Abstract
Peripheral nerve grafts (PNG) into the rat spinal cord support axon regeneration after acute or chronic injury, with synaptic reconnection across the lesion site and some level of behavioral recovery. Here, we grafted a peripheral nerve into the injured spinal cord of cats as a preclinical treatment approach to promote regeneration for eventual translational use. Adult female cats received a partial hemisection lesion at the cervical level (C7) and immediate apposition of an autologous tibial nerve segment to the lesion site. Five weeks later, a dorsal quadrant lesion was performed caudally (T1), the lesion site treated with Chondroitinase ABC two days later to digest growth inhibiting extracellular matrix molecules, and the distal end of the PNG apposed to the injury site. After 4–20 weeks, the grafts survived in 10/12 animals with several thousand myelinated axons present in each graft. The distal end of 9/10 grafts was well apposed to the spinal cord and numerous axons extended beyond the lesion site. Intraspinal stimulation evoked compound action potentials in the graft with an appropriate latency illustrating normal axonal conduction of the regenerated axons. Although stimulation of the PNG failed to elicit responses in the spinal cord distal to the lesion site, the presence of c-Fos immunoreactive neurons close to the distal apposition site indicates that regenerated axons formed functional synapses with host neurons. This study demonstrates the successful application of a nerve grafting approach to promote regeneration after spinal cord injury in a non-rodent, large animal model.
Keywords: spinal cord injury, regeneration, peripheral nerve graft, cat, c-Fos, chondroitinase
INTRODUCTION
Traumatic insult to the spinal cord induces both immediate mechanical damage and subsequent tissue degeneration leading to substantial physiological, biochemical and functional alteration of the spinal cord. Various interventions have shown the adaptive potential of the spinal cord but also its limitations when descending axons are injured and spinal cord neurons are deprived of adequate supraspinal drive.
CNS injured axons fail to spontaneously regenerate after SCI resulting in persistent functional impairment. In an elegant series of studies, A.J. Aguayo showed that adult CNS axons do have the potential to re-grow after being severed if provided with an appropriate environment (reviewed in Aguayo 1985). Thus, regeneration is a promising repair strategy after SCI if attempted in the right conditions. The major impediment to CNS axon growth beyond a supportive substratum is the clustering of inhibitory molecules that forms part of the glial scar (David and Lacroix, 2003; Silver and Miller, 2004; Yiu and He, 2006; Fawcett, 2006), and this is even more pronounced in the chronic state of the injury (Houlé and Tessler, 2003). Recovery of function after SCI will most likely result from a combination approach involving regeneration, neuroprotection, neurotrophic factors, neutralization of inhibitory molecules, rehabilitation and/or electrical stimulation of muscles and spinal networks (Thuret et al., 2006; Schwab et al., 2006; Bunge, 2008). Transplants designed to provide a growth-permissive environment or to enhance the regenerative effort of axotomized CNS neurons (Schwab and Bartholdi, 1996; Stichel and Muller, 1998) when grafted into the site of injury promote the growth of axotomized axons and rescue neurons destined to undergo retrograde cell death. Even a small amount of spared or rescued connections can provide significant functional gain (Blight and DeCrescito, 1986; Kloos et al., 2005).
Axonal growth into a peripheral nerve graft and formation of synaptic contact with host neurons has been illustrated in different animal models but growth beyond the PNG is limited to a small number of axons over a short distance (Vidal-Sanz et al., 1987; Carter et al., 1989; Keirstead et al., 1989; Houlé, 1991). A key factor is the presence of chondroitin sulfate proteoglycans (CSPG) (Busch and Silver, 2007; Galtrey and Fawcett, 2007) that are potent inhibitors of neurite outgrowth. Neutralizing CSPGs after SCI injury has been shown to enhance axon regeneration (Bradbury et al., 2002; Houlé et al., 2006; Massey et al., 2008) and improve functional recovery in rats (Caggiano et al., 2005; Fouad et al., 2005) and cats (Tester and Howland, 2008). In our previous work with rats we showed that combining a PNG with chondroitinase ABC (ChABC), a CSPG digesting enzyme, promotes axonal regeneration, growth beyond the distal end of the graft and formation of functional synapses across the lesion with some degree of motor recovery (Houlé et al., 2006; Tom and Houlé, 2008; Tom et al., 2009).
According to NSCISC, SCI individuals present most frequently an incomplete cervical injury (http://www.nscisc.uab.edu). The objective of this study was to address the clinical potential of a combined treatment of PNG and ChABC after an incomplete cervical SCI using a large animal model where the spinal cord and PNG are closer in size to humans. We show that this combined approach promotes axonal regeneration and synaptic activity across a spinal cord lesion site in the cat.
MATERIAL AND METHODS
Surgical procedures (spinal cord injury, ChABC treatment, peripheral nerve graft)
All procedures were performed in accordance with protocols approved by Drexel University College of Medicine Institutional Animal Care and Use Committees and followed National Institutes of Health guidelines for the care and use of laboratory animals.
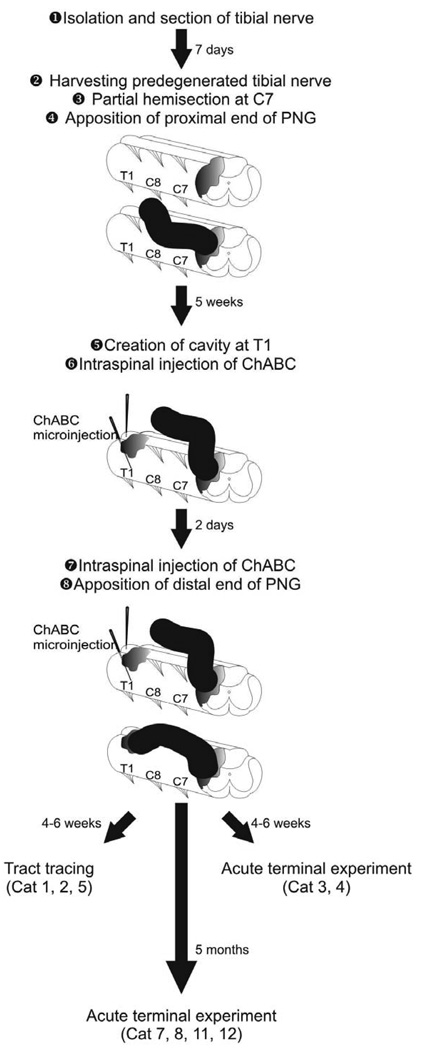
Twelve adult female cats (domestic short hair, 2.5–3.5 kg) underwent multiple surgical procedures under aseptic conditions according to the timeline presented in Figure 1 and as described below.
Figure 1.
Sequence and timeline for the surgical procedures by which a peripheral nerve graft is used to bridge a partial cervical hemisection.
Pre- and post-operative care
Protocols for pre and post-operative care were analogous to those described previously (Boyce et al., 2007). Briefly, anesthesia was initiated with ketamine (25 mg/kg, IM) in combination with atropine sulfate (0.05 mg/kg, IM) and inhaled isoflurane (5%). An endotracheal intubation was then performed and anesthesia subsequently maintained with isoflurane (1–3%). The cephalic vein was catheterized for intravenous administration of drugs and fluids (~20 ml/hr). Blood pressure, heart rate, end-tidal CO2, respiratory rate and body temperature were monitored and kept within physiological limits. Ampicillin (15 mg/kg) was administered intramuscularly 12 hrs prior to surgery, 1 hr prior to surgery and at the completion of the surgical procedure and subcutaneously every 12 hrs for 10 days post-operatively. Analgesia was provided by Buprenorphin (0.01 mg/kg, IM) prior to surgery and through a transdermal fentanyl patch (Duragesic, 25 µg/hr) (Franks et al., 2000) for continuous and stable delivery over a 72h period.
Isolation and section of the tibial nerve in the distal right hindlimb
The nerve was ligated and cut as it emerged between the medial gastrocnemius and flexor digitorum longus muscles in order to have a segment of ~4 cm available for harvesting 7 days later, after Wallerian degeneration of the cut axons occurred. Degeneration of the peripheral nerve before grafting is necessary as fresh cut nerves do not support ingrowth by a high number of regenerating axons. Autologous nerve grafts were used in this experiment to avoid immune suppression that would be necessary if a heterologous graft was used.
Creation of a partial cervical hemisection of the spinal cord and grafting of a segment of the PN into the lesion site
Seven days later, the predegenerated tibial nerve was dissected free and a ~4 cm segment removed and immersed in Hank’s Balanced Salt Solution on ice. The incision of the right hindlimb was then sutured. A laminectomy was performed at the C7 vertebral level and the dura opened to expose the dorsal surface of the spinal cord. Spinal cord injury was performed using microscissors to cut through the dorsal and lateral portions of the right side of the spinal cord. Using a fine glass micropipette with gentle aspiration, the dorsal-lateral funiculus and adjacent gray matter were removed to form a 2–3 mm long lesion cavity (Figure 1). Saline-soaked gel foam was used to achieve hemostasis. The perineurium was stripped from the proximal end of the tibial nerve segment and this end was apposed to the rostral lesion cavity wall. To secure the graft, the perineurium of the proximal end of the tibial nerve was sutured to the dura mater. The remaining length of the tibial segment remained extra-dural, lying alongside the lateral vertebral processes and enveloped in a PVA/PVP (polyvinyl alcohol/poly-N-vinylpyrrolidone) film to isolate the graft from surrounding connective tissue. The distal graft end was left unopposed to spinal cord tissue so that regenerating axons would have time to extend through the graft and be in a growth phase when apposed to the distal lesion site. We allowed 5 weeks for axons to grow through the PNG to reach the distal end.
Creation of a lesion cavity as a site for apposition of the distal end of the PNG
Five weeks later, the nerve graft was identified within its synthetic film and reflected from the surgical field. A laminectomy was performed at the T1 vertebral level to expose the spinal cord (Figure 1). A cavity of approximately 3 mm wide and 5–6 mm deep was created by aspiration in the right dorsal quadrant of the T1 spinal cord. Hemostasis was achieved with saline-soaked gel foam. To initiate degradation of inhibitory components (chondroitin sulfate proteoglycans, CSPGs) of the extracellular matrix that form around the injury site, 5µl of chondroitinase (ChABC, 20 U/ml, Seikagaku America, Falmouth, MA) was injected into the spinal cord ventral to the lesion cavity and another 5 µl into the spinal cord 1–2 mm rostral and 1–2 mm caudal to the lesion cavity using a glass micropipette attached to a Hamilton syringe. The depth of microinjection was approximately 5 mm for these latter areas. Dura mater was sutured and the PNG placed on top of the dorsal surface of the spinal cord and adjacent vertebral processes.
Apposition of the distal end of the PNG
Because of temporally restricted enzymatic activity of ChABC a second set of intraspinal microinjections of ChABC was provided 2 days after the initial exposure, as described above. This treatment strategy was designed to digest CSPGs that had formed after creation of the distal cavity, as determined from experimental work with this approach in injured rats (Tom and Houle, 2008). The perineurium from the distal end of the PNG was stripped and the PNG placed into the lesion cavity in apposition to the ventral floor. The distal graft end was secured by suturing the perineurium to the dura. This procedure completed the bridging across the lesion site and provided an opportunity for axons to exit the nerve graft and grow back into the spinal cord.
Tract tracing
Four to six weeks after bridging the SCI, 3 cats underwent a tract tracing procedure. The PNG was carefully exposed and sectioned at its midpoint. Gel foam saturated with True Blue (TB, 2%, Sigma, St. Louis, MO) was applied to the cut end of the proximal nerve stump for 60 min to label neurons that had grown their axon into the graft (retrograde transport). The cut end of the distal nerve stump was covered with gel foam saturated with fluorescein dextran (Fluoro-Emerald, FE, 10%, Molecular Probes, Eugene, OR) for 60 minutes to label axons growing through the distal graft back into the spinal cord. The graft was wrapped by a PVA/PVP film and covered by suturing overlying muscles and skin. These animals were euthanized 5–7 days later.
Acute electrophysiological experiment
To assess conductivity through the PNG, a terminal electrophysiological experiment was performed ~6 wks (n=2) or 5 months (n=4) after bridging the SCI. The PNG and 20 mm of spinal cord rostral and caudal to the graft apposition sites were exposed. The animal was then transferred to a stereotaxic frame and a pool of warm mineral oil formed with skin flaps surrounding the exposed spinal cord and PNG to prevent desiccation and current spread during stimulation.
Stimulation and recordings
In 1 cat (#11), electrical stimulation was delivered into the spinal cord (1000, 2000 and 3000 µm deep) approximately 15 mm rostral of the proximal end of the PNG and 1/3 way between midline and the right dorsal roots. Stimulation (10 s train, 10–400 µA, 100 µs pulse duration, 2.0 Hz) was delivered using a single sharp platinum iridium electrode (FHC, Bowdoinham, ME). Electrical activity being conducted through the PNG was recorded using a hook electrode. The distance between the stimulating and recording electrodes was about 25 mm.
In 2 cats (#7,8), two Teflon-coated wire electrodes with 3 mm exposed stainless steel tips (AS633, Cooner Wire, Chatsworth, CA) were inserted through the skin into the belly of the triceps muscle using a 25-gauge hypodermic needle. The PNG was directly stimulated using a bipolar hook electrode (2 s train, 0.5–1.0 mA, 1 ms pulse duration, 10 Hz) and EMG activity of the triceps recorded, as well as the forearm monitored for visible contractions in any muscle. In 4 cats (#3,4,8,12), the PNG was stimulated with a hook electrode with a 2–3 mm interelectrode distance (FHC) while recordings were done in the spinal cord caudal to the PNG re-entry using 16 channel electrode (Neuronexus, Ann Arbor, MI). This arrangement yielded a recording region with a height of 400 mm and width of 500 mm. The arrays were mounted on a custom electrode holder driven by a manual linear micromanipulator (M-633, Physik Instrumente, Germany). In 2 cats (#11,12), the graft was stimulated for 1h (1 mA, 100 µs pulse duration, 50 Hz) at the end of the terminal experiment. A common electrode was inserted in the axial musculature to serve as a ground. Intraspinal and EMG signals were amplified, bandpass filtered and sampled on a computer (10–40 kHz). Data acquisition was controlled by customized software (Labview, National Instruments, Austin, TX). These animals were perfused 1 hour later and spinal cord tissue was processed for immunocytochemical detection of c-Fos in neurons distal to the nerve graft as an indicator of synaptic activation during electrical stimulation of the graft (see below).
Histology and Immunocytochemistry
Animals were anaesthetized with an overdose of pentobarbital (390 mg/kg, IP) and transcardially perfused with 4% paraformaldehyde.
Animals undergoing tract tracing procedures
Blocks of brainstem and spinal cord tissue surrounding the distal apposition site were harvested, immersed in 30% sucrose for cryoprotection and sectioned on a cryostat for detection of TB-labeled neurons or FE-labeled axons respectively. For animals with the graft labeled with TB, transverse sections (50µm) through the medulla and pons were prepared and mounted in serial order on glass slides and cover slipped with Vectashield hard set mounting medium for fluorescence (Vector Laboratories, Burlingame, CA). Sections were imaged using a Zeiss Axioskop microscope to examine the presence of retrogradely labeled brain stem neurons. For animals with a FE labeled graft, four sets of alternating transverse sections (30 µm) through the proximal and distal nerve apposition sites were collected. One set was mounted on glass slides and coverslipped with Vectashield hard set mounting medium. The second set of sections was processed for immunocytochemical detection of medium size neurofilaments (NF) using a rabbit primary antibody (1:400, Millipore) and a fluorescein-tagged goat anti-rabbit secondary antibody (1:500, Sigma). Sections were rinsed, mounted on glass slides and cover slipped with Vectashield hard set mounting medium.
The fluorescent microscope was used to examine the first set of sections for the presence of labeled axons distal to the graft-host interface. Using MetaView software (Molecular Devices, Downingtown, PA) a line was drawn between the distal graft end and the apposed spinal cord. A counting program was used to quantitate the number of axons observed to cross this interface. The mean number of axons from 3 sections per cat was computed. For a general qualitative indication of axon growth into the nerve grafts, sections in the second set were examined microscopically. Quantitation of graft axons was performed in semi-thin sections described in the next section. The third set of sections was mounted on glass slides, processed for Nissl-myelin staining and examined microscopically to determine the positioning of the proximal and distal ends of the grafts and the extent of graft-host apposition. The fourth set of sections was kept in reserve for use as needed.
Animals undergoing a terminal electrophysiological experiment
Blocks of the distal apposition site in thoracic spinal cord were harvested and three sets of alternating transverse sections (30 µm) through the distal nerve apposition site were collected. The first set was processed for immunocytochemical detection of NF (as above) and the second set was processed for detection of c-Fos by incubating with the primary antibody (1:10,000, Sigma) for 24h at room temperature followed by 24h at 4°C. Goat anti-rabbit IgG was used as the secondary antibody and a diaminobenzidine (DAB, Sigma) reaction was carried out for colorimetric detection. MetaView software was used to set threshold for the density of cFos immunoreactive (ir) spinal motoneurons contralateral to the lesion-graft site (present because these cats are actively walking around the caged area). The program counted number of cFos-ir neurons on the uninjured side of spinal cord separate from the number on the lesioned/grafted side and we compared the mean number per side per 3 sections per cat. Blocks of PNG from the rostral apposition end and from the graft midpoint were collected for analysis of the number of axons within the graft.
Blocks were processed for embedding in Epon and 1 µm transverse sections were cut, mounted on glass slides and stained with Toluidine Blue (see Tom et al., 2009). Using a MetaView counting program the number of myelinated axons in 3 sections from each portion (proximal and middle) of each graft was counted under a light microscope. The mean number of axons per section was figured and comparison between the different regions was made.
The third set of sections was kept in reserve for use as needed.
RESULTS
Graft survival and apposition, growth into graft
Table 1 provides an overview of the outcome measures applied to all 12 cats involved in this study. There was an 83% (10/12) success rate for survival of the nerve graft with healthy grafts being well vascularized (Figure 2B) and with a full, rounded appearance. The success rate for good apposition of both proximal and distal ends being secured into the spinal cord was 90% (9/10 healthy grafts). Graft-host apposition was confirmed further by examination of histological sections (Figure 3). There was 1 cat that died during the electrophysiological testing procedure but we deemed the graft to have a healthy appearance (i.e. well vascularized with a white exterior) and to be apposed to the spinal cord at both rostral and caudal ends. Functional apposition in this cat was well supported by EMG signals recorded in the triceps when stimulating the peripheral nerve graft (detailed below, see Fig. 6a). Of the 8 animals with good grafts that survived to the end of the experiment we obtained axon tracing data from 3 cats and electrophysiological data from 5 cats. Graft stimulation for the test of synaptic activation and subsequent immunoreaction for c-Fos was performed for 3/5 of these latter cats. Neurofilament immunoreactivity was applied to sections through the graft for all 8 of these animals.
Table 1.
Overview of the outcome measures for each animal
| Cat # | Distal graft apposition |
Electrophysiology | FE | TB | c-Fos | NF | Nissl Stain |
Axon count |
|---|---|---|---|---|---|---|---|---|
| 1 | Good | − | + | + | − | + | + | − |
| 2 | Good | − | + | + | − | + | + | − |
| 3 | Good | + | − | − | − | + | + | + |
| 4 | Good | + | − | − | − | + | + | + |
| 5 | Good | − | + | − | − | + | + | − |
| 6 | NA | |||||||
| 7 | Good | + | − | − | − | − | − | − |
| 8 | Good | + | − | − | − | + | − | − |
| 9 | Poor | |||||||
| 10 | NA | |||||||
| 11 | Good | + | − | − | + | + | + | + |
| 12 | Good | + | − | − | + | + | + | + |
Overview of the outcome measures performed on the 12 cats involved in this study. First column, the apposition of the distal end of the PNG was successful in 9/10 cats. Cat #6 and #10 had a necrotic PNG due to poor rostral apposition, so the distal end of the PNG was not apposed. The distal end of the PNG was poorly apposed for cat #9. Therefore no further testing was performed on these 3 animals. +, indicates that the experiment/staining procedure was performed; −, indicates no data collection. FE, Fluoro-emerald; TB, True blue; NF, neurofilament immunocytochemistry.
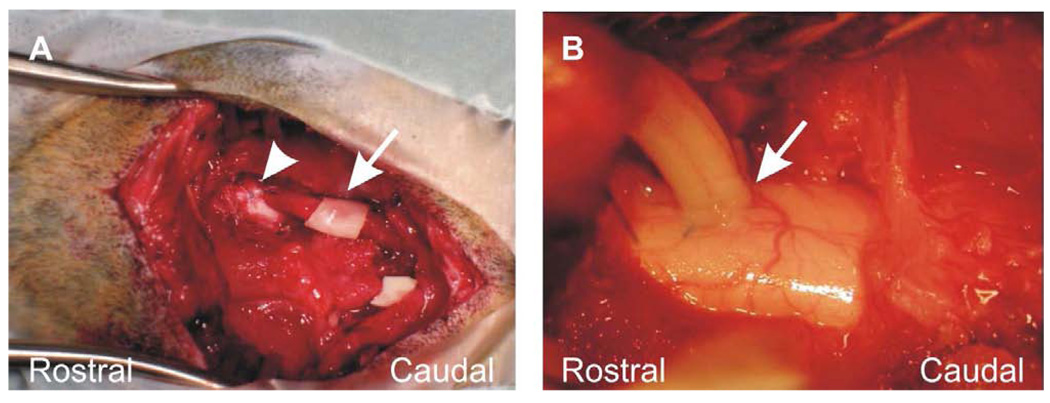
Figure 2.
Apposition of the peripheral nerve graft. (A) Rostral (arrowhead) and caudal ends are apposed to the spinal cord. Protective PVA/PVP film (arrow) was placed around the mid portion of the graft. The piece of PVA/PVP film at the lower right of the field is not associated with the graft. (B) Demonstration of the distal apposition (arrow) of the PNG to the right side of the upper thoracic cat spinal cord.
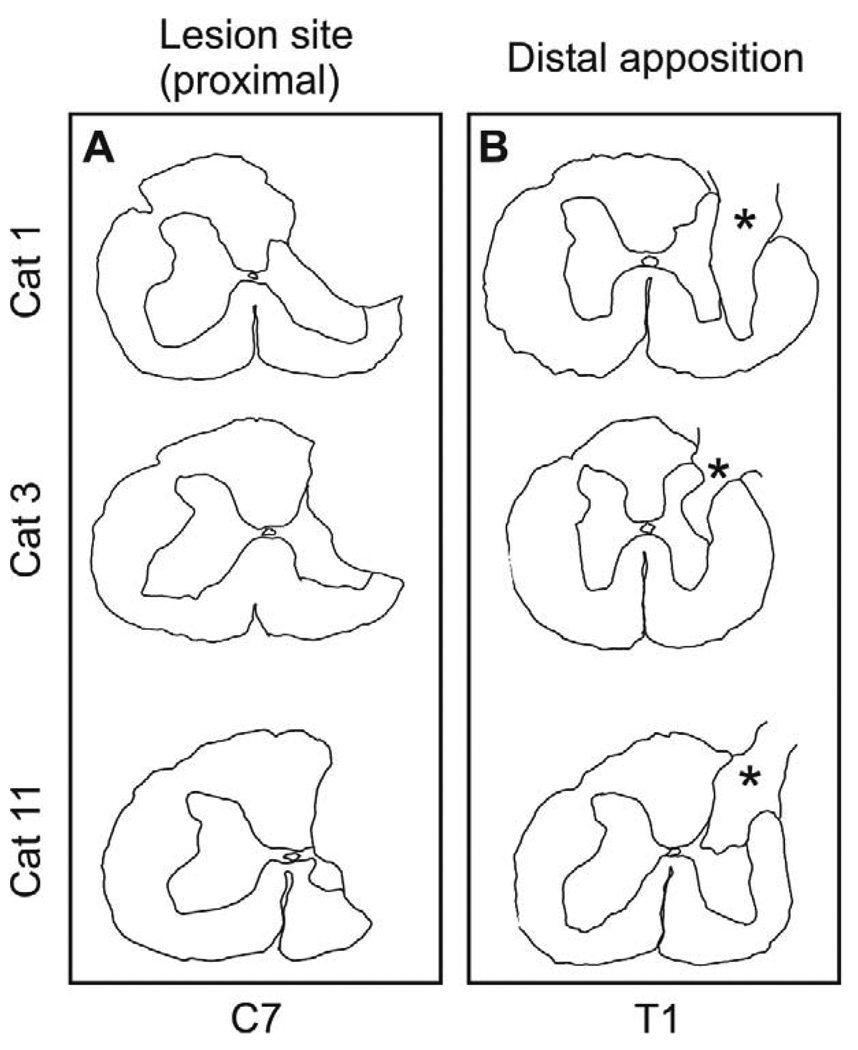
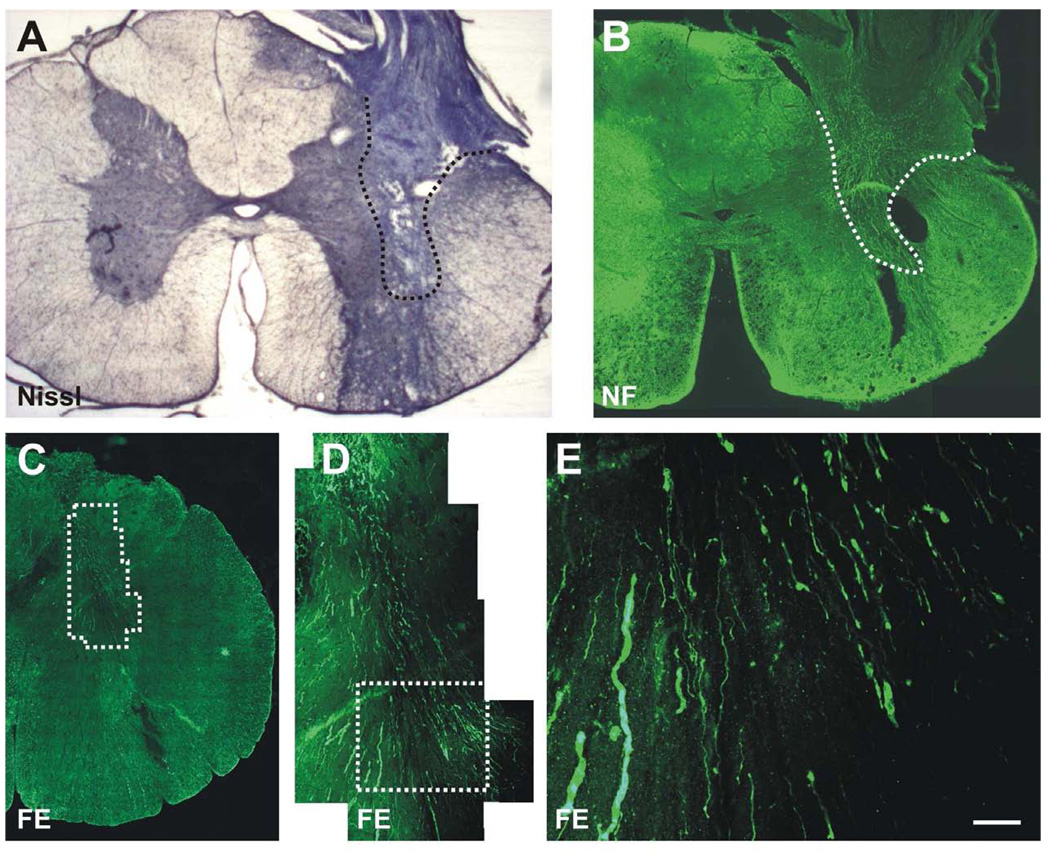
Figure 3.
Proximal and distal apposition sites for PNG. (A) The proximal, partial cervical hemisection encompassed most of the dorsal funiculus, the lateral funiculus, the dorsal horn and a small portion of the intermediate gray. (B) The distal apposition site at the thoracic level indicated that the graft (asterisk) filled a dorsal quadrant lesion cavity that included a portion of the dorsolateral funiculus and the dorsal horn-intermediate gray.
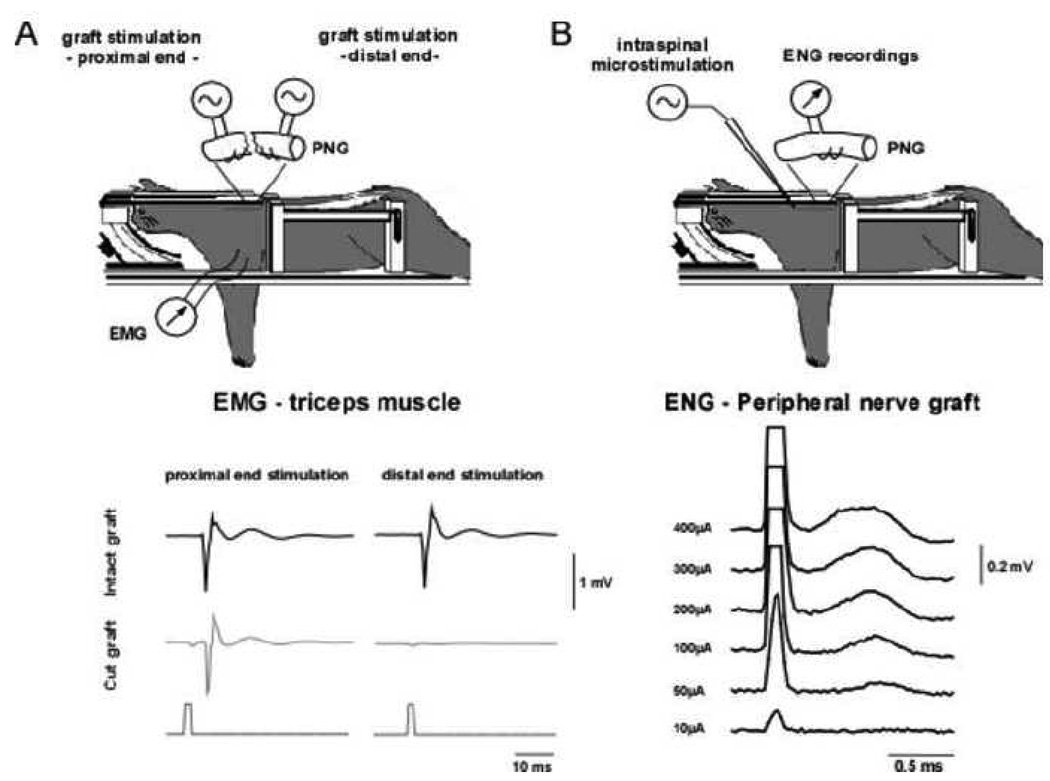
Figure 6.
Axonal conduction in the peripheral nerve graft. (A) Experimental set-up and EMG activity recorded in the triceps muscle with stimulation delivered to the PNG. Black traces show recordings obtained with the PNG intact, while traces in gray show recordings after cutting through the graft midsection. (B) Experimental set-up and recordings obtained with electrical stimulation delivered intraspinally at a depth of 1000µm in the lateral grey matter, 2.5 cm rostral to the PNG (100µsec duration pulse). The PNG was mounted on a bipolar hook electrode and recordings were obtained at increasing stimulation levels. A compound action potential obtained at about 50µA threshold, with a latency of 0.5ms increased in size with increased in stimulation amplitude.
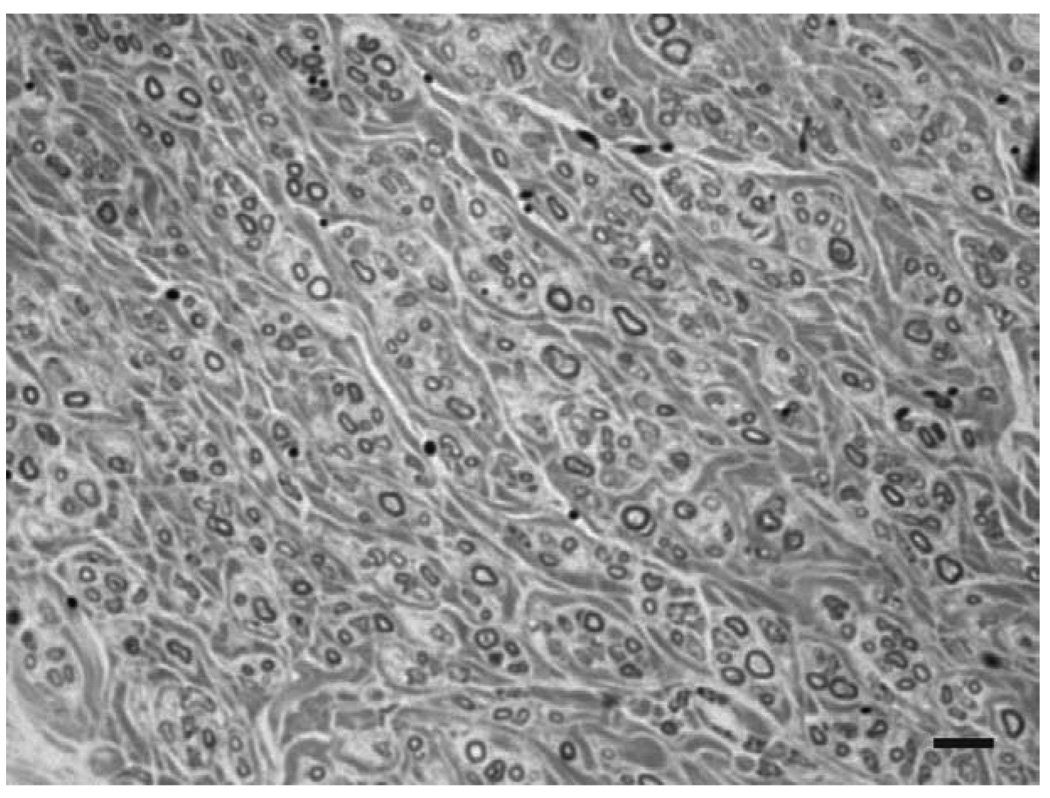
The initial injury to the spinal cord encompassed most of the dorsal funiculus, the lateral funiculus, the dorsal horn and a small portion of the intermediate gray (Fig. 3a). With this extent of damage, animals demonstrated a clenched forepaw, little movement of the forelimb and no weight bearing immediately after injury. There was gradual improvement in forelimb use with weight supported stepping by 7–10 days and only modest deficits at 3–4 weeks after injury when cats walked on a treadmill. This level of spontaneous recovery prior to placement of the distal end of the graft precluded the use of kinematics during locomotor episodes as an indication of functional recovery. Examination of the distal apposition site indicated that the grafts filled a dorsal quadrant lesion cavity that included a portion of the dorsolateral funiculus and a portion of the dorsal horn-intermediate gray (Figure 3b). At both rostral and caudal sites the graft was in contact with spinal cord tissue and only occasional small cysts were observed at the point of apposition. Spinal cord tissue surrounding the graft exhibited healthy with neurons present directly adjacent to the grafts (Fig. 4a, b). There was no obvious pathology in the spinal cord between the proximal and distal apposition sites aside from myelin degeneration resulting from disruption of spinal pathways in the dorsal and lateral funiculi. The morphology of spinal cord tissue contralateral to the lesion was unremarkable. All PNGs were filled with NF+ fibers that reached the distal graft apposition site (Fig. 4b). The extent of re-entry of these fibers into the host spinal cord was uncertain as the host spinal cord also contains NF+ fibers and there was mixing of immunostained fibers at the interface. Semi thin sections through the midpoint of grafts demonstrated myelinated axons spread throughout (Fig. 5). It was not possible to discern unmyelinated axons by the staining techniques used, but large and small caliber axons appeared as single fibers or were grouped in small fascicles separated by bundles of collagen. The mean number of myelinated axons in 4 grafts was 2476 ± 1254, with a range of 1343 to 4234.
Figure 4.
Distal apposition site of the PNG. (A) Apposition of the graft with gray and white matter regions of the host spinal cord is evident. Nissl stain. (B) Axonal growth is throughout the dorsal to ventral extent of the graft. Neurofilament (NF) immunocytochemistry. (C) Transverse section demonstrating the apposition of the PNG to the spinal cord. (D–E) Higher magnification of the boxed areas with Fluoro-Emerald (FE) labeled axons projecting from the PNG into the dorsal and intermediate gray of the spinal cord. FE was applied to the mid-point of the PNG 2 days prior to sacrifice. Scale bar for E is 50µm.
Figure 5.
Myelinated axons that regenerated into a peripheral nerve graft. Transverse section through the midpoint of a PNG processed for semi thin sectioning, with numerous myelinated axons of small and large caliber are scattered throughout this small portion of the graft. Scale bar, 10µm.
Regenerated axons extend beyond a peripheral nerve bridge
To determine if axons present in the PNG extended beyond the graft into the spinal cord, we labeled regenerated axons by diffusion fill with Fluoro-Emerald (FE). FE+ axons were present in the distal portion of the PNG as well as in the gray matter ventral to the graft (Fig. 4c, d). The mean number of axons per section crossing the graft-host interface was 11, 37 and 39 for the three FE labeled cats. These results suggest that regenerated axons from the PNG re-enter the spinal cord since the only source of labeled axons was through the PNG itself. TB was used to attempt to identify neurons that contributed an axon to the PNG but we were not able to detect TB retrogradely labeled neurons in the brainstem or cerebral cortex. An occasional TB labeled neuron was found in the cervical spinal cord close to the lesion site but this was rare. We interpret this absence of long distance retrograde transport as a technical problem since we have been successful using a similar protocol in rats. Perhaps the PNG was not in contact with TB for a long enough period or the 1 week post labeling period was insufficient to allow transport over a long distance.
Signal transmission into the graft
We performed different procedures to assess axonal conduction into and through the PNG. We first stimulated the PNG to assess possible transmission of signal back into the host spinal cord and ensure that the stimulation was restricted to the axons present in the graft. We performed recordings either in the region adjacent to the distal apposition of the PNG or in the triceps muscle ipsilateral to the lesion. We failed to induce any response in the spinal cord of 4 cats, but could elicit an EMG response in the triceps muscle in one out of 2 cats (Fig. 6a). The EMG response was consistent with a 4 ms delay post-stimulation. As a control test, the graft was cut and either the proximal end or the distal end of the graft was stimulated separately. The EMG response was maintained when stimulating the proximal end of the graft but disappeared when stimulating the distal end. These results suggest that activation of the triceps occurred via antidromic activation of axons that had grown into the PNG but not necessarily out of the graft.
In the third experiment, electrical activity in the PNG was recorded after stimulation of the spinal cord 15mm upstream of the proximal insertion of the PNG. The stimulating electrode was placed into lateral gray matter ipsilateral to the lesion, at a depth of 1000µm, 2000µm or 3000µm. The response was maximal at a depth of 1000µm (Fig. 6b). Hence, a compound action potential with a very short latency of 0.3ms (interelectrode distance 25mm) could be observed with a ~50µA threshold. The response increased in size with increased stimulation amplitude.
Simulation of a PNG induces cFos expression in the spinal cord caudal to a lesion site
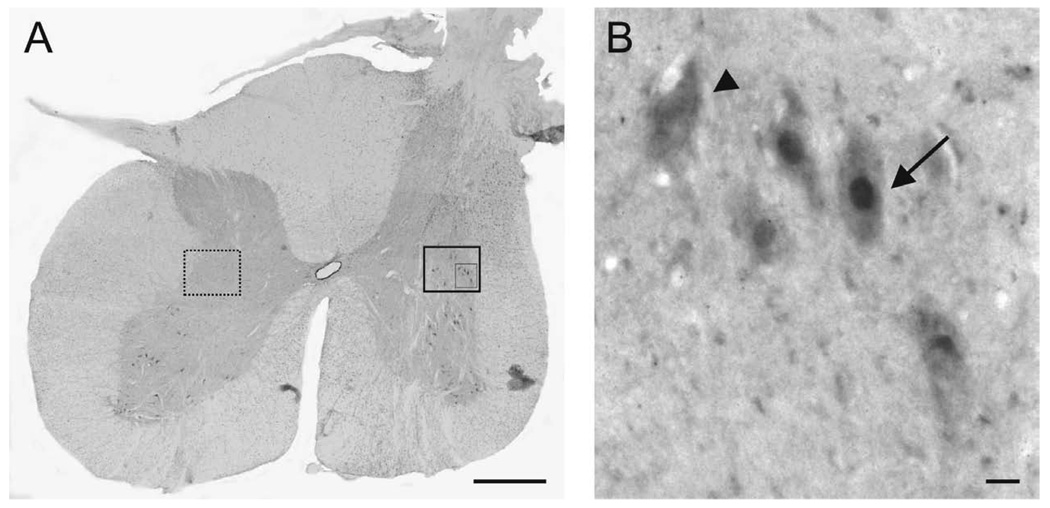
To assess whether axons that exit the PNG make functional synapses with neurons of the host spinal cord, we stimulated the PNG for 1h and sacrificed the animals 1h later for detection of c-Fos-ir neurons. The immediate early gene cfos is rapidly expressed in neurons in response to stimulation (Morgan and Curran, 1991) and cFos-ir is a marker of transynaptic neuronal activation (Luckman et al., 1994). This approach has been used widely as an indicator of neuronal excitation in the cat and rat spinal cord (Ahn et al., 2006; Ichiyama et al., 2008; Noga et al., 2009). We found cFos-ir neurons in the intermediate gray adjacent to the PNG-spinal cord interface (Figure 7) indicating synaptic activation through connections made with regenerated axons. No cFos-ir neurons were present in comparable regions of the uninjured, contralateral spinal cord. In these same sections cFos-ir motoneurons also were present in the ventral horn on both sides of the spinal cord. This is consistent with the near absence of functional deficit in these animals. The mean number of cFos-ir neurons on the injured side was significantly higher (Students t test) compared to the uninjured, ungrafted side (24±4.4 vs. 15.1±3.2 (p<0.002).
Figure 7.
Induction of c-Fos in host neurons after stimulation of the PNG. (A) Representative transverse section of the spinal cord from a cat at the distal apposition site for the PNG. c-Fos-ir neurons (large boxed area) are located close to the distal apposition site in the intermediate gray, but no c-Fos-ir neurons are present in a comparable area (dashed box) on the contralateral side. Scale bar, 1mm. (B) Higher magnification of the inset boxed area. Arrow: c-Fos-ir neuron. Arrowhead: neuron not expressing c-Fos. Scale bar, 10µm.
DISCUSSION
Several promising therapies for SCI that have emerged from the laboratory since the late 1960s have entered into prospective randomized clinical trials, but it is disappointing that few have had convincing success leading to a significant gain for SCI individuals (Tator, 2006). Maximizing the chances of success prior to translation into expensive and lengthy clinical trials is critical. The importance of the type of injury model used, reproducibility of positive results, and incorporation of large animal studies into SCI research has been emphasized recently (Blesch and Tuszynski, 2009). A recent survey undertaken by the SCI research community demonstrated unequivocally that the efficacy of treatments in large animal models (e.g. cat/dog/rabbit/sheep) and concentration on cervical injury models will best provide preclinical evidence that is necessary before moving forward with human SCI trials (Kwon et al., 2009). In the present study, we used our extensive experience with rat SCI and nerve grafting (Houlé, 1991; Houlé et al., 2006; Tom et al., 2009) to advance to a cat model of incomplete cervical SCI. We addressed the potential of a combined treatment, PNG and proteoglycan digestion with ChABC, in higher mammals to provide evidence of axon regeneration and functional connectivity, as an essential pre-clinical step towards future human application. The cat’s size allowed us to assess the potential for regeneration in a spinal cord model closer in size to humans, as the mean length of the feline spinal cord is 34 cm (Blinkov and Glezer, 1968) versus 40–45 cm in humans (Perese and Fracasso, 1959). Not only were there several thousand axons within most grafts but we provide evidence of axonal growth beyond the distal graft back into the spinal cord and the presence of c-Fos immunoreactive neurons that are indicative of synaptic activity during electrical stimulation of the graft. These results indicate the tremendous opportunity provided by a peripheral nerve grafting approach to support and direct axon regeneration across a spinal cord injury site.
Axonal Regeneration in a large animal model
The limited success of repair strategies after SCI can be attributed to several factors, including a poor intrinsic regenerative capacity of CNS neurons (Neumann and Woolf, 1999), inhibitory factors associated with CNS myelin (Filbin, 2003; Schwab, 2004) and glial scar-associated inhibitors such as chondroitin sulfate proteoglycans (CSPGs) (Silver and Miller, 2004). The presence of Schwann cells in the PNG provides a more permissive environment that modifies the cellular response of axotomized supraspinal neurons (Storer et al., 2002) and enhances growth cone motility, possibly due to the release of multiple neurotrophic factors and the presence of laminin sheaths surrounding the Schwann cells (Bunge et al., 1990; Lacroix and Tuszynski, 2000; Oudega et al., 2005). Our anatomical data indicate myelination of a great number of axons in each graft and electrophysiological data indicates fast conduction through the graft, again a measure of the degree of myelination of regenerated axons (Radtke and Vogt, 2009). This is one of the main advantages to using a PNG to bridge the lesion compared to other cellular or tissue grafts that do not have the cells necessary to form myelin. It is well established that adequate signal propagation and motor recovery depend on myelin integrity (Keirstead et al., 2005; Arvanian et al., 2009). Although axon regeneration into a PNG is relatively successful, the number of axons that leave the distal end is small and likely limited by inhibitory properties of the glial scar. Treatment with ChABC, a bacterial enzyme that digests glycosaminoglycan (GAG) side chains disrupts the inhibitory activity of CSPGs and renders the environment more permissive to extension of regrowing axons (Bradbury et al., 2002; Chau et al., 2004; Fouad et al., 2005). ChABC treatment also promotes axonal sprouting (Barritt et al., 2006), neuroplasticity (Cafferty et al., 2008) and neuroprotection (Carter et al., 2008). In the rat, the combination of PNG and ChABC successfully promotes regeneration by axotomized neurons with formation of functional synapses that facilitate return of functional activity (Houlé et al., 2006; Tom and Houlé, 2008; Tom et al., 2009). We show here that thousands of axons regenerate into an autologous PNG apposed to the rostral wall of a cat cervical SCI and extend for distances close to 40mm. Many axons appear to extend beyond the graft back into the spinal cord distal to the injury and we provide evidence for some functional connectivity (see below). Cat PNGs appear less densely filled by regenerated axons relative to what is seen in rats: the mean number of myelinated axons/graft in the rat is 3953 ± 737 with ChABC and 2741±677 without ChABC (Tom et al., 2009) whereas the average was 2476 ± 1254 in the cat. With a larger graft diameter, we expected a higher number of axons to grow into the cat PNGs, so these results indicate a need to improve apposition procedures.
Propriospinal activation of the PNG and spinal host neurons
Electrophysiological experiments showed that a compound action potential can be recorded from the graft following intraspinal stimulation rostral to the PNG, indicating the presence of “conductive” axons in the bridge. It is not known if axons which have grown in the graft belong to neurons in close proximity (propriospinal) to the graft or if they are of brainstem origin. Our attempts at retrogradely labeling neurons contributing to the PNG were unsuccessful, even though a similar technique is routine for our comparable rat experiments. The activation of the triceps muscle by stimulation of the PNG confirms that regenerated axons in the PNG effectively conduct a signal although in this situation antidromic activation of neurons located rostral to the injury is the probable route of transmission. We failed to elicit any intraspinal recordable activity after stimulation of the PNG; but c-Fos activated cells observed caudal to the distal apposition of the PNG demonstrate the presence of functional synapses formed by axons originating from the bridge and re-entering the cord on the distal side, similar to what has been shown in rats (Tom et al., 2009).
Importantly, we illustrate the potential for regeneration of axons and the formation of functional synapses in a large animal model. Although we have modest evidence for conduction of signal back into the spinal cord, a small amount of spared or rescued connections has the potential to provide a significant functional improvement (Blight and DeCrescito, 1986; Kloos et al., 2005). Directing axonal regeneration to appropriate targets is challenging, and regenerated pathways may not regain their original function. There is evidence that regenerated axons will synapse on new targets in spinal networks whose configuration has been modified to provide maximal functional benefits (Bareyre et al., 2004; Courtine et al., 2008). Whether rhythmic activity of paralyzed limbs has a role in enhancing regeneration past the graft-spinal cord interface will be the center of study for future experiments and the cat injury-graft model lends itself well to the evaluation of training on recovery of function (Barbeau and Rossignol, 1987; Bélanger et al., 1996; de Leon et al., 1998).
Approaches to increase axonal growth from a PNG
Therapies aiming at promoting regeneration are potentially promising, but the modest improvement in functional outcomes in our rat experiments (Tom et al., 2009) suggest that further support will be required to optimize anatomical and functional recovery. Combinations of therapies that will address the heterogeneity of the processes triggered by the injury are needed to achieve a meaningful recovery of function (Thuret et al., 2006; Schwab et al., 2006). Exercise has been shown to improve locomotor recovery after SCI (de Leon et al., 1998; Harkema, 2001; Dietz, 2008). Activity-dependent processes triggered by exercise include an increase in neurotrophin expression in the brain, spinal cord and muscles (Dupont-Versteegden et al., 2004; Vaynman and Gomez-Pinilla, 2005) that helps define the level of motor recovery after SCI (Ying et al., 2008). We are currently testing whether forced exercise of the impaired forelimb (Sandrow-Feinberg et al., 2009) will lead to improved functional recovery in rats that receive a PNG and ChABC treatment.
In our cats rapid and spontaneous recuperation of forelimb movement before the distal end of the graft had been apposed excluded any motor testing as an indicator of functional improvement. Apparently the severity of injury was not sufficient to provide a stable deficit for long term study. The better situation would be to have a more severe injury to replicate more realistically the severity of most human injuries (Blesch and Tuszynski, 2009) and involve a longer lasting deficit that can take advantage of training and kinematic analysis. Detailed kinematic analysis and EMG recordings are standard procedures in adult cats and the benefits of step-training on a treadmill are well established (Barbeau and Rossignol, 1987; Bélanger et al., 1996; de Leon et al., 1998; Côté et al., 2003; Côté and Gossard, 2004). All of these features provided the motivation to extend our studies into this large animal model and will be used in future studies incorporating treadmill training for SCI cats.
Given the difficulty of providing therapeutic treatment to individuals in the early hours-days after injury, repair of the spinal cord at the chronic state is critical and there are encouraging results with different combination approaches that demonstrated the potential for repair in rats long after SCI has occurred (Tom et al., 2009; Kadoya et al., 2009). One of the next challenges will be to advance our success with acute PNGs into a chronic SCI model for cats.
Acknowledgements
We are grateful to Dr. Noelle Comolli (Villanova University) for providing biomaterials used for nerve graft protection. This study was supported by NIH grants NS 26380 (JDH) and NS 007440 (KOL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aguayo AJ. Axonal regeneration from injured neurons in the adult mammalian central nervous system. In: Cotman CW, editor. Synaptic Plasticity. New York: Guilford Press; 1985. pp. 457–484. [Google Scholar]
- Ahn SN, Guu JJ, Tobin AJ, Edgerton VR, Tillakaratne NJ. Use of c-fos to identify activity-dependent spinal neurons after stepping in intact adult rats. Spinal Cord. 2006;44:547–559. doi: 10.1038/sj.sc.3101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanian VL, Schnell L, Lou L, Golshani R, Hunanyan A, Ghosh A, Pearse DD, Robinson JK, Schwab ME, Fawcett JW, Mendell LM. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp Neurol. 2009;216:471–480. doi: 10.1016/j.expneurol.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Blight AR, DeCrescito V. Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience. 1986;19:321–341. doi: 10.1016/0306-4522(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Blinkov SM, Glezer II. The human brain in figures and tables: a quantitative handbook. New York: Plenum Press; 1968. [Google Scholar]
- Boyce VS, Tumolo M, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31:262–269. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Clark MB, Dean AC, Eldridge CF, Bunge RP. Schwann cell function depends upon axonal signals and basal lamina components. Ann N Y Acad Sci. 1990;580:281–287. doi: 10.1111/j.1749-6632.1990.tb17937.x. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, McMahon SB. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28:11998–12009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- Carter DA, Bray GM, Aguayo AJ. Regenerated retinal ganglion cell axons can form well-differentiated synapses in the superior colliculus of adult hamsters. J Neurosci. 1989;9:4042–4050. doi: 10.1523/JNEUROSCI.09-11-04042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci. 2008;28:14107–14120. doi: 10.1523/JNEUROSCI.2217-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Côté M-P, Gossard J-P. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M-P, Ménard A, Gossard J-P. Spinal cats on the treadmill: changes in load pathways. J Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–440. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Dietz V. Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull. 2008;76:459–463. doi: 10.1016/j.brainresbull.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Houlé JD, Dennis RA, Zhang J, Knox M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle & Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks JN, Boothe HW, Taylor L, Geller S, Carroll GL, Cracas V, Boothe DM. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onychectomy. J Am Vet Med Assoc. 2000;217:1013–1020. doi: 10.2460/javma.2000.217.1013. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Houlé JD. Demonstration of the potential for chronically injured neurons to regenerate axons into intraspinal peripheral nerve grafts. Exp Neurol. 1991;113:1–9. doi: 10.1016/0014-4886(91)90139-4. [DOI] [PubMed] [Google Scholar]
- Houlé JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182:247–260. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Houlé JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den BR, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M. Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science. 1989;246:255–257. doi: 10.1126/science.2799387. [DOI] [PubMed] [Google Scholar]
- Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Hillyer J, Tetzlaff W. Translational Research in Spinal Cord Injury: A Survey of Opinion from the SCI Community. J Neurotrauma. 2010;27:21–33. doi: 10.1089/neu.2009.1048. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Tuszynski MH. Neurotrophic factors and gene therapy in spinal cord injury. Neurorehab Neural Repair. 2000;14:265–275. doi: 10.1177/154596830001400403. [DOI] [PubMed] [Google Scholar]
- Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209:426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Ann Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Noga BR, Johnson DM, Riesgo MI, Pinzon A. Locomotor-activated neurons of the cat. I. Serotonergic innervation and co-localization of 5-HT7, 5-HT2A, and 5-HT1A receptors in the thoraco-lumbar spinal cord. J Neurophysiol. 2009;102:1560–1576. doi: 10.1152/jn.91179.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Moon LD, de Almeida Leme RJ. Schwann cells for spinal cord repair. Braz J Med Biol Res. 2005;38:825–835. doi: 10.1590/s0100-879x2005000600003. [DOI] [PubMed] [Google Scholar]
- Perese DM, Fracasso JE. Anatomical considerations in surgery of the spinal cord: a study of vessels and measurements of the cord. J Neurosurg. 1959;16:314–325. doi: 10.3171/jns.1959.16.3.0314. [DOI] [PubMed] [Google Scholar]
- Radtke C, Vogt PM. Peripheral nerve regeneration: a current perspective. Eplasty. 2009;9:e47. [PMC free article] [PubMed] [Google Scholar]
- Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houlé JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma. 2009;26:721–731. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Muller HW. Experimental strategies to promote axonal regeneration after traumatic central nervous system injury. Prog Neurobiol. 1998;56:119–148. doi: 10.1016/s0301-0082(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Storer PD, Houlé JD, Oblinger M, Jones KJ. Combination of gonadal steroid treatment and peripheral nerve grafting results in a peripheral motoneuron-like pattern of BII-tubulin mRNA expression in axotomized hamster rubrospinal motoneurons. J Comp Neurol. 2002;449:364–373. doi: 10.1002/cne.10304. [DOI] [PubMed] [Google Scholar]
- Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Howland DR. Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp Neurol. 2008;209:483–496. doi: 10.1016/j.expneurol.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Houlé JD. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol. 2008;211:315–319. doi: 10.1016/j.expneurol.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houlé JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehab Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M, Bray GM, Villegas-Perez MP, Thanos S, Aguayo AJ. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987;7:2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]