Abstract
N-acyl phosphatidylethanolamines (NAPEs) are synthesized in response to stress in a variety of organisms from bacteria to man. More recently, non-enzymatic modification of the ethanolamine headgroup of PE by various aldehydes including levuglandin/isoketals, which are γ-ketoaldehydes (γKAs) derived from arachidonic acid, has also been demonstrated. The levels of these various N-modified PEs formed during stress and their biological significance remain to be fully characterized. Such studies require an accurate, facile, and cost-effective method for quantifying N-modified PEs. Previously, NAPE and some of the non-enzymatically N-modified PE species have been quantified by mass spectrometry after hydrolysis to their constituent N-acylethanolamine by enzymatic hydrolysis, most typically with S. chromofuscus phospholipase D (PLD). However, enzymatic hydrolysis is not cost-effective for routine analysis of large number of samples and hydrolytic efficiency may vary for different N-modified PEs, making quantitation more difficult. We therefore sought a robust and inexpensive chemical hydrolysis approach. Methylamine (CH3NH2) mediated deacylation has previously been used in headgroup analysis of phosphatidylinositol phosphates. We therefore developed an accurate assay for NAPEs and γKA-PEs using CH3NH2 mediated deacylation and quantitation of the resulting glycerophospho-N-modified ethanolamines by LC/MS/MS.
Keywords: phosphatidylethanolamine, N-acyl ethanolamine, lipid peroxidation, aldehydes, lipid adducts, levuglandins, isoketals, LC/MS/MS
INTRODUCTION
Biosynthesis of N-acyl phosphatidylethanolamines (NAPEs) in response to stressors has been reported in a wide variety of organisms including bacteria [1; 2], yeast [3], plants [4; 5; 6; 7], and mammals [8; 9; 10; 11] (Fig 1). NAPEs are well-established precursors of biologically active N-acylethanolamides (NAEs), including C20:4NAE (anandamide) and C18:1NAE (oleoylethanolamine). Recent work has also raised the possibility that NAPEs are themselves biologically active mediators of protective responses to stressors [12; 13]. Increased levels of NAPEs or their metabolites reduce glutamate excitotoxicity, ischemic damage, inflammation, hunger and food intake [12; 14].
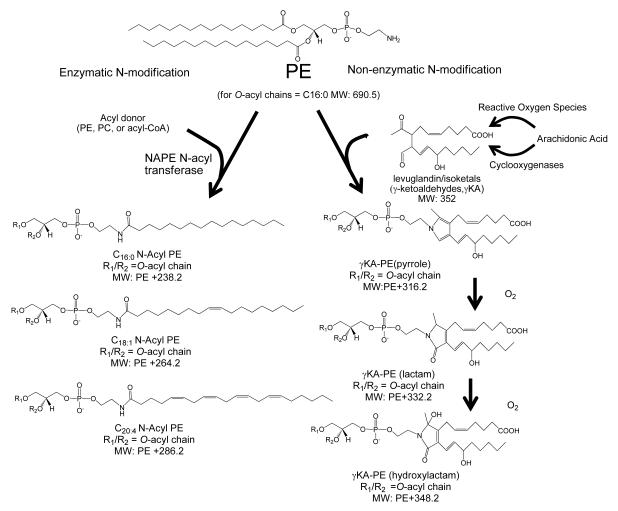
Figure 1.
Formation of N-modified phosphatidylethanolamines by enzymatic and non-enzymatic pathways in response to stressors. Phosphatidylethanolamine (PE) is enzymatically N-acylated to form N-acyl PE (NAPE) by NAPE- specific acyltransferases (NAPE-AT) in a variety of organisms in response to stressors. In mammals, these stressors include ischemia reperfusion and glutamate excitotoxicity. PE can also be modified non-enzymatically by lipid aldehydes produced by peroxidation of polyunsaturated fatty acids during free radical stress. For example, peroxidation of arachidonic acid forms γ-ketoaldehydes (γKAs), given the trivial name of levuglandins or isoketals, that react with PE to form stable N-modified PE pyrrole adducts. In the presence of oxygen, these pyrrole adducts are converted to lactam and hydroxylactam adducts.
In addition to enzymatic N-acyl modification of PEs, non-enzymatic N-modification of PE can occur by lipid aldehydes generated during lipid peroxidation. These lipid aldehydes include γ-ketoaldehydes derived from arachidonic acid (γKAs) [15; 16; 17] (Figure 1), as well as α,β-unsaturated carbonyls such as 4-hydroxynonenal and acrolein [18; 19; 20]. The γKAs form as non-enzymatic rearrangement products of bicyclic endoperoxides generated from arachidonic acid (AA) either by the action of cyclooxygenases (levuglandins) [21] or non-enzymatically via free radical mediated mechanisms (isoketals)[22; 23]. Free radical mediated peroxidation of phospholipid-esterified arachidonic acid generates γKA adducted to adjacent amines while still esterified [24]. The initial stable reaction product of γKAs with amines are pyrrole adducts [25]; however, in the presence of molecular oxygen and oxidizing conditions, the pyrrole adducts mature into lactam and hydroxylactam adducts[23] (Fig 1). Although very little is known about the non-enzymatically N-modified PEs, elevated levels of γKA modified PE (γKA-PE), particularly the hydroxylactam adducts, were recently found in plasma of patients with age-related macular degeneration as well as in the liver of ethanol-fed mice [16]. Furthermore, exposure of cultured endothelial cells to γKA-PE induced cell death, suggesting that γKA-PEs are biologically active [17]. Thus, the effects of γKA-PE appear to oppose those of NAPEs, so that the relative balance of these two classes of N-modified PEs may be important determinants of health and disease. To study the roles of N-modified PE in stress responses, an accurate, facile, and cost-effective method to measure both NAPEs and γKA-PEs simultaneously is needed.
NAPEs have been previously directly measured by two methods: TLC [26] and mass spectrometry [2; 26; 27; 28; 29]. While TLC is readily available and inexpensive, it lacks specificity for individual N-acyl chains and is less sensitive and more difficult to accurately quantify than mass spectrometry methods. Mass spectrometry, particularly LC/MS/MS, has several advantages. Not only can internal standards be added to ensure that variations in extraction efficiency do not affect measured levels, but chromatography can be used to separate NAPE from other phospholipids and the exact species of NAPE can be identified by its measured mass. However, accurate quantitation of NAPEs by LC/MS/MS requires measurement of more than 50 individual masses because each NAPE contains two O-acyl chains in addition to its N-acyl chain, each of which can potentially differ in chain length and unsaturation,. Hydrolysis of NAPE to their constituent NAE using either S. chromofuscus PLD or mammalian NAPE-PLD has been employed as an alternative to measuring each individual NAPE because this procedure removes the differences in mass related to the O-acyl chains [3; 10; 12]. These methods still allow differentiation between the various species of N-acyl chains, but greatly simplify the number of total masses that must be quantified. Monitoring of N-acyl chain species is important because C20:4NAE, clearly differs in biological activity from C18:1NAE, so that relative levels of the individual precursors may be important. We have also employed hydrolysis by S. chromofuscus PLD to measure γKA-PE [17]. Unfortunately, S. chromofuscus PLD is expensive to use because relatively large amounts are required to ensure complete hydrolysis of all NAPE and γKA-PE in the sample. Whether S. chromofuscus PLD can hydrolyze other non-enzymatically N-modified PEs such as HNE-PE is also unknown. Although NAPE-PLD is an efficient lipase of NAPEs, NAPE-PLD is not commercially available and whether it can utilize γKA-PEs as a substrate is unknown. Therefore, alternative strategies are needed for a facile and inexpensive method to quantify N-modified PEs.
One potential low cost alternative to phospholipases is chemical deacylation. A four hour incubation with sodium hydroxide has been used to convert NAPEs to their constituent glycerophosphate-N-acylethanolamines (GP-NAEs)[30]. This method hydrolyzes O-acyl chains but not N-acyl chains. However, sodium hydroxide mediated deacylation requires significant additional sample clean-up to remove the excess sodium salt prior to LC/MS/MS analysis. Furthermore, we have observed that γKA adducts appear to be somewhat labile during incubation with strong acids and bases for extended periods, so we sought a milder deacylation reagent that would also be sufficiently volatile to use directly in LC/MS/MS. Deacylation mediated by methylamine (CH3NH2) has been employed for headgroup analysis of phosphatidyl inositols [31]. CH3NH2 serves as both the base and the acyl acceptor, preventing secondary reactions other than deacylation [32]. Furthermore, CH3NH2 is volatile, so that hydrolyzed samples can potentially be directly injected into LC/MS/MS without further preparation. Finally, because CH3NH2 mediates deacylation via transesterification, the deacylation product of esterified γKA adducts is expected to be a methylamide with a mass 13 amu greater than the deacylation product of non-esterified γKA adducts, making it possible to distinguish the two γKA-PE products in the mass spectrometer. Therefore, we tested whether CH3NH2 mediated deacylation could be used for quantitation of both NAPEs and γKA-PEs. We found that deacylation of NAPEs and γKA-PEs with CH3NH2 and subsequent measurement of the resulting glycerophosphates (GPs) by LC/MS/MS was a facile and cost-effective method to accurately quantify NAPE and γKA-PE levels in biological samples.
MATERIALS and METHODS
Materials
1,2 dioleoyl-sn-glycero-3-phosphoethanolamine-N-arachidonoyl ammonium salt (C20:4NAPE), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (C32:0PE), and porcine brain PE were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Palmitoyl chloride, heptadecanoyl chloride, stearoyl chloride, and CH3NH2 (40 wt. % in water) were purchased from Sigma-Aldrich (St. Louis, MO). Organic solvents including methanol, chloroform, dichloromethane and acetonitrile were HPLC grade. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. E. coli C41 cells transformed with either empty vector (C41-Vec) or with the expression vector for Arabidopsis thaliana NAPE N-acyltransferase, (C41pDEST), were a kind gift from Denis Coulon and Lionel Faure from Université Victor Segalen Bordeaux 2. Sep-Pak silica cartridges was purchased from the Waters Corporation (Milford, MA)
Synthesis of NAPEs
The synthesis of NAPEs was adapted from [12] with a modified purification method. In summary, to a 50 ml flask cooled in an ice-water bath, 0.208 gram (0.3 mmol) of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) was suspended in 20 ml chloroform containing 0.1 g triethylamine with a magnetic stirring bar. An appropriate acyl chloride (1.1 mmol) in 7 ml chloroform was added in a dropwise fashion. After the addition was complete, the reaction mixture was heated to 40°C for 2 h and allowed to stir overnight at room temperature. The reaction was quenched by adding saturated NaHCO3 solution (10 mL) and the organic layer was collected and washed with 30 mL of 0.01 M HCl and 30 mL of brine. After dehydrated over anhydrous Na2SO4, the product was dried with a rotary evaporator and purified using silica gel chromatography (methanol/ethyl acetate/ ammonium hydroxide 20:80:2). The purified NAPE was weighed and the reaction yields 65-80%. The purified NAPE was analyzed by TLC using silica plates and chloroform/methanol/ethanol/ethyl acetate/0.25% KCl (10:4:10:10:3.6) as solvent. Plates were visualized with phosphomolybdic acid.
Synthesis of γKA-PE and esterified γKA-PE
γKA (15-E2-isoketal, MW 352) and methyl ester γKA (methyl ester 15-E2-isoketal, MW 366) were prepared as previously described by organic synthesis [33]. The structure of 15-E2-isoketal is shown in Figure 1. The synthesis of γKA-PE (or esterified γKA-PE) was performed with slight modification from our previously described procedure [17]. DPPE and γKA (or methyl ester of γKA) were suspended in a mixture of 1 M triethylammonium acetate/chloroform/ethanol (1:1:3) with a final concentration of 0.25 mM and 0.083 mM for DPPE and γKA, respectively. After incubation at 37°C overnight, the pyrrole formation of the product was identified by LC-MS analysis and quantified by Ehrlich’s reagent with the absorbance at 580 nm measured by spectrometer.
Bacterial and mammalian cell culture
C41-Vec and C41pDEST were cultured according to [4] with the following modifications to produce optimal amount of NAPEs. After overnight growth in LB medium with 0.1 mg/mL ampicillin at 37 °C at 250 rpm, cultures were diluted (1:50) and continued at 37 °C at 250 rpm until they reached an optical density (A600) of 0.5-0.6. Then isopropyl β-D-thiogalactoside (1 mM) was added to induce the expression and the culture was allowed to grow overnight at 30 °C at 200 rpm. After measurement of the optical density, the cells were harvested by centrifugation at 5000 g for 15 min and the pellet was resuspended in 500 uL of water.
Human umbilical vein endothelial cells (HUVEC; passage 6) were cultured in 100 mm dishes coated with 0.2% gelatin for 4 to 6 days, allowing to grow to >90% confluence. Plates were washed three times with HBSS and then duplicate plates were incubated with 5mL of vehicle (0.1% DMSO in HBSS), synthetic γKA (1 μM in HBSS with 0.1% DMSO), or arachidonic acid (AA, 1 mM in HBSS with 0.1% DMSO) at 37 °C for 4 h. The treatment medium was carefully removed after incubation and 2 mL HBSS was added to the cells on ice. Cells were scraped and collected with the wash media into clean centrifuge tubes.
Isolation and deacylation of N-modified PEs
The overall work flow for analysis of N-modified PE is shown in Figure 2. The bacterial pellet from a 25 ml culture of bacterial cells or human plasma (200 uL) were mixed with 3 mL pre-chilled Folch solution (chloroform/methanol, 2:1 containing 0.01% BHT), followed with addition of 1 nmol C17:0 NAPE (10uL in CHCl3) and 1 mL of water. For HUVEC cells, 6 ml of Folch solution and 1 nmol C17:0 NAPE were added to the 2 ml of cell solution. The mixture was vortexed for 5 min and centrifuged at 4000 g for 5 min. The resulting lower (chloroform) layer was carefully collected and dried under nitrogen. The extracted lipids were reconstituted in 1 mL chloroform and loaded onto a Sep-Pak silica cartridge preequilibrated with chloroform. The cartridge was washed with 8 mL chloroform and N-modified PEs were eluted by 8 mL 50% methanol/chloroform. The lipids were dried under N2 and saved at −20 °C before methylamine hydrolysis.
Figure 2.
Schematic of work-flow for analysis of N-modified PE by LC/MS/MS using CH3NH2 mediated deacylation.
Methylamine hydrolysis was adapted from those previously described for phosphoinositides [31]. Dried lipid extracts were dissolved in 200 μL methylamine reagent (CH3NH2 (40 wt.%)/CH3OH/1-butanol (4:4:1)) and mixed well. In the initial experiments, the reaction was incubated at 53°C for 0-2 h and aliquots removed at appropriate time points for TLC analysis and mass spectrometry. TLC was performed using silica plates and chloroform/methanol/ethanol/ethyl acetate/0.25% KCl (10:4:10:10:3.6) as solvent. Subsequently, all reactions were incubated at 53°C for 1 h and the resulting solution was injected for MS analysis.
Mass spectrometry analysis
Samples were analyzed on a ThermoFinnigan Quantum electrospay ionization triple quadrapole mass spectrometer operating in negative ion mode, equipped with Surveyor autosampler. Volume of injection was 5 uL. The mobile phase consisted of solvent A, 10mM t-butyl ammonium acetate in water/acetonitrile (96:4), and solvent B, 10mM t-butyl ammonium acetate in water/acetonitrile (6:94). The lipids were chromatographed on a Kinetex C18 column (50 mm × 2.10 mm, 2.6 um, 100 Å; Phenomenex, Torrance, CA) with a constant flow rate of 250 uL/min. After 0.5min hold at 1% solvent B, the solvent was gradient ramped to 99% B over 4 minutes, held at 99% B for 1.5 min and return to 1%B over 1 min and held for 2 min before the next injection. The electrospray needle was maintained at −3300 V. The ion-transfer tube was operated at −35 V and 270 °C. The tube lens voltage was set to −180 V. Product ion spectra were generated by collision-induced decomposition of the precursor ion (m/z 500.3 for C20:4 GP-NAE, m/z 530.3 for GP-γKA-Etn and m/z 543.3 for GP-γKA- MA). Only the precursor ion was allowed to pass through the first quadrupole; the ion was activated by collision with argon in the second quadrupole (10 eV; argon 1.5 mTorr). Product ion spectra were recorded for m/z 50 to 500 over 1 s. The mass spectral resolution of both quadrapoles was set to a peak width of 0.7 μm (full width at half maximum).
When the mass spectrometer was operated in the multiple reaction monitoring (MRM) mode, mass transitions with the product ion of m/z 79.1 at the specified parent ion (Table 1) at a collision energy of 50 eV were monitored. The chromatographic results were processed in Xcaliber software (ThermoFinnigan) using 11-point Gaussian smoothing. Linear regression and correlation analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
Table 1.
Multiple Reaction Monitoring Parameters
| Starting species | Species measured after CH3NH2 hydrolysis |
Precursor ion |
Product ion |
|---|---|---|---|
| C16:1NAPE | C16:1GP-NAE | 450.30 | 79.1 |
| C16:0NAPE | C16:0GP-NAE | 452.30 | 79.1 |
| C18:2NAPE | C18:2GP-NAE | 476.30 | 79.1 |
| C18:1NAPE | C18:1GP-NAE | 478.30 | 79.1 |
| C18:0NAPE | C18:0GP-NAE | 480.30 | 79.1 |
| C20:4NAPE | C20:4GP-NAE | 500.30 | 79.1 |
| γKA-PE (pyrrole) | GP-γKA-Etn (pyrrole) | 530.30 | 79.1 |
| γKA-PE (lactam) | GP-γKA-Etn (lactam) | 546.30 | 79.1 |
| γKA-PE (lactam-OH) | GP-γKA-Etn (lactam-OH) | 562.30 | 79.1 |
| Esterified γKA-PE (pyrrole) | GP-γKA-MA (pyrrole) | 543.30 | 79.1 |
| Esterified γKA-PE (lactam) | GP-γKA-MA (lactam) | 559.30 | 79.1 |
| Esterified γKA-PE (lactam-OH) | GP-γKA-MA (lactam-OH) | 575.30 | 79.1 |
Sensitivity studies
Various amount (0.2 pmol to 20 nmol) of the synthetic N-modified PE (C20:4NAPE, γKA-PE or methyl ester γKA-PE) were added to 200 μl PBS. C17:0NAPE (1 nmol) was added as the internal standard. After methylamine treatment, the samples were analyzed by LC/MS/MS using the appropriate MRM transitions. The amount of the synthetic compound was quantified as the ratio of peak height with the C17:0NAPE internal standard and plotted against the actual amount added.
RESULTS
Validation of methylamine-based MS assays for NAPEs
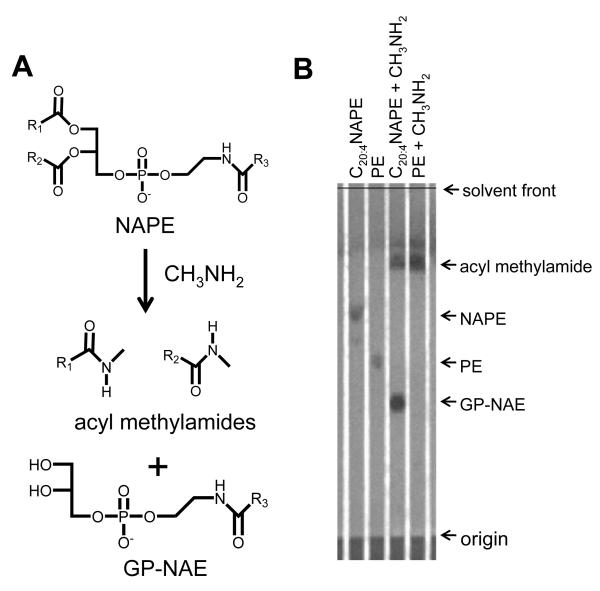
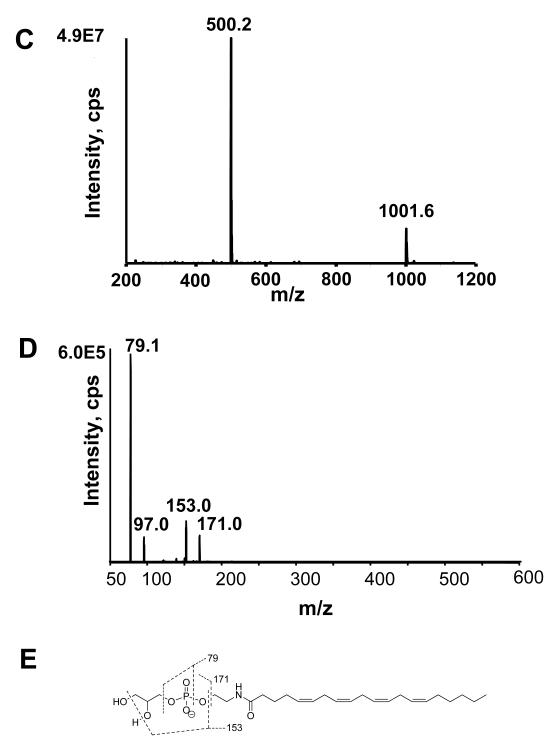
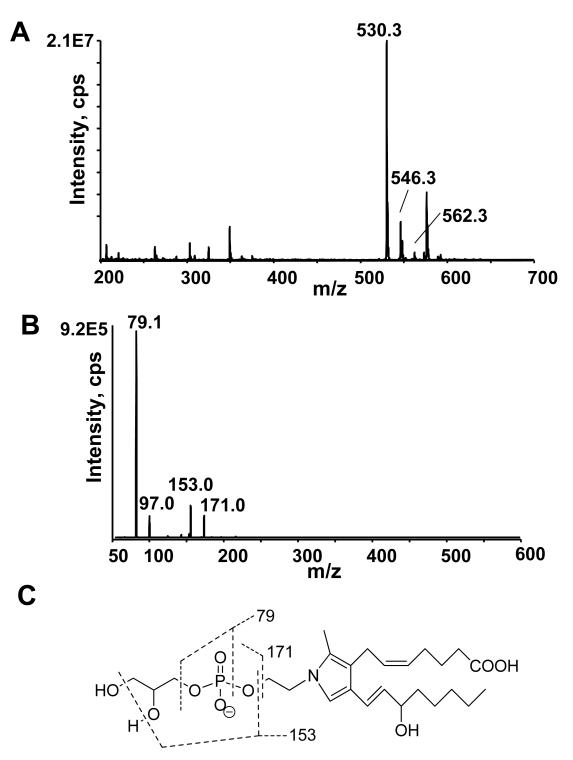
The efficacy of CH3NH2 mediated deacylation was analyzed using synthetic 2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-arachidonoyl (C20:4NAPE) which was expected to produce acyl methylamides and glycerol-phospho- N-C20:4 ethanolamine (Figure 3A). CH3NH2 mediated deacylation of 1-palmitoyl-2-oleoyl-sn-glycero-3-PE (PE) was as used a control. The reaction was monitored by TLC. C20:4NAPE and C34:1PE each migrated as a single spot (Rf 0.64 and 0.49, respectively) prior to CH3NH2 treatment. After 1 h incubation at 53 °C with CH3NH2, the starting NAPE was no longer visible and two new spots appeared with Rf 0.38 and 0.78 (Fig 3B). CH3NH2 treated PE also produced a new spot with Rf 0.78, consistent with this being the expected acyl methylamide products. Negative ion LC/MS analysis of the lower spot (Rf 0.38) primarily showed a peak at m/z 500.3, which is consistent with the expected glycerolphosphate C20:4N-acyl ethanolamine (C20:4GP-NAE) product (Fig 3C). The collision induced dissociation (CID) produced ions with m/z 79, 97, 153, and 171 (Figure 3D), which are consistent with a lipid phosphate compound (Figure 3E).
Figure 3.
Deacylation of NAPE to glycerophospho-N-acyl-ethanolamines (GP-NAE) by CH3NH2. A. Expected products after CH3NH2 mediated deacylation of NAPE with CH3NH2. Schematic of CH3NH2 reaction with NAPE B. TLC of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-arachidonoyl (C20:4NAPE) without CH3NH2 treatment (Rf 0.64); 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (PE) without CH3NH2 treatment (Rf 0.49); C20:4NAPE with CH3NH2 treatment to form C20:4GP-NAE (Rf 0.38) and oleoyl methylamide (Rf 0.78); and PE with CH3NH2 treatment to form oleoyl methylamide and palmitoyl methylamide (Rf 0.78) and glycerolphosphate. TLC solvent system was chloroform/methanol/ethanol/ethyl acetate/0.25% KCl (10:4:10:10:3.6). Plates were visualized with phosphomolybdic acid. C. Mass spectrum scan from negative ion limited scan of products from CH3NH2 hydrolysis of C20:4NAPE. The product with m/z 500 is consistent with C20:4GP-NAE. D. The collision induced disassociation (CID) spectrum of m/z 500. E. Interpretation of major product ions. Similar fragmentation ions were found for other synthetic GP-NAEs (C16:0GP-NAE, C17:0GP-NAE and C18:0GP-NAE).
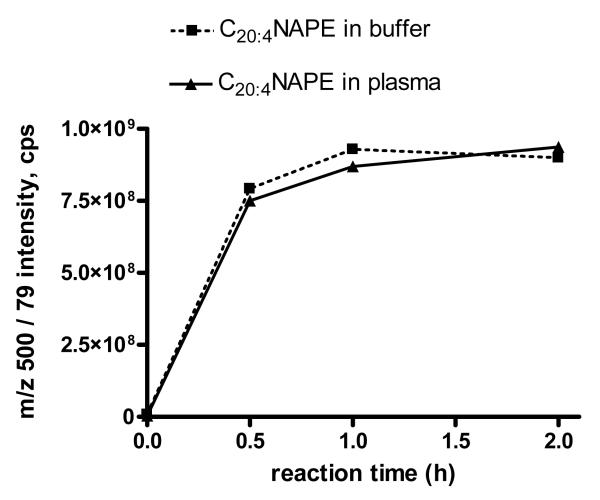
The time course for CH3NH2 deacylation of C20:4NAPE was further investigated. Plasma was used as a source of additional phospholipids that might compete for hydrolysis with C20:4NAPE. C20:4NAPE was spiked into buffer (PBS) or human plasma and the phospholipids extracted by chloroform/methanol (2:1) and then further isolated by a solid phase exchange (SPE) silica cartridge. After treating the lipids with CH3NH2 at 53 °C for 0-2 h, the amount of C20:4GP-NAE was monitored by LC/MS/MS using the MRM transition m/z 500.to 79.1. The C20:4GP-NAE signal maximized by 1 h, indicating the reaction had reached completion (Figure 4).
Figure 4.
Time course for CH3NH2 deacylation of C20:4 NAPE spiked in buffer and plasma. 20 nmoles C20:4NAPE was added to either 250 ul buffer or 250 ul plasma, the lipids extracted and treated with CH3NH2 for 0-2 hrs and the peak height for the MRM transition from m/z 500 to 79 measured.
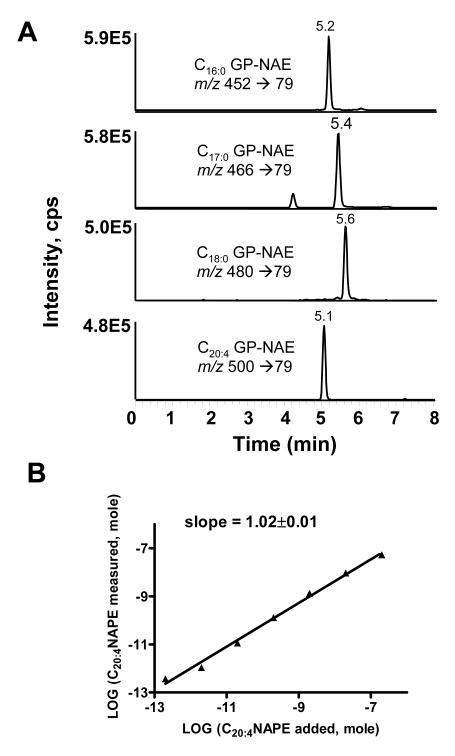
To determine if other NAPE species could be assayed in a similar manner, we synthesized C16:0, C17:0 and C18:0 NAPEs from their respective acyl chloride and DPPE. CH3NH2 treatment of C16:0, C17:0, and C18:0NAPEs gave products with ions with m/z 452, 466, and 480, respectively. CID spectrum of these products were very similar to that of C20:4 GP-NAE with the major product ion of m/z 79 (data not shown). C17:0NAPE has been previously used as an internal standard for measurement of endogenous NAPEs based on its similar ionization as endogenous NAPE species and its absence in most biological samples. To test whether deacylation and ionization efficiency was similar for all GP-NAEs, we deacylated and analyzed a solution containing equimolar concentration of each of the four NAPEs. Analysis of the solution by MRM using the m/z 79.1 product ion for each GP-NAE produced peaks with similar peak heights and areas (Figure 5A). The individual GP-NAE eluted separately, with increasing chain length correlating with increasing retention times for the saturated GP-NAEs. Because CH3NH2 hydrolysis could potentially generate isobaric species from some ether linked PEs (i.e. hydrolysis of C18:0 alkylacyl PE would form a lyso-alkylPE (m/z 466) that is isobaric to C17:0 GP-NAE,) we hydrolyzed a commercial preparation of brain PE containing ether linked PEs (alkenylacyl and alkylacyl PE) and analyzed them using our LC/MS/MS system. We found that lyso ether PEs eluted with retention times of 6.7-7.5 minutes, so that they do not interfere with the analysis of GP-NAEs (data not shown).
Figure 5.
LC/MS/MS assay for NAPEs. A. MRM chromatographs of synthetic NAPEs deacylated to their glycerophosphate N-acylethanolamines (GP-NAEs). 0.1 nmol of C16:0NAPE, C17:0NAPE, C18:0NAPE, and C20:4NAPE were treated with CH3NH2 for 1 h and injected on LC/MS/MS as described in the experimental methods. B. Correlation between amount of C20:4NAPE added to sample (buffer with 1nmol C17:0NAPE as internal standard) and C20:4NAPE measured using peak height for MRM of m/z 500 → 79 (C20:4GP-NAE) versus m/z 466 → 79 (C17:0GP-NAE). Limit of detection was less than 0.2 pmol C20:4NAPE in samples.
To determine the linearity and sensitivity of the assay, we analyzed 200 μl PBS solutions containing 0-20 nmol C20:4NAPE and 1 nmol C17:0NAPE as internal standard. The ratio of the resulting peak heights in the LC/MS/MS analysis were used to calculate the measured amount of C20:4NAPE. There was a highly linear correlation between the amount of C20:4NAPE added and the C20:4GP-NAE measured (Figure 5B). The limit of detection was 0.2 pmol in the sample.
Measurement of NAPEs in biological samples
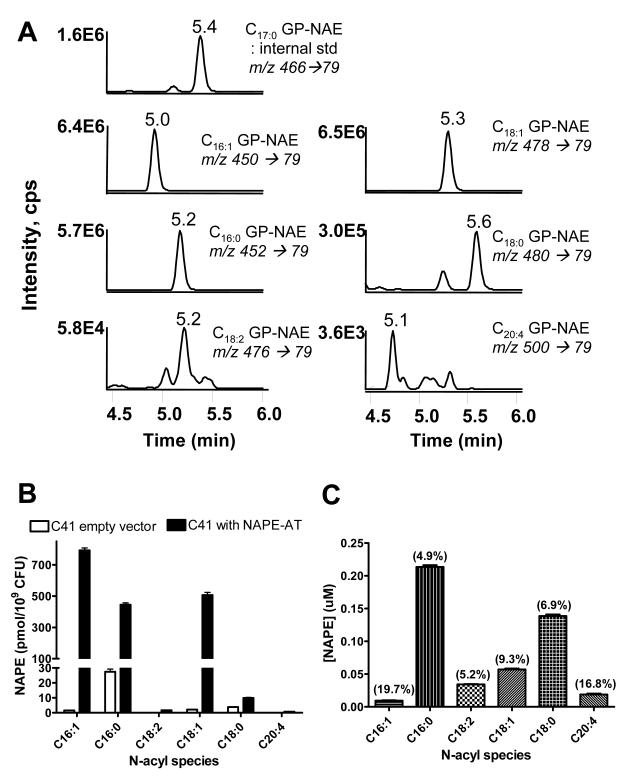
After confirming that C17:0NAPE was not present in common biological samples and could therefore be used as an internal standard, we analyzed NAPE in several biological samples. The Arabidopsis thaliana NAPE N-acyl transferase (NAPE-AT) was recently cloned and heterologously expressed in E. coli C41 cells resulting in the substantial levels of C16:0NAPE, C16:1NAPE, and C18:1NAPE. We therefore analyzed levels of NAPE in C41 cells transformed either with empty vector or with the expression vector for NAPE-AT. LC/MS/MS analysis of bacterial pellets from cells expressing NAPE-AT produced MRM chromatographs essentially identical to those produced from the analysis of synthetic NAPEs (Figure 6A). Monitoring of MRM for the predicted precursor ions for C16:1GP-NAE, C18:1GP-NAE, and C18:2GP-NAE also produced peaks with retention times consistent with what would be expected for unsaturated GP-NAEs. The two monounsaturated NAPEs were the most abundant NAPE in the NAPE-AT cells, followed by C16:0NAPE (Figure 6B). In contrast, E. coli C41 transformed with empty vector expressed very little NAPE and this was primarily C16:0NAPE.
Figure 6.
Measurement of NAPEs in biological samples. A. MRM chromatographs of various species of GP-NAEs derived by CH3NH2 hydrolysis of NAPEs extracted from C41 E. coli heterologously expressing Arabidopsis thaliana NAPE acyltransferase. B. Amount of each NAPE species expressed by C41 E. coli transformed with either empty vector or the NAPE-AT expression vector. C. Amount of each NAPE species in normal human plasma ((%CV) shown on each bar).
Circulating levels of NAPE change very modestly (~60%) in response to stimuli such as fat feeding[12], but even these modest changes may be biological significant. Therefore accurate detection of biologically significant variations in NAPE levels requires method with low intra-assay variability. To determine the intra-assay variability of our analysis method, we measured NAPE levels in five identical aliquots of human plasma. In human plasma, C16:0NAPE and C18:0NAPE were the most abundant NAPEs and were present at nanomolar concentrations (Figure 6C). The coefficient of variation for these two NAPEs were 4.9% and 6.9%, respectively, so that changes in NAPE levels in response to stressors should be readily detectable by our methods.
Validation of methylamine-based MS assays for γKA-PEs or esterified γKA-PEs
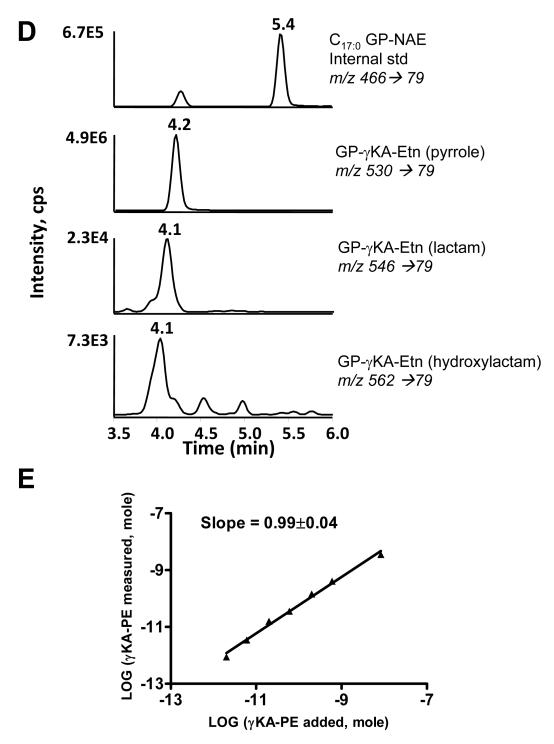
A major impetus in developing these methodologies was to enable the simultaneous measurement of both NAPE and non-enzymatically N-modified PEs including γKA-PE. We therefore synthesized γKA-PE and performed a similar analysis as with NAPEs. Deacylation by CH3NH2 of a preparation of γKA-PE containing primarily γKA-PE pyrrole adducts generated products ions with m/z 530.3, 546.3, and 562.3 when analyzed by negative ion mass spectrometry (Figure 7A). These ions are consistent with the formation glycerophospho-N-γKA-ethanolamines (GP-γKA-Etn) pyrrole, lactam and hydroxylactam (lactam-OH) species. The CID spectrum of the putative pyrrole product (m/z 530) showed a similar fragmentation pattern as those for GP-NAEs with a most dominant product ion of m/z 79 (Figure 7B). The similarity in fragmentation patterns suggests that most N-modified glycerophosphate ethanolamine could be monitored by use of the m/z 79 product ion (Figure 7C). CID spectrum of the lactam and lactam-OH adducts (m/z 546 and m/z 562, respectively) gave similar results (data not shown). When these samples were analyzed by the same LC/MS/MS system as for NAPEs and using MRM with transition from the appropriate precursor ion and the m/z 79 product ion, the GP-γKA-Etn adducts eluted substantially earlier than C17:0GP-NAE (Figure 7D). This is consistent with the greater polarity of GP-γKA-Etn compared to GP-NAEs. To determine the sensitivity of the assay for measurement of γKA-PE, we analyzed 200 μl PBS solutions containing 0-20 nmol γKA-PE and 1 nmol C17:0NAPE as internal standard. The ratio of the resulting peak heights for all three adducts and C17:0NAPE in the LC/MS/MS analysis were used to calculate the measured amount of total GP-γKA-Etn adduct. There was a highly linear correlation between the amount of γKA-PE added and the GP-γKA-Etn measured (Figure 7E). The limit of detection was 2 pmol in the sample.
Figure 7.
Hydrolysis of γKA-PEs to glycerophospho-N-γKA-ethanolamines (GP-γKA-Etn) by CH3NH2. A. Mass spectrum from negative ion scan of products from CH3NH2 hydrolysis of γKA-PE. The product with m/z 530, 546 and 562 are consistent with the pyrrole, lactam and hydroxylactam species of GP-γKA-Etn. B. The CID spectrum of the major product (pyrrole, m/z 530). CID spectrum of m/z 546 and of m/z 562 gave similar results. C. Interpretation of major product ions. Similar fragmentation ions were found for GP-γKA-Etn as those for GP-NAEs. D. MRM chromatographs of various forms of γKA-PE adducts hydrolyzed to their glycerophosphate γKA-ethanolamines (GP-γKA-Etn) products. 25 nmol of γKA-PE spiked with 0.5 nmol C17:0NAPE was treated for 1 h with CH3NH2 and injected on LC/MS/MS as described in the experimental methods. E. Correlation between γKA-PE added to sample (buffer with 1nmol C17:0NAPE as internal standard) and the sum total GP-γKA-Etn measured. Limit of detection was less than 2 pmol γKA-PE in the sample.
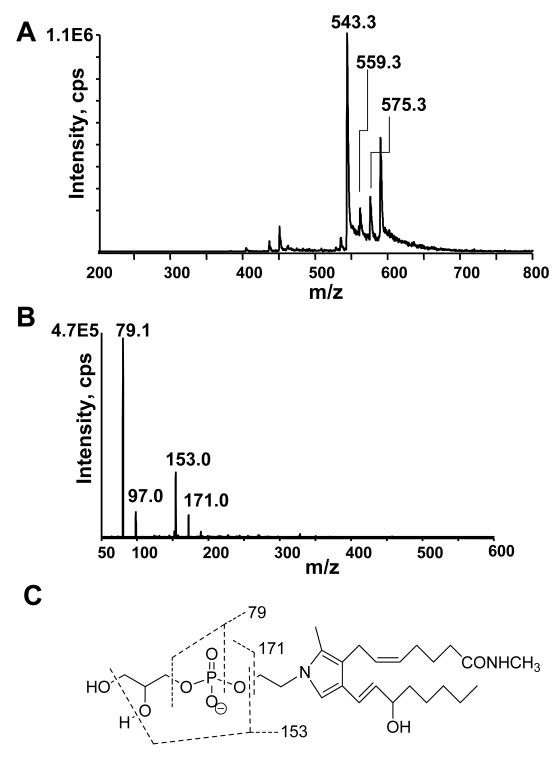
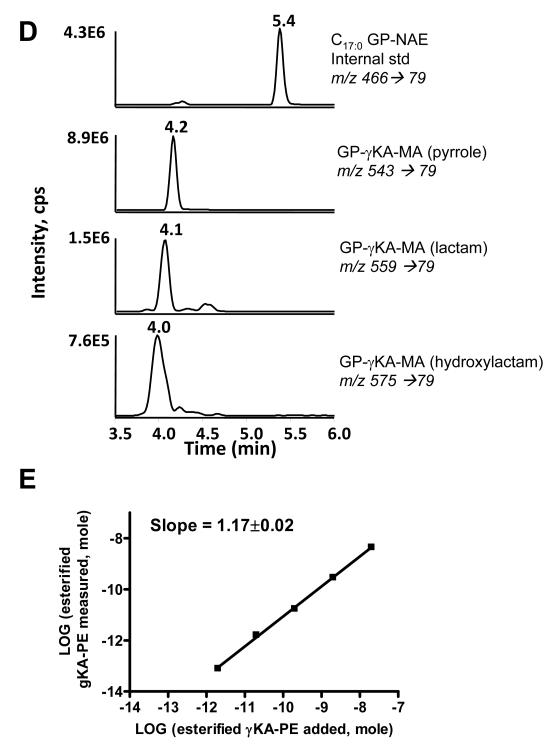
One potential advantage of CH3NH2 deacylation compared to standard base hydrolysis is that deacylation products of γKA-PE and esterified γKA-PE are predicted to differ by 13 amu, due to methyl amide formation at the γKA phospholipid ester. Thus the contribution of esterified γKA-PE versus non-esterified γKA-PE could be analyzed simultaneously. While the cyclooxygenase mediated pathway of γKA formation (i.e. levuglandins) occurs exclusively using non-esterified arachidonic acid, free radical mediated formation of γKA can occur while arachidonic acid is still esterified to phospholipids[24]. Therefore the formation of glycerophospho-N- γKA-ethanolamine methylamide (GP-γKA-MA) after CH3NH2 mediated deacylation should be a clear indicator of non-cyclooxygenase mediated γKA formation. To test whether CH3NH2 mediated deacylation results in formation of GP-γKA-MA, we reacted PE with the methyl ester γKA and then subjected the resulting product to CH3NH2 treatment. Mass spectrum from negative ion scan of CH3NH2 treated methyl ester γKA-PE resulted in ions with m/z 543.3, 559.3 and m/z 575.3, consistent with the pyrrole, lactam, and hydroxylactam species of GP-γKA-MA (Fig 8A). As with other GP species, CID generated primarily a product ion with m/z 79.1 (Fig 8B), as would be anticipated from the structure of this compound (Figure 8C). When analyzed by LC/MS/MS, the GP-γKA-MA eluted with similar retention times as GP-γKA-Etn (Figure 8D). When we analyzed solution containing 0-20 nmol methyl ester γKA-PE and 1 nmol C17:0NAPE as internal standard, we found a highly linear correlation between the amount of methyl ester γKA-PE added and the GP-γKA-MA measured (Figure 8E). However, the ionization efficiency of GP-γKA-MA appeared to be significantly less than that of NAPE because the x- and y-intercept deviate significantly from zero. Therefore, the ratio of GP-γKA-MA to C17:0GP-NAE must be corrected by a factor of 8.1 to accurately calculate actual esterified γKA-PE in the sample. The limit of detection for esterified γKA-PE was 2 pmol in the sample.
Figure 8.
Hydrolysis of esterified γKA-PE to glycerophospho-N- γKA-ethanolamine methylamides (GP-γKA-MA) by CH3NH2. A. Mass spectrum from negative ion scan after CH3NH2 hydrolysis of methylester-γKA-PE. The product with m/z 543, 559 and m/z 575 are consistent with the pyrrole, lactam, and hydroxylactam species of GP-γKA-Etn-methylamides. B. The collision induced disassociation (CID) spectrum of the major pyrrole product (m/z 543). CID spectrum of m/z 559 and of m/z 575 gave similar results. C. Interpretation of major product ions. Similar fragmentation ions were found for GP-γKA-MA as those for GP-NAEs. D. MRM chromatographs of various forms of methylester γKA-PE adducts hydrolyzed to their glycerophosphate γKA-ethanolamine methylamine (GP-γKA-MA) products. 20 nmol of methylester γKA-PE spiked with 1 nmol C17:0NAPE was treated for 1 h and injected on LC/MS/MS as described in the experimental methods. E. Correlation between methyl ester γKA-PE added to sample (buffer with 1nmol C17:0NAPE as internal standard) and the sum total GP-γKA-MAs measured. Limit of detection was less than 20 pmol γKA-PE in the sample.
Measurement of N-modified PE in HUVEC cells
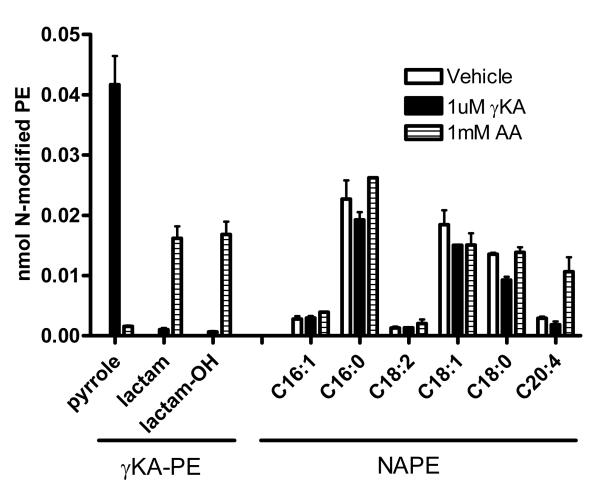
Oxidative injury to endothelial cells has been postulated to be an important component of cardiovascular disease. High levels of plasma free fatty acids, as occurs in metabolic syndrome and diabetes, induces oxidative stress in endothelial cells [34] and may therefore lead to formation of γKAs. NAPE is present in cultured endothelial cells, suggesting that they have the capacity to synthesize NAPE in response to stress. We therefore measured levels of N-modified PE in HUVEC generated in response to 1 mM arachidonic acid. As a positive control, we also measured the levels of N-modified PEs formed in response to 1 μM γKA. Consistent with our previous reports, treatment with 1 μM γKA resulted in significantly increased levels γKA-PE adducts compared to vehicle treated cells (Figure 9). The γKA-PE adducts were primarily pyrrole adducts. No significant changes in NAPE levels were detected. Treatment with 1 mM arachidonic acid also significantly increased levels of γKA-PE adducts, but in this case the adducts were primarily lactam and hydroxylactam adducts. No esterified γKA-PE was detected. While levels of most NAPEs did not change significantly, levels of C20:4NAPE increased about three-fold.
Figure 9.
Measurement of γKA-PE in human umbilical vein endothelial cells (HUVEC). Confluent HUVEC in 6-well plates were treated with either vehicle, 1 μM synthetic γKA, or 1 mM arachidonic acid (AA) for 4 hours and the resulting γKA-PEs and NAPEs were measured simultaneously by LC/MS/MS after CH3NH2 hydrolysis. Levels of γKA-PE pyrrole, lactam, and hydroxylactam differed significantly after treatment with either γKA or AA compared to vehicle. Levels of C20:4NAPE, but not other NAPE species, significantly increased after AA treatment.
DISCUSSION
Our studies show that deacylation with CH3NH2 and subsequent analysis by LC/MS/MS is an accurate, facile, and cost-effective method to measure NAPEs and various species of γKA-PE. The same methods are likely to be adaptable to a broad-range of non-enzymatically N-modified PE that have previously been described including those formed by hydroxyalkenals [18; 19; 35; 36], acrolein [20], malondialdehyde[37; 38], oxysterols[39], and advanced glycation end products [40; 41]. Simultaneous measurement of the various N-modified PEs should facilitate studies to understand the relative rates of formation under various conditions and how these various biologically active lipids control cell fate during stress.
Our results with HUVEC illustrate the value of simultaneous measurement of both enzymatically and non-enzymatically N-modified PEs in understanding these processes. First, they demonstrate that γKA-PE forms endogenously under conditions of oxidative stress. Interestingly, the species of γKA-PE formed under these conditions are primarily lactam and hydroxylactam adduct, while direct addition of γKA produces primarily pyrrole adducts. Second, our results suggest that formation of γKA-PE is by itself insufficient to trigger synthesis of NAPE as we saw no increase in NAPE concentrations in response to γKA treatment. Third, our results show that while oxidative stress induced by high levels of arachidonate lead to formation of both types of N-modified PEs, it substantially favored formation of γKA-PE over NAPE. Because of the cytotoxicity of γKA-PE, such imbalances are likely to result in endothelial dysfunction. While the implications of these results require further investigation beyond the scope of these current studies, they do confirm the potential value of simultaneously measuring multiple N-modified PEs in gaining a greater understanding of the role of these bioactive lipids in the response to stress.
In summary, we have developed an accurate, facile, and relatively inexpensive assay to simultaneously measure both enzymatically and non-enzymatically N-modified PEs. Because of the potential biological importance of these N-modified PEs, the ability to simultaneously measure their levels in biological tissues is a critical tool that will facilitate studies to unravel their contributions to health and disease.
Acknowledgements
We are grateful for financial support from the National Institutes of Health (OD003137) and Vanderbilt University Department of Pharmacology. We thank Denis Coulon and Lionel Faure for their generous gift of the C41DEST expressing the A. thaliana NAPE N-acyltransferase, and Zhongyi Chen and Xavier Stien for their excellent technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Hazlewood GP, Dawson RM. Intermolecular transacylation of phosphatidylethanolamine by a Butyrivibrio sp. Biochem J. 1975;150:521–5. doi: 10.1042/bj1500521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mileykovskaya E, Ryan AC, Mo X, Lin CC, Khalaf KI, Dowhan W, Garrett TA. Phosphatidic acid and N-acylphosphatidylethanolamine form membrane domains in Escherichia coli mutant lacking cardiolipin and phosphatidylglycerol. J Biol Chem. 2009;284:2990–3000. doi: 10.1074/jbc.M805189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Merkel O, Schmid PC, Paltauf F, Schmid HH. Presence and potential signaling function of N-acylethanolamines and their phospholipid precursors in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2005;1734:215–9. doi: 10.1016/j.bbalip.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [4].Faure L, Coulon D, Laroche-Traineau J, Le Guedard M, Schmitter JM, Testet E, Lessire R, Bessoule JJ. Discovery and characterization of an Arabidopsis thaliana N-acylphosphatidylethanolamine synthase. J Biol Chem. 2009;284:18734–41. doi: 10.1074/jbc.M109.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chapman KD, Moore TS., Jr. Catalytic Properties of a Newly Discovered Acyltransferase That Synthesizes N-Acylphosphatidylethanolamine in Cottonseed (Gossypium hirsutum L.) Microsomes. Plant Physiol. 1993;102:761–769. doi: 10.1104/pp.102.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cai SJ, McAndrew RS, Leonard BP, Chapman KD, Pidgeon C. Rapid purification of cotton seed membrane-bound N-acylphosphatidylethanolamine synthase by immobilized artificial membrane chromatography. J Chromatogr A. 1995;696:49–62. doi: 10.1016/0021-9673(94)01113-s. [DOI] [PubMed] [Google Scholar]
- [7].McAndrew RS, Leonard BP, Chapman KD. Photoaffinity labeling of cottonseed microsomal N-acylphosphatidylethanolamine synthase protein with a substrate analogue, 12-[(4-azidosalicyl)amino]dodecanoic acid. Biochim Biophys Acta. 1995;1256:310–8. doi: 10.1016/0005-2760(95)00038-e. [DOI] [PubMed] [Google Scholar]
- [8].Schmid PC, Schmid HH. N-Acylation of ethanolamine phospholipids by acyl transfer does not involve hydrolysis. Biochim Biophys Acta. 1987;922:398–400. doi: 10.1016/0005-2760(87)90066-x. [DOI] [PubMed] [Google Scholar]
- [9].Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–42. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–42. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Fonseca F. Rodriguez, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–12. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- [12].Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135:813–24. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shiratsuchi A, Ichiki M, Okamoto Y, Ueda N, Sugimoto N, Takuwa Y, Nakanishi Y. Inhibitory effect of N-palmitoylphosphatidylethanolamine on macrophage phagocytosis through inhibition of Rac1 and Cdc42. J Biochem. 2009;145:43–50. doi: 10.1093/jb/mvn139. [DOI] [PubMed] [Google Scholar]
- [14].Schmid HHO, Schmid PC, Natarajan V. N-Acylated glycerophospholipids and their derivatives. Progress in Lipid Research. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- [15].Bernoud-Hubac N, Fay LB, Armarnath V, Guichardant M, Bacot S, Davies SS, Roberts LJ, II, Lagarde M. Covalent binding of isoketals to ethanolamine phospholipids. Free Radic Biol Med. 2004;37:1604–11. doi: 10.1016/j.freeradbiomed.2004.07.031. [DOI] [PubMed] [Google Scholar]
- [16].Li W, Laird JM, Lu L, Roychowdhury S, Nagy LE, Zhou R, Crabb JW, Salomon RG. Isolevuglandins covalently modify phosphatidylethanolamines in vivo: Detection and quantitative analysis of hydroxylactam adducts. Free Radic Biol Med. 2009;47:1539–52. doi: 10.1016/j.freeradbiomed.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sullivan CB, Matafonova E, Roberts LJ, II, Amarnath V, Davies SS. Isoketals form cytotoxic phosphatidylethanolamine adducts in cells. J. Lipid Res. 2010;51:999–1009. doi: 10.1194/jlr.M001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bacot S, Bernoud-Hubac N, Baddas N, Chantegrel B, Deshayes C, Doutheau A, Lagarde M, Guichardant M. Covalent binding of hydroxy-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J Lipid Res. 2003;44:917–26. doi: 10.1194/jlr.M200450-JLR200. [DOI] [PubMed] [Google Scholar]
- [19].Guichardant M, Taibi-Tronche P, Fay LB, Lagarde M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic Biol Med. 1998;25:1049–56. doi: 10.1016/s0891-5849(98)00149-x. [DOI] [PubMed] [Google Scholar]
- [20].Berry K.A. Zemski, Murphy RC. Characterization of acrolein-glycerophosphoethanolamine lipid adducts using electrospray mass spectrometry. Chem Res Toxicol. 2007;20:1342–51. doi: 10.1021/tx700102n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salomon RG, Miller DB, Zagorski MG, Coughlin DJ. Solvent Induced Fragmentation of Prostaglandin Endoperoxides. New Aldehyde Products from PGH2 and Novel Intramolecular 1,2-Hydride Shift During Endoperoxide Fragmentation in Aqueous Solution. J Am Chem Soc. 1984;106:6049–6060. [Google Scholar]
- [22].Salomon RG, Subbanagounder G, Singh U, O’Neil J, Hoff HF. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem Res Toxicol. 1997;10:750–9. doi: 10.1021/tx970016b. [DOI] [PubMed] [Google Scholar]
- [23].Brame CJ, Salomon RG, Morrow JD, Roberts LJ., 2nd Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274:13139–46. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- [24].Brame CJ, Boutaud O, Davies SS, Yang T, Oates JA, Roden D, Roberts LJ., 2nd Modification of proteins by isoketal-containing oxidized phospholipids. J Biol Chem. 2004;279:13447–51. doi: 10.1074/jbc.M313349200. [DOI] [PubMed] [Google Scholar]
- [25].DiFranco E, Subbanagounder G, Kim S, Murthi K, Taneda S, Monnier VM, Salomon RG. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem Res Toxicol. 1995;8:61–7. doi: 10.1021/tx00043a008. [DOI] [PubMed] [Google Scholar]
- [26].Rawyler AJ, Braendle RA. N-Acylphosphatidylethanolamine accumulation in potato cells upon energy shortage caused by anoxia or respiratory inhibitors. Plant Physiol. 2001;127:240–51. doi: 10.1104/pp.127.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hansen HH, Hansen SH, Bjornsdottir I, Hansen HS. Electrospray ionization mass spectrometric method for the determination of cannabinoid precursors: N-acylethanolamine phospholipids (NAPEs) J Mass Spectrom. 1999;34:761–7. doi: 10.1002/(SICI)1096-9888(199907)34:7<761::AID-JMS832>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [28].Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–28. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Astarita G, Ahmed F, Piomelli D. Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain. J Lipid Res. 2008;49:48–57. doi: 10.1194/jlr.M700354-JLR200. [DOI] [PubMed] [Google Scholar]
- [30].Hansen HH, Hansen SH, Schousboe A, Hansen HS. Determination of the phospholipid precursor of anandamide and other N-acylethanolamine phospholipids before and after sodium azide-induced toxicity in cultured neocortical neurons. J Neurochem. 2000;75:861–71. doi: 10.1046/j.1471-4159.2000.0750861.x. [DOI] [PubMed] [Google Scholar]
- [31].Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- [32].Clarke NG, Dawson RM. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981;195:301–6. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Amarnath V, Amarnath K, Matherson T, Davies S, Roberts LJ. A Simplified Synthesis of Diastereomers of Levuglandin E2. Synthetic Communications. 2005;35:397–408. [Google Scholar]
- [34].Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- [35].Guichardant M, Bernoud-Hubac N, Chantegrel B, Deshayes C, Lagarde M. Aldehydes from n-6 fatty acid peroxidation. Effects on aminophospholipids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:147–9. doi: 10.1054/plef.2002.0412. [DOI] [PubMed] [Google Scholar]
- [36].Bacot S, Bernoud-Hubac N, Chantegrel B, Deshayes C, Doutheau A, Ponsin G, Lagarde M, Guichardant M. Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals. J Lipid Res. 2007;48:816–25. doi: 10.1194/jlr.M600340-JLR200. [DOI] [PubMed] [Google Scholar]
- [37].Jain SK. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J Biol Chem. 1984;259:3391–4. [PubMed] [Google Scholar]
- [38].Bhuyan DK, Master RW, Bhuyan KC. Crosslinking of aminophospholipids in cellular membranes of lens by oxidative stress in vitro. Biochim Biophys Acta. 1996;1285:21–8. doi: 10.1016/s0005-2736(96)00142-3. [DOI] [PubMed] [Google Scholar]
- [39].Bach D, Epand RF, Epand RM, Wachtel E. Interaction of 7-ketocholesterol with two major components of the inner leaflet of the plasma membrane: phosphatidylethanolamine and phosphatidylserine. Biochemistry. 2008;47:3004–12. doi: 10.1021/bi702070b. [DOI] [PubMed] [Google Scholar]
- [40].Requena JR, Ahmed MU, Fountain CW, Degenhardt TP, Reddy S, Perez C, Lyons TJ, Jenkins AJ, Baynes JW, Thorpe SR. Carboxymethylethanolamine, a biomarker of phospholipid modification during the maillard reaction in vivo. J Biol Chem. 1997;272:17473–9. doi: 10.1074/jbc.272.28.17473. [DOI] [PubMed] [Google Scholar]
- [41].Oak JH, Nakagawa K, Oikawa S, Miyazawa T. Amadori-glycated phosphatidylethanolamine induces angiogenic differentiations in cultured human umbilical vein endothelial cells. FEBS Lett. 2003;555:419–23. doi: 10.1016/s0014-5793(03)01237-7. [DOI] [PubMed] [Google Scholar]