Abstract
Production of antimicrobial peptides by epithelia is an essential defense against infectious pathogens. In this study we evaluated whether the commensal microorganism Staphylococcus epidermidis may enhance production of antimicrobial peptides by keratinocytes and thus augment skin defense against infection. Exposure of cultured undifferentiated human keratinocytes to a sterile nontoxic small molecule of < 10 kDa from S. epidermidis conditioned culture medium (SECM), but not similar preparations from other bacteria, enhanced human β-defensin 2 (hBD2) and hBD3 mRNA expression and increased the capacity of cell lysates to inhibit the growth of group A Streptococcus (GAS) and S. aureus. Partial gene silencing of hBD3 inhibited this antimicrobial action. This effect was relevant in vivo as administration of SECM to mice decreased susceptibility to infection by GAS. Toll-like receptor 2 (TLR2) was important to this process as a TLR2-neutralizing antibody blocked induction of hBDs 2 and 3, and Tlr2-deficient mice did not show induction of mBD4. Taken together, these findings reveal a potential use for normal commensal bacterium S. epidermidis to activate TLR2 signaling and induce antimicrobial peptide expression, thus enabling the skin to mount an enhanced response to pathogens.
INTRODUCTION
Skin, as the first line of defense against microbial invasion, is exposed to a myriad of microbial organisms, a group that has been collectively referred to as the “microbiome.” The barrier produced by the skin to these organisms consists of the physical barrier presented by the stratum corneum and epidermis, and a chemical barrier composed of antimicrobial lipids, peptides, proteins, and reactive oxygen products produced by keratinocytes and other cells in the skin (Schwarz, 2003; Goodarzi et al., 2007; Meyer et al., 2007). Keratinocytes, comprising the epidermis of the skin, normally use the defensive barrier to effectively prevent pathogen entry or long-term survival on the skin while permitting survival of the diverse microbial ecosystem of the skin microbiome (Dekio et al., 2007; Gao et al., 2007; Fierer et al., 2008; Grice et al., 2008; Krutmann, 2009). The mechanisms responsible for selective detection and response to the microbial ecosystem of the skin are incompletely understood but involve elements such as the Toll-like receptors (TLRs) to enable recognition of microbial invasion (Braff et al., 2005a; Schauber et al., 2007; Lai and Gallo, 2008). The activation of TLR in the cutaneous pathogen recognition system in turn triggers the release of soluble effectors such as the antimicrobial peptides that maintain sterility in the dermis (Schauber et al., 2007).
Antimicrobial peptides of skin are a diverse collection of molecules that serve as important countermeasures to contend with microbial invasion and/or infection (Lai and Gallo, 2009). Cathelicidin and β-defensins are two major antimicrobial peptides in the skin (Braff et al., 2005a). These cationic peptides directly kill a broad spectrum of bacteria, fungi, and viruses. They are present in normal skin at low levels but can be induced at sites of microbial entry to act as an impediment to infection. On the basis of their location at the environmental surface, antimicrobial peptides are thought to participate in maintenance of mutually beneficial host–microbial relationships at epithelial surfaces by restricting growth of resident microbes and opportunistic pathogens (Hooper et al., 2003, 2004; Cash et al., 2006).
Staphylococcus epidermidis is the most commonly isolated bacterial species from healthy human skin and generally has a benign relationship with its host (Evans et al., 1950; Marples, 1969; Otto, 2004). As a normal skin inhabitant, S. epidermidis has been hypothesized to have a beneficial role in human nutrition and health by promoting nutrient supply and preventing pathogen colonization (Bibel et al., 1983). However, the mechanisms through which S. epidermidis could provide this beneficial effect have been incompletely studied. Recent work has revealed that S. epidermidis may benefit the skin in several ways: it can produce its own antimicrobial peptides that inhibit pathogenic organisms (Cogen et al., 2010), and lipoteichoic acid produced by S. epidermidis served to modulate inflammation through a TLR-crosstalk phenomenon between TLR2 and TLR3 (Lai et al., 2009). In this study we sought to determine whether S. epidermidis might also benefit normal skin by stimulating the expression of antimicrobial peptides from keratinocytes. Our findings provide further insight into how this skin resident microbe could act to minimize skin infections and increase innate immune defense.
RESULTS
S. epidermidis induces β-defensins in epidermal keratinocytes
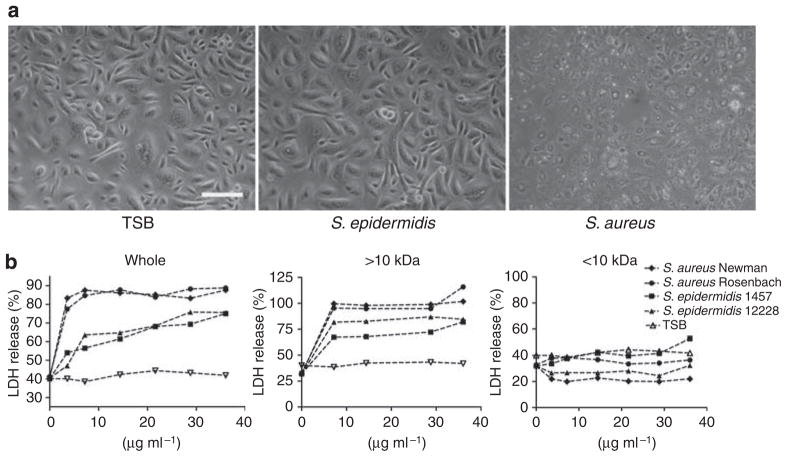
Keratinocytes actively participate in skin immune defense by the release of antimicrobial peptides such as β-defensins and cathelicidin (Braff and Gallo, 2006; Liu et al., 2006; Lai and Gallo, 2008). To examine how molecules released into the overnight growth medium of staphylococci influence keratinocyte innate immune function, we first explored the cytotoxicity of different molecular weight fractions derived from Staphylococci when exposed to undifferentiated normal human epidermal keratinocytes (NHEKs). The morphology of undifferentiated NHEKs after exposure to 3.6 μgml−1 sterile supernatant of culture medium conditioned by overnight growth of Staphylococcus aureus Newman or S. epidermidis 1457 showed that S. aureus Newman conditioned medium was toxic to undifferentiated NHEKs, whereas S. epidermidis 1457 had no detectable effect (Figure 1a). Lactate dehydrogenase (LDH) release assay revealed that sterile whole supernatants and large molecular weight retentive form 10 kDa microdialysis prepared from two S. aureus strain-induced maximal membrane damage (Figure 1b). Whole supernatants and the large dialysis fractions from S. epidermidis strains were less toxic, but some membrane damage was also observed by LDH release. However, the sterile <10 kDa ultrafiltrates of both bacterial species did not induce an increase in LDH release (Figure 1b). Therefore, the small molecular weight fraction was selected for further study as it was nontoxic. Furthermore, this fraction was a logical choice as it would be predicted to be more diffusible through the stratum corneum. Such a process could be reflective of the microbial components that contact basal keratinocytes under normal physiological conditions as an intact stratum corneum functions as natural barrier to exclude larger molecules from passively penetrating into the lower epidermis (Potts and Guy, 1992; Rappersberger et al., 1996; Ruzicka et al., 1997; Bos and Meinardi, 2000; Karande et al., 2004).
Figure 1. Toxicity of staphylococci to cultured human keratinocytes.
(a) The morphology of undifferentiated cultured normal human epidermal keratinocytes (NHEKs) after exposure to a sterile supernatant of culture medium conditioned by the overnight growth of S. aureus Newman or S. epidermidis 1457. (b) Sterile filtered whole conditioned medium (0–36 μgml−1), the large molecular weight > 10 kDa dialysis retentate, or the small molecular weight < 10kDa ultrafiltrate of conditioned culture medium from two S. aureus strains (S. aureus Newman and S. aureus Rosenbach) and two S. epidermidis strains (S. epidermidis 1457 and S. epidermidis 12228) were used to treat undifferentiated NHEKs for 24 hours, followed by lactate dehydrogenase (LDH) assay. Scale bar = 50 μm. TSB, tryptic soy broth.
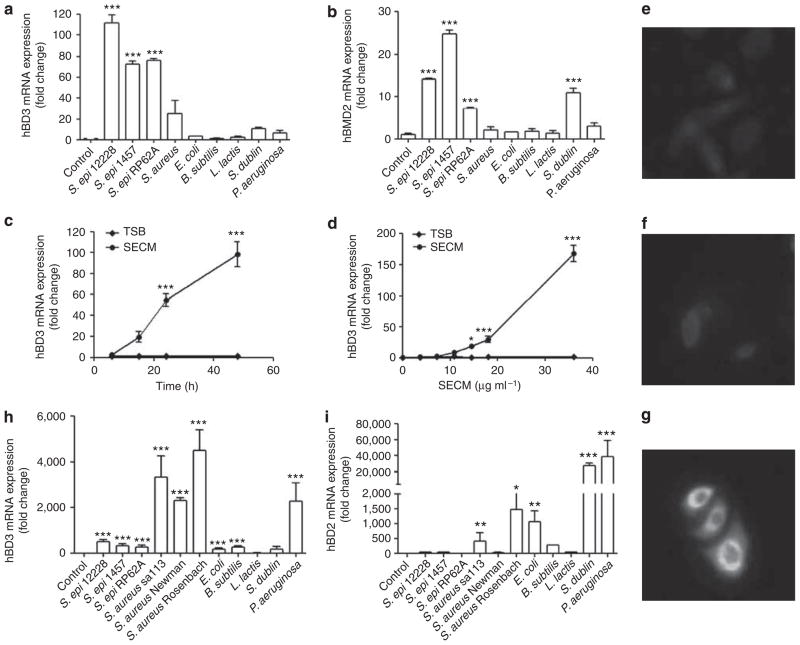
The capacity of <10 kDa ultrafiltrates from a panel of bacterial culture supernatants to induce antimicrobial peptide expression in undifferentiated NHEKs was evaluated by quantitative real-time reverse transcriptase PCR (RT-PCR). Human β-defensin 3 (hBD3) and human β-defensin 2 (hBD2) mRNAs were significantly increased after exposure to the <10 kDa ultrafiltrates of three S. epidermidis strains (Figure 2a and b). Interestingly, this response to S. epidermidis was greater than the response to similarly prepared conditioned media from other common Gram-positive and Gram-negative bacteria; S. aureus induced less hBD3 and did not induce hBD2, Salmonella dublin only induced hBD2 but not hBD3, and four other bacteria grown under similar conditions (E. coli, Bacillus subtilis, Lactococcus lactis, and Pseudomonas aeruginosa) did not significantly induce either antimicrobial peptide in undifferentiated NHEKs (Figure 2a and b).
Figure 2. Staphylococcus epidermidis induces human β-defensin (hBDs) 2 and 3 in normal human keratinocytes.
(a) hBD3 and (b) hBD2 expression of undifferentiated human keratinocytes stimulated by 36 μgml−1 of sterile 10 kDa ultrafiltrates from nine bacteria conditioned culture media for 24 hours. (c) The time- and (d) dose-dependent curves of hBD3 induced by sterile <10 kDa products from S. epidermidis cultured media (SECM). (e–g) Immunofluorescent staining of hBD3 induced by 36 μg ml−1 of SECM in cultured undifferentiated keratinocytes. (e) Rabbit IgG, (f) tryptic soy broth (TSB), and (g) SECM. (h) hBD3 and (i) hBD2 expression of differentiated human keratinocytes stimulated by 36 μgml−1 of sterile 10 kDa ultrafiltrates from nine bacteria conditioned culture media for 24 hours. *P<0.05; **P<0.01; P<0.001. P-values were determined using one-way analysis of variance (ANOVA) (a, b, h, and i) or two-way ANOVA (c, d). Data shown represent two independent experiments with n = 3 per group. Scale bar = 10 μm.
The increase of hBD3 mRNA in response to sterile <10 kDa products from S. epidermidis cultured media (SECM) occurred in both time- and dose-dependent manners (Figure 2c and d) in undifferentiated NHEKs, and was accompanied by an increase in the abundance of hBD3 protein as detected by immunofluorenscent staining (Figure 2e–g). Unlike these two β-defensins, the human cathelicidin gene CAMP did not significantly increase in response to SECM (data not shown). Furthermore, in differentiated NHEKs the response was different. The induction of hBD3 and hBD2 mRNAs by the <10 kDa ultrafiltrates of S. aureus or P. aeruginosa conditioned media was much greater than that induced by the <10 kDa ultrafiltrates of three S. epidermidis strains (Figure 2h and i), suggesting that keratinocytes under different conditions have different responses to commensal and pathogenic bacteria. This is consistent with previous reports that the differentiation state of keratinocytes changed their profile of hBD expression (Liu et al., 2002).
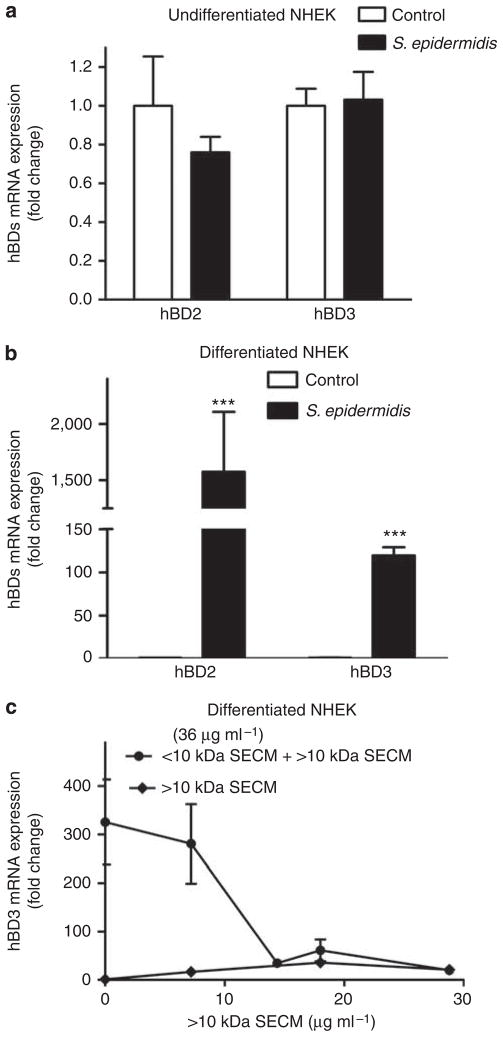
In contrast to the low molecular weight molecules in SECM, live S. epidermidis added to undifferentiated NHEKs did not increase hBD3 expression, whereas it slightly inhibited hBD2 expression (Figure 3a). However, in differentiated keratinocytes both hBD2 and hBD3 expressions were greatly increased by live S. epidermidis (Figure 3b). Although the increase of hBD3 by live S. epidermidis was observed in differentiated NHEKs, this increase (120-fold) was much lower than the increase induced by SECM (310-fold; Figure 3c). Furthermore, the ability of SECM to induce hBD3 was eliminated by the > 10 kDa fraction of S. epidermidis when the > 10 kDa fraction was recombined with the < 10 kDa fraction (Figure 3c), suggesting that S. epidermidis may produce inhibitory factors in the large molecular weight fraction and also partially explaining the lack of induction of hBD2 or hBD3 in undifferentiated keratinocytes exposed to whole S. epidermidis.
Figure 3. Effects of differentiation, live S. epidermidis, and S. epidermidis extract on hBD2 and 3 expression by keratinocytes.
The expression of hBD2 and hBD3 by live S. epidermidis in undifferentiated versus differentiated normal human keratinocytes, and the inhibitory effect of S. epidermidis > 10 kDa fraction on hBD3 induced by SECM in differentiated normal human keratinocytes. (a, b) Colony-forming units, 106, of live S. epidermidis 1457 was added to (a) undifferentiated normal human epidermal keratinocytes (NHEKs) or (b) differentiated NHEKs for 24 hours. The expression of human β-defensin 3 (hBD3) and hBD2 was analyzed using real-time RT-PCR. (c) Combining the > 10 kDa dialysis retentate of S. epidermidis 1457 with the < 10 kDa ultrafiltrate from the conditioned culture medium (SECM) abrogated the capacity of SECM to induce hBD3. P<0.001. P-values were determined using two-way analysis of variance (ANOVA). Data shown represent two independent experiments with n = 3 per group.
S. epidermidis protects normal human epidermal keratinocytes against multiple skin pathogens
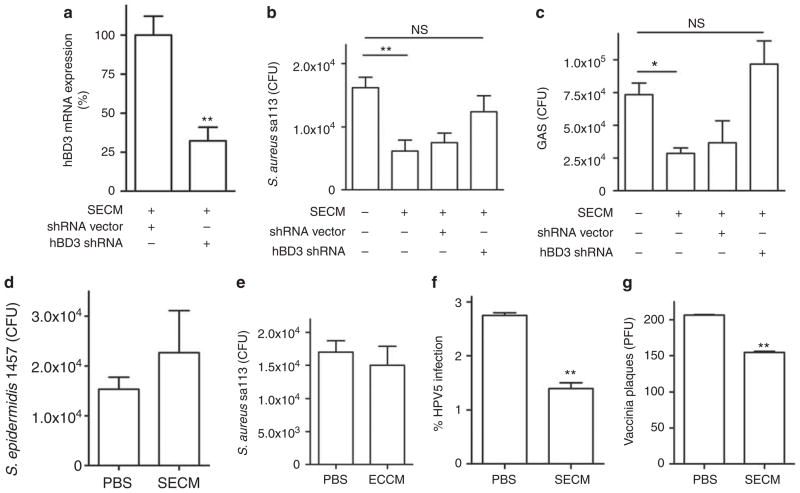
As hBD2 and hBD3 act against multiple pathogens and viruses that infect the skin (Howell et al., 2006; Kisich et al., 2007; Grice et al., 2008), we next evaluated whether the expression of these genes in undifferentiated NHEKs stimulated by SECM could inhibit bacterial or viral replication or infection. Treatment of undifferentiated NHEKs with short hairpin RNA (shRNA) targeted to hBD3 was effective in partially inhibiting hBD3 expression (70% decrease; Figure 4a). A lysate from undifferentiated NHEKs pretreated for 24 hours with SECM inhibited the growth of S. aureus sa113 and group A Streptococcus (GAS; Figure 4b and c), but did not inhibit S. epidermidis 1457 (Figure 4d). After transfection with shRNA targeted to the hBD3 gene, lysates from undifferentiated keratinocytes treated with SECM lost the capacity to inhibit bacterial growth (Figure 4b and c). However, a similar lysate from undifferentiated NHEKs pretreated with a < 10 kDa ultrafiltrate of E. coli conditioned media did not inhibit the growth of S. aureus sa113 (Figure 4e), which confirmed the earlier observation that the expression of hBDs was not induced by E. coli conditioned media in undifferentiated NHEKs (Figure 2a and b). In addition to inhibiting bacterial growth, SECM also enabled keratinocytes to inhibit human papillomavirus 5 pseudovirus survival (Figure 4f) and vaccinia virus plaque-forming capacity in keratinocyte monolayers (Figure 4g).
Figure 4. Staphylococcus epidermidis increases antimicrobial activity of undifferentiated keratinocytes against multiple skin pathogens.
(a) The expression of human β-defensin 3(hBD3) after treatment with hBD3 short hairpin RNA (shRNA). (b) The growth of S. aureus sa113 and (c) group A Streptococcus (GAS) after exposure to lysates from undifferentiated human keratinocytes pretreated with hBD3 shRNA and then stimulated by sterile < 10 kDa products from S. epidermidis cultured media (SECM). (d) The growth of S. epidermidis 1457 after exposure to lysates of SECM-treated keratinocytes. (e) The growth of S. aureus sa113 after exposure to lysates of ECCM-treated keratinocytes. (f) The survival of human papillomavirus 5 (HPV5) in HaCaT and (g) vaccinia virus in undifferentiated human keratinocytes after 24-hour SECM pretreatment. *P<0.05; **P<0.01. NS, no significance. P-values were evaluated using two-tailed t-tests (a and f–g) or one-way analysis of variance (ANOVA) (b, c). Data are the mean±SD of triplicate cultures and are representative of two independent experiments.
S. epidermidis protects mice from GAS infection
To validate that the induction of antimicrobial peptides in keratinocytes by SECM would be relevant to protection against infection of the skin, SECM was intradermally injected into mice 24 and 2 hours before an infectious challenge at the site with GAS. Mice treated with SECM showed significantly smaller infectious skin lesions when compared with control mice injected with a similar sterile < 10 kDa ultrafiltrate of tryptic soy broth (TSB) that was not exposed to S. epidermidis (Figure 5a and b). Mouse skin treated with SECM also had much less surviving GAS at the local site of infection (99% decrease; Figure 5c) and less systemic survival of GAS measured in the liver (90% decrease; Figure 5d).
Figure 5. Staphylococcus epidermidis protects mice from group A Streptococcus (GAS) infection, and Malp2 induces hBDs 2 and 3 against GAS infection in a manner similar to S. epidermidis.
(a) Photograph of skin lesions caused by GAS at 3 days after GAS injection. (b) ImageJ analysis of the lesion size of a. (c) Local GAS survival in skin and (d) systemic GAS survival in liver of SECM- and tryptic soy broth (TSB)-pretreated mice. (e) The expression of human β-defensins (hBDs) 2 and 3 by Toll-like receptor 2 (TLR2) ligands in undifferentiated normal human keratinocytes. (f) ImageJ analysis of skin lesion size of Malp2- and phosphate-buffered saline (PBS)-pretreatment mice. (g) The survival of local GAS in skin and (h) systemic survival of GAS in the livers of Malp2- and PBS-pretreatment mice. The injection of SECM, TSB, Malp2, or PBS at 24 and 2 hours before live GAS injection did not cause skin lesions. *P<0.05; P<0.001. P-values were determined using two-tailed t-tests. Data shown are representative of two independent experiments with n = 3–6 per group.
S. epidermidis induces β-defensins through TLR2 signaling
We next sought to identify the mechanism involved in the recognition of SECM that leads to an increase in antimicrobial expression and function. Pretreatment of SECM with papain completely abrogated the capacity of SECM to induce hBD2-3 mRNA (data not shown). On the basis of this observation, and previous observations that TLR2 is a major receptor on NHEKs for lipopeptides produced by Gram-positive bacteria (Schroder et al., 2003; Takeda et al., 2003; Sumikawa et al., 2006), we hypothesized that lipopeptides that activate TLR2 might initiate a response similar to SECM. To test this we treated undifferentiated NHEKs with purified lipopeptides in a manner similar to SECM and observed that only a TLR2/6 ligand, the diacylated lipopeptide Malp2, increased the expression of hBDs 2 and 3 (Figure 5e). Furthermore, Malp2 also acted similarly in mice, as injection into the skin 24 hours before challenge decreased the skin lesion caused by GAS (Figure 5f) as well as local GAS survival in skin (Figure 5g) and decreased systemic spread of GAS to liver (Figure 5h). Other TLR2 ligands, such as Pam3CSK4 (TLR2/1 ligand), and lipoteichoic acid (TLR2 ligand), did not induce gene expression of hBD2-3 (Figure 5e). These data suggested that a TLR2/6 ligand present in SECM may be the active factor.
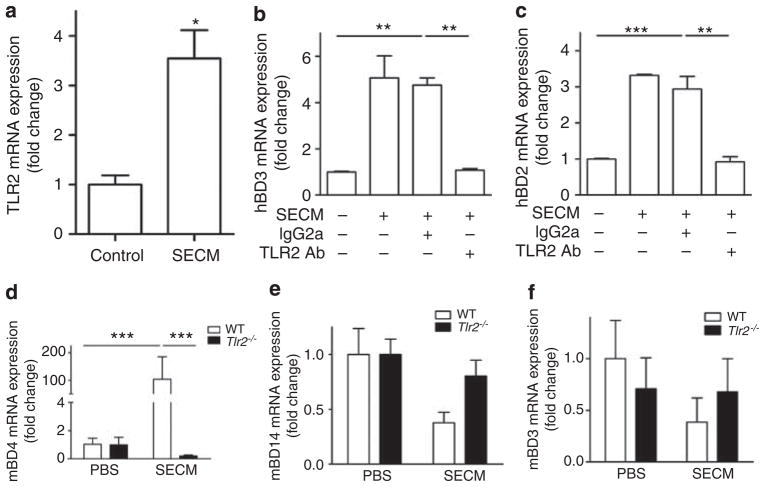
Analysis of undifferentiated NHEKs revealed that after a 24-hour stimulation with SECM, the expression of TLR2 was significantly increased (Figure 6a). To test our hypothesis that hBD2-3 expression was being induced by a TLR2 ligand from S. epidermidis, two independent approaches to block TLR2-dependent activation were used. First, a monoclonal TLR2-neutralizing antibody completely blocked the expression of SECM-induced hBD2 and hBD3 in undifferentiated NHEKs (Figure 6b and c). Second, the role of TLR2 in the induction of β-defensins by SECM was confirmed in mice as SECM was observed to significantly increase the expression of mBD4 mRNA (mouse ortholog of hBD2) in the skin of wild-type mice, but not in TLR2-deficient mice (Figure 6d). Interestingly, SECM did not induce the expression of mouse β-defensin 14 (mBD14), a potential ortholog of hBD3, or mouse β-defensin 3 (mBD3; Figure 6e and f).
Figure 6. Staphylococcus epidermidis activates Toll-like receptor 2 (TLR2) to induce β-defensins.
(a) TLR2 expression in undifferentiated normal human keratinocytes after a 24-hour SECM (10 μgml−1) stimulation. (b) Human β-defensin 3 (hBD3) expression by SECM (10 μgml−1) in undifferentiated normal human epidermal keratinocytes (NHEKs) after TLR2-neutralizing antibody treatment. (c) The expression of hBD2 by SECM (10 μgml−1) in undifferentiated NHEKs after TLR2-neutralizing antibody treatment. (d) Murine β-defensin 4 (mBD4) expression induced by 12 μg of SECM in wild-type and TLR2-deficient mice. (e) mBD14 expression induced by SECM in vivo. (f) mBD3 expression induced by SECM in vivo. *P<0.05; **P<0.01; P<0.001. P-values were evaluated using two-tailed t-tests (a), one-way analysis of variance (ANOVA) (b, c), or two-way ANOVA (d). Data shown represent two independent experiments with n = 3 (a–c) or n = 6 (d–f) per group.
DISCUSSION
Human skin is exposed to a large variety of microbial organisms that can be detected by a diverse array of recognition systems including the TLRs. The keratinocyte is the major cell type in the epidermis, constituting 95% of the cells of this structure, and responsible in part for the release of antimicrobial peptides such as β-defensins, cathelicidin, and many others including psoriasin (Harder and Schroder, 2005; Braff et al., 2005a, 2005b; Abtin et al., 2008). The production of antimicrobial peptides is crucial to innate defense against skin infection. Our results in this study show that <10 kDa molecules of S. epidermidis will increase expression of antimicrobial peptides in murine skin or isolated undifferentiated human keratinocytes and render them more resistant to infection by selected bacterial and viral pathogens. Thus, this study suggests that under some conditions the normal skin resident bacterium S. epidermidis could function in a beneficial way to enhance innate antimicrobial defense against invading pathogens.
The capacity of extracts from S. epidermidis to confer protection was selective as extracts from other bacteria, including S. aureus, L. lactis, P. aeruginosa, and E. coli, did not induce hBD2 and hBD3 in undifferentiated human keratinocytes to the same degree as S. epidermidis. Furthermore, undifferentiated keratinocytes activated by S. epidermidis showed selective antimicrobial action against GAS, S. aureus, vaccinia virus, and human papillomavirus pseuedovirus infection, but did not show increased antimicrobial action against S. epidermidis. The molecule(s) produced by S. epidermidis that are responsible for triggering the antimicrobial response remains unknown and is the subject of ongoing investigations. However, our present data clearly show that the overall resistance to infection in murine skin is dependent on TLR2, and recognition by keratinocytes is dependent on TLR2 pathways for the production of β-defensins. The enhanced antimicrobial responses are likely TLR2 ligands produced by this microbe, as purified lipopeptides that activate TLR2 (such as Malp2) partially reproduced this effect.
Administration of SECM as an intradermal injection is an experimental model to test physiological relevance of the observations in vitro with undifferentiated NHEKs, but this may also partially mimic events that normally occur in vivo. It is reasonable to hypothesize that commensal microflora on the skin surface secrete small molecular weight products that will penetrate the stratum corneum and directly contact basal keratinocytes. Alternatively, the antimicrobial peptide stimulating potential of commensal microflora may become important when they directly contact basal keratinocytes when the barrier is disrupted after injury. This effect of S. epidermidis has also been recently reported by us in the context of inhibition of inflammation after injury (Lai et al., 2009).
Although antimicrobial peptide production by keratinocytes has been shown to have an important role in defense against invasive bacterial infection (Braff et al., 2005b), the overall enhanced defense to infection by SECM observed in vivo likely involves the response of cell types in addition to keratinocytes. Certainly, in the model used in this study, several other resident cell types of the skin in addition to keratinocytes above and below the injection were involved. These cells were also exposed to S. epidermidis, and dendritic cells and mast cells are known to perform essential functions in response to infection. Therefore, although the data of this paper have focused on the keratinocyte response, the relative function of other cellular elements in coordination with the epidermal keratinocyte remains to be determined.
The regulation of antimicrobial peptide production has been an active subject of investigation because of the importance of this event in the control of infection. A variety of factors influence the expression of specific antimicrobial peptides. Cathelicidin expression in humans can be abundantly induced by the administration of vitamin D both in vitro and in vivo (Wang et al., 2004; Gombart et al., 2005; Liu et al., 2006; Schauber et al., 2007, 2008; Hata et al., 2008). The expression of β-defensins is influenced by TLR ligands, tumor necrosis factor, IL-1β, IFN-γ, and phorbol myristate acetate (Harder et al., 1997, 2000; Wehkamp et al., 2003; Selsted and Ouellette, 2005). More recently, it has been shown that commensal bacteria can regulate antimicrobial peptide expression in some tissues. For example, in the gut, commensal bacteria have been suggested to provide protection by chronically stimulating expression of antimicrobial peptides at levels that kill pathogens (Boman, 2000; Cash et al., 2006). In the gingival epithelium, Fusobacterium nucleatum stimulates the inducible defensin hBD2, whereas Porphyromonas gingivalis, the anaerobe that destroys gum tissue, does not, behaving as a silent invader (Krisanaprakornkit et al., 2000). The observations of this study show that commensal bacteria of human skin can selectively induce antimicrobial peptides and provide a protective effect in vivo when administered before infectious challenge.
Although S. epidermidis suppresses pathogens by stimulating the host to secrete antimicrobial peptides, it also develops some countermeasures against the action of endogenous antimicrobial peptides. For example, S. epidermidis produces metalloprotease sepA to degrade anionic antimicrobial peptide Dermcidin (Lai et al., 2007). In addition to the production of protease, S. epidermidis has evolved another powerful strategy against hBDs. Under stimulation of hBDs, S. epidermidis controls a three-component antimicrobial peptide-sensing system to modify the net negative charge of the membrane that will repel cationic antimicrobial peptides (Peschel et al., 2001; Li et al., 2007; Schauber et al., 2007). Our data that the cell lysate from keratinocytes treated with SECM did not kill S. epidermidis itself further confirmed that S. epidermidis has evolved strategies for survival on skin to exist as a symbiote. Our observations are also consistent with a previous report that the expression of hBD2 was induced in keratinocytes upon stimulation by S. epidermidis (Dinulos et al., 2003). However, our data contrast with another report (Holland et al., 2009) that live S. epidermidis inhibited or did not induce hBDs expression in undifferentiated keratinocytes or in a living skin equivalent model. Although we could not confirm these previous observations, the difference between our results and those of Holland et al. (2009) may be because of several factors, including: (1) bacteria grown in rich medium such as TSB may produce factors different than live bacteria in contact with host cells; (2) live S. epidermidis on the skin surface may produce some molecules to facilitate the penetration of these factors through stratum corneum and inhibit the expression of β-defensins; and (3) when in contact with lower layer keratinocytes S. epidermidis may express molecules that directly inhibit β-defensin expression. Unfortunately, under special circumstances, these strategies may help S. epidermidis evade innate defense systems and make it an opportunistic pathogen.
The traditional concept of TLR recognition events is that this pattern recognition strategy lacks the capacity to discriminate between pathogenic and commensal micro-organisms. Responses to commensals are undesirable and limited by physical separation from the cell type expressing the receptor. Recent work has shown that recognition of commensal microflora by TLRs or their adaptor MyD88 is required for protection of the gut from injury and the control of bacterial load in intestine (Rakoff-Nahoum et al., 2004; Vaishnava et al., 2008). In this study our results showed that S. epidermidis-induced hBD 2 and 3 gene expression in cultured undifferentiated keratinocytes is TLR2 dependent (Figure 6b and c) and leads to an increased activity of keratinocytes against microbial infection. These observations suggest that the ability to recognize commensal bacterial products may be important in maintaining skin homeostasis. Understanding this relationship thereby provides a new direction for the study of the skin immune response, and further highlights concerns related to indiscriminant use of both systemic and topical antimicrobial products. Furthermore, the activation of TLRs results in the initiation of intracellular signaling cascades involving diverse elements such as the mitogen-activated protein kinase family, the NF-κB, and activator protein-1 transcription families (Lai and Gallo, 2008), as well as phosphoinositide 3-kinases (Medzhitov, 2001; Fukao et al., 2002; Fukao and Koyasu, 2003; Takeda et al., 2003). However, whether these downstream signaling families are involved in the regulation of hBDs 2 and 3 expression by S. epidermidis remains to be determined.
MATERIALS AND METHODS
Mice
Mice (C57Bl/6 wild-type and TLR2 knockout) were housed in VA San Diego Healthcare System Veterinary Medical Unit. All animal experiments were approved by the institutional animal care and use committee of the VA San Diego Healthcare System.
Plasmid, virus, bacterial strains, and cell line
S. epidermidis 1457, S. aureus Sa113, B. subtilis Cohen, L. lactis NZ9700, GAS NZ131, E. coli ATCC29522, and S. dublin Lane, P. aeruginosa Migula were either stored in our laboratory or obtained from Dr Victor Nizet (University of California, San Diego). p5sheLL plasmid, pfwB (GFP plasmid), and 293 TT cells were kind gifts from Drs John Schiller and Chris Buck (National Cancer Institute, Bethesda, MD) and Martin Müller (German Cancer Research Center, Heidelberg, Germany).
Bacterial extract preparation
Bacteria from glycerol stock were inoculated and grown in TSB at 37 °C overnight. The overnight cultures were diluted 1:100 into TSB and grown for 15 to 16 hours. Bacterial supernatants were collected and filtered by 0.22 μm Stericup (Fisher Scientific, Pittsburgh, PA). MacroSep 10K OMEGA column (VWR International, Brisbane, CA) was used to collect the fraction < 10 kDa from bacterial supernatants. The concentration of these bacterial fractions was determined by BCA Protein Assay Kit (Pierce, Rockford, IL). There was no difference of AMP induction by SECM from one overnight culture or SECM from two overnight cultures.
Primary cell culture and stimulation
NHEKs (Invitrogen, Carlsbad, CA) were cultured in EpiLife medium supplemented with EpiLife Defined Growth Supplement and 60 μM CaCl2 (Invitrogen). Subconfluent cells were seeded in 6-, 12-, or 24-well plates to grow to 70% confluence. To induce keratinocyte differentiation, fresh EpiLife medium containing EpiLife Defined Growth Supplement and 1mM CaCl2 was added to stimulate NHEKs when cells grew to 90% confluence. After 2 days, the medium was removed and new fresh EpiLife medium containing EpiLife Defined Growth Supplement and 1.6mM CaCl2 was added to stimulate NHEKs for another 2 days. To test the cytotoxicity of different fractions of bacterial growth media, 0, 3.6, 7.2, 14.4, 21.6, 28.8, and 36 μgml −1 of TSB culture medium conditioned by overnight growth of bacteria was used to stimulate undifferentiated NHEKs. After 24 hours, LDH release from the cells was tested by cytotoxicity detection kit (LDH) (Roche, Pleasanton, CA) according to the manufacture’s introduction. To compare the capacity of different bacteria to induce hBDs 2 and 3 in parallel, 36 μgml −1 of < 10 kDa bacterial ultrafiltrates or 106 colony-forming units of live S. epidermidis 1457 were added to stimulate undifferentiated or differentiated NHEKs for 24 hours. To test whether the induction of hBDs by SECM is in both time- and dose-dependent manner, 36 μgml −1 of SECM was used to stimulate undifferentiated NHEKs for various periods; 0, 3.6, 7.2, 10.8, 14.4, 18.0, and 36 μgml −1 of SECM was used to stimulate undifferentiated NHEKs. To test the inhibitory effect of the > 10 kDa fraction of S. epidermidis on the induction of hBD3 by SECM, 36 μgml −1 of SECM was added into differentiated NHEKs 10 minutes before 0, 7.2, 14.4, 18.0, and 28.8 μgml −1 of the > 10 kDa fraction was added. For TLR2 blocking assay, 10 μgml −1 of SECM was used to activate TLR2 or to induce hBDs. To test the expression of hBDs induced by TLR ligands, 1 μgml −1 Pam3CSK4 (InvivoGen, San Diego, CA), 10 μgml −1 lipoteichoic acid (Sigma, St Louis, MO), or 100 ngmL −1 Malp2 (Invitrogen) was used. Cells were harvested and the expression of genes was analyzed using real-time RT-PCR.
Real-time quantitative RT-PCR
Total RNA was extracted after a 24-hour stimulation with different stimuli using Trizol Reagent (Invitrogen). The amount of RNA was measured using spectrophotometer. Total RNA (1 μg) was used for complementary DNA synthesis by the iSCRIPT complementary DNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Real-time RT-PCR was conducted in an ABI PRISM 7000 sequence detector (PE Applied Biosystems, Foster City, CA). The primers and probes used for real-time RT-PCR were purchased from Applied Biosystems. RNA analysis was performed using TaqMan Master Mix reagents kit (Applied Biosystems). The quantification of gene expression was determined by the comparative ΔΔCT method. The target gene expression in the test samples was normalized to the endogenous reference glyceraldehyde-3-phosphate dehydrogenase level and was reported as the fold difference relative to glyceraldehyde-3-phosphate dehydrogenase gene expression. All the assays were performed in triplicate and repeated at least two times.
Immunofluorenscent staining
Second passage neonatal human epidermal keratinocytes were grown in Lab-Tek Chamer Slide (Fisher Scientific). SECM were added in final concentration of 36 μgml −1 to stimulate cells for 24 hours. After cold acetone fixation and subsequent washing by phosphate-buffered saline (PBS), cells were blocked with 3% BSA (Sigma) for 30 minutes at room temperature and stained with polyclonal rabbit anti-hBD3 (Orbigen, San Diego, CA) and negative control rabbit immunoglobulin fraction (Dako, Carpinteria, CA) at 4°C overnight. After washing by PBS, the cells were reprobed with anti-rabbit IgG FITC- conjugated antibody (Sigma). After subsequent washing by PBS, the slides were mounted in ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole (Invitrogen) and visualized using Olympus BX41 microscope (Tokyo, Japan).
RNA interference
Human epidermal keratinocytes at first or second passage were seeded in six-well plates. To knock down hBD3 in NHEKs, four individual shRNA lentiviral constructs (Sigma) targeting different regions of hBD3 were used according to the manufacturer’s instructions. Two multiplicity of infection for each constructs or non-target shRNA control vector (Sigma) was added for 24-hour transfection. SECM (18 μgml −1) was added to stimulate cells for 24 hours after hBD3 shRNA transfection. The lysates from undifferentiated NHEKs were collected for bacterial killing assay or RNA was isolated for real-time RT-PCR.
Bacterial killing assay
After undifferentiated NHEKs were treated with hBD3 mission lentiviral transduction particles (Sigma) and/or SECM or undifferentiated NHEKs were only treated with 18 μgml−1 of the <10 kDa ultrafiltrate of E. coli conditioned media, 100 μl of PBS containing protease inhibitor cocktail (Roche) was added into wells. Cells were collected by cell scraper and then sonicated on ice-cold water. After removing cell debris by centrifugation, the concentration of the lysates was determined by BCA Protein Assay Kit. GAS was grown in Todd Hewitt Broth, whereas S. aureus sa113 and S. epidermidis 1457 were grown in TSB. Colony-forming units (105) of GAS, S. aureus sa113, and S. epidermidis 1457 were incubated with 10 μg of cell lysates for 3 hours, and then bacteria were serially diluted. Bacteria (10 μl) were plated on agar plates and grown at 37 °C overnight. Next day, colonies were counted.
Viral inhibition assay
Human papillomavirus 5 pseudoviruses were produced in 293 TT cells according to the protocol provided by Drs Schiller and Buck at the National Cancer Institute, National Institutes of Health (http://home.ccr.cancer.gov/lco/pseudovirusproduction.htm). HaCaT cells were pretreated with 36 μgml −1 of SECM for 24 hours, and then inoculated with 10 μg of human papillomavirus 5 pseudovirus. After a 2-day infection, HaCaT cells were trypsinized and the infection was analyzed by the flow cytometric analysis.
For vaccinia virus infection, 250 plaque-forming units of vaccinia virus were added in undifferentiated normal human keratinocytes pretreated with 36 μgml −1 of SECM for 24 hours. After a 1-hour incubation, viruses were removed and fresh medium was added. Cells were grown for another 24 hours. Crystal violet (0.1%) was used to show plaques.
GAS infection in vivo
Invasiveness of GAS into mouse skin was measured as previously described (Nizet et al., 2001). The backs of age-matched adult littermates were shaved and hair was removed by chemical depilation (Nair; Church & Dwight, Princeton, New Jersey). A total of 30 μg of SECM and a sterile 10 kDa ultrafiltrate from TSB, or 5 μg of Malp2 and 100 μl of PBS as control, were intradermally injected into the back skin of mice. The next day, 50 μl of live GAS (OD600 = 0.7–0.8) complexed to cytodex beads (Sigma) as carrier was intradermally injected 2 hours after second injection of SECM or a sterile 10 kDa ultrafiltrate from TSB or Malp2 or 100 μl PBS. Lesion sizes were measured daily. At day 3, skin around the lesion and liver were homogenized for GAS culture.
Inactivation of TLR2
Mouse monoclonal anti-human TLR2 antibody (5 μgml −1 Abcam, Cambridge, MA) and mouse IgG2a (Dako) was added 10 minutes before undifferentiated normal human keratinocytes were treated with 10 μgml −1 of SECM. After a 24-hour coincubation, RNA was isolated and complementary DNA was synthesized using the iSCRIPT complementary DNA Synthesis Kit. The expression of hBDs was tested using real-time RT-PCR.
mBDs induction in vivo
SECM (12 μg) was intradermally injected in the ear of 7- to 10-week-old C57BL/6 wild-type (or BALB/c wild-type mice) and Tlr2-deficient mice. After 24 hours, ears were cut and homogenized by bead beater. RNA was isolated from ears using Trizol Reagent. The expressions of mBDs were analyzed using real-time RT-PCR.
Statistical analysis
To determine significances between groups, comparisons were made using two-tailed t-tests. Analyses of multiple groups were carried out using one-way or two-way analysis of variance with Bonferroni post-test of GraphPad Prism Version 4 (San Diego, CA). For all statistical tests, a P-value of <0.05 was accepted for statistical significance.
Acknowledgments
We thank Dr Victor Nizet for helpful discussion. This work was supported by the NIH grants, NIH R01AR052728, NIH R01AI052453, and NIH R01AI083358, a VA Merit Award to RLG, and the NIH/NIDCD grant DC006279 to AFR.
Abbreviations
- GAS
group A Streptococcus
- hBD
human β-defensin
- LDH
lactate dehydrogenase
- mBD
murine β-defensin
- NHEK
normal human epidermal keratinocyte
- PBS
phosphate-buffered saline
- SECM
sterile <10 KDa products from S. epidermidis cultured media
- S. epidermidis
Staphylococcus epidermidis
- S. aureus
Staphylococcus aureus
- shRNA
short hairpin RNA
- TLR
Toll-like receptor
- TSB
tryptic soy broth
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Abtin A, Eckhart L, Mildner M, et al. Flagellin is the principal inducer of the antimicrobial peptide S100A7c (psoriasin) in human epidermal keratinocytes exposed to Escherichia coli. FASEB J. 2008;22:2168–76. doi: 10.1096/fj.07-104117. [DOI] [PubMed] [Google Scholar]
- Bibel DJ, Aly R, Bayles C, et al. Competitive adherence as a mechanism of bacterial interference. Can J Microbiol. 1983;29:700–3. doi: 10.1139/m83-114. [DOI] [PubMed] [Google Scholar]
- Boman HG. Innate immunity and the normal microflora. Immunol Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- Braff MH, Bardan A, Nizet V, et al. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005a;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- Braff MH, Zaiou M, Fierer J, et al. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005b;73:6771–81. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekio I, Sakamoto M, Hayashi H, et al. Characterization of skin microbiota in patients with atopic dermatitis and in normal subjects using 16S rRNA gene-based comprehensive analysis. J Med Microbiol. 2007;56:1675–83. doi: 10.1099/jmm.0.47268-0. [DOI] [PubMed] [Google Scholar]
- Dinulos JG, Mentele L, Fredericks LP, et al. Keratinocyte expression of human beta defensin 2 following bacterial infection: role in cutaneous host defense. Clin Diagn Lab Immunol. 2003;10:161–6. doi: 10.1128/CDLI.10.1.161-166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CA, Smith WM, Johnston EA, et al. Bacterial flora of the normal human skin. J Invest Dermatol. 1950;15:305–24. doi: 10.1038/jid.1950.105. [DOI] [PubMed] [Google Scholar]
- Fierer N, Hamady M, Lauber CL, et al. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA. 2008;105:17994–9. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–63. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Pei Z, et al. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA. 2007;104:2927–32. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25- dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Goodarzi H, Trowbridge J, Gallo RL. Innate immunity: a cutaneous perspective. Clin Rev Allergy Immunol. 2007;33:15–26. doi: 10.1007/s12016-007-0037-4. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–50. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Teran LM, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin- 2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–21. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- Harder J, Schroder JM. Antimicrobial peptides in human skin. Chem Immunol Allergy. 2005;86:22–41. doi: 10.1159/000086650. [DOI] [PubMed] [Google Scholar]
- Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–31. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland DB, Bojar RA, Farrar MD, et al. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol Lett. 2009;290:149–55. doi: 10.1111/j.1574-6968.2008.01402.x. [DOI] [PubMed] [Google Scholar]
- Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–34. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–73. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- Howell MD, Boguniewicz M, Pastore S, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006;121:332–8. doi: 10.1016/j.clim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high throughput screening. Nat Biotechnol. 2004;22:192–7. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- Kisich KO, Howell MD, Boguniewicz M, et al. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J Invest Dermatol. 2007;127:2368–80. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- Krisanaprakornkit S, Kimball JR, Weinberg A, et al. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–15. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutmann J. Pre- and probiotics for human skin. J Dermatol Sci. 2009;54:1–5. doi: 10.1016/j.jdermsci.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Tolllike receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–82. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. Toll-like receptors in skin infections and inflammatory diseases. Infect Disord Drug Targets. 2008;8:144–55. doi: 10.2174/1871526510808030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–41. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Villaruz AE, Li M, et al. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol. 2007;63:497–506. doi: 10.1111/j.1365-2958.2006.05540.x. [DOI] [PubMed] [Google Scholar]
- Li M, Lai Y, Villaruz AE, et al. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci USA. 2007;104:9469–74. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY, Destoumieux D, Wong AV, et al. Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Marples MJ. The normal flora of the human skin. Br J Dermatol. 1969;81(Suppl 1):2–13. doi: 10.1111/j.1365-2133.1969.tb12827.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Meyer T, Stockfleth E, Christophers E. Immune response profiles in human skin. Br J Dermatol. 2007;157(Suppl 2):1–7. doi: 10.1111/j.1365-2133.2007.08264.x. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Otto M. Virulence factors of the coagulase-negative staphylococci. Front Biosci. 2004;9:841–63. doi: 10.2741/1295. [DOI] [PubMed] [Google Scholar]
- Peschel A, Jack RW, Otto M, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–76. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts RO, Guy RH. Predicting skin permeability. Pharm Res. 1992;9:663–9. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rappersberger K, Meingassner JG, Fialla R, et al. Clearing of psoriasis by a novel immunosuppressive marolide. J Invest Dermatol. 1996;106:701–10. doi: 10.1111/1523-1747.ep12345542. [DOI] [PubMed] [Google Scholar]
- Ruzicka T, Bieber T, Schopf E, et al. A short-term trial of tacrolimus ointment for atopic dermatitis. N Engl J Med. 1997;337:816–21. doi: 10.1056/NEJM199709183371203. [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Buchau AS, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–24. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- Schroder NW, Morath S, Alexander C, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–94. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- Schwarz T. Skin immunity. Br J Dermatol. 2003;149(Suppl 66):2–4. doi: 10.1046/j.0366-077x.2003.05624.x. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–7. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Sumikawa Y, Asada H, Hoshino K, et al. Induction of beta-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2. Microbes Infect. 2006;8:1513–21. doi: 10.1016/j.micinf.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Weichenthal M, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–23. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]