochemistry. 1996;35(47):14684–14688. doi: 10.1021/bi9621627. [DOI] [PubMed] [Google Scholar]
- 26.Langedijk JP, Meloen RH, Taylor G, Furze JM, van Oirschot JT. Antigenic structure of the central conserved region of protein G of bovine respiratory syncytial virus. J Virol. 1997;71(5):4055–4061. doi: 10.1128/jvi.71.5.4055-4061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190(2):373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 28.Tebbey PW, Hagen M, Hancock GE. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188(10):1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci U S A. 2005;102(25):8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Graaff PM, Heidema J, Poelen MC, van Dijk ME, Lukens MV, van Gestel SP, et al. HLA-DP4 presents an immunodominant peptide from the RSV G protein to CD4 T cells. Virology. 2004;326(2):220–230. doi: 10.1016/j.virol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 31.de Waal L, Yuksel S, Brandenburg AH, Langedijk JP, Sintnicolaas K, Verjans GM, et al. Identification of a common HLA-DP4-restricted T-cell epitope in the conserved region of the respiratory syncytial virus G protein. J Virol. 2004;78(4):1775–1781. doi: 10.1128/JVI.78.4.1775-1781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]