Abstract
Alzheimer’s disease (AD) currently has over 6 million victims in the USA, alone. The recently FDA approved drugs for AD only provide mild, transient relief for symptoms without addressing underlying mechanisms to a significant extent. Basic understanding of the activities of the amyloid β peptide (Aβ) and associated proteins such as β–site AβPP-cleaving enzyme 1 (BACE1) is necessary to develop effective medical responses to AD. In this issue, Tabaton et al have presented a model of both non–pathological and pathological Aβ activity and suggest potential therapeutic pathways based on their proposed framework of Aβ acting as the signal that induces a kinase cascade, ultimately stimulating transcription factors that upregulate genes such as BACE1. We respond by presenting evidence of Aβ’s other activities, including protection against metal–induced reactive oxidizing species (ROS), modification of cholesterol transport, and potential activity as a transcription factor in its own right. We touch upon clinical implications of each of these functions and highlight the currently unexplored implications of our suggested novel function of Aβ as a transcription factor. Aβ appears to be a highly multifunctional peptide, and any or all of the pathways it engages in is a likely candidate for anti–AD drug development.
Keywords: Aging, Alzheimer’s disease, amyloid beta peptide, clinical trial, dementia, drug discovery, gene regulation, molecular pharmacology, signaling, transcription factor, treatment
Introduction
Alzheimer’s disease (AD) currently afflicts 6.5 million Americans (5.1 million over the age of 65) and is projected to increase to between 11 and 16 million by 2050 (“2009 Alzheimer’s disease facts and figures,” 2009). Over $4 billion in revenues are currently generated by the five US federal drug agency (FDA)–approved drugs: Aricept (Donepezil, Pfizer), Cognex (Tacrine, Parke–Davis), Razadyne (Galantamine, Ortho–McNeil–Janssen), Exelon (Rivastigmine, Novartis), and Namenda (Memantine, Forest). These current therapies for AD provide only mild, transient symptomatic relief. A significant unmet need exists for improved drugs, which are based on novel molecular targets that modify the underlying course and address the etiology of the disease. To design drugs for this end, the fundamental activities of molecules such as amyloid–β peptide (Aβ), Aβ precursor protein (AβPP), β–site AβPP–cleaving enzyme 1 (BACE1)—the β–secretase molecule, and presenilin–1 (PSEN1)—a necessary component of γ–secretase activity—must be elucidated. The Aβ peptide is of particular interest, as it is the center of the “amyloid cascade” hypothesis—the currently dominant model of AD etiology. In addition, understanding of normal Aβ clearance pathways, such as insulin degrading enzyme (IDE), is important for therapeutic use Aβ metabolism (Eckman and Eckman, 2005).
The processing of AβPP into Aβ requires two enzymatic activities. AβPP is first cleaved by β–secretase, producing soluble AβPP and a cell–membrane bound fragment (Lahiri, et al., 2003). This fragment is further cleaved by γ–secretase to produce Aβ and the AβPP intracellular domain (AICD). AICD has been shown to function in regulation of gene transcription (Konietzko, et al., 2010), indicating an important non pathogenic role for γ–secretase. However, the Aβ generation is a minority AβPP processing pathway. The majority of AβPP is cleaved by α–secretase, a large molecule complex that includes members of the ADAM protein family (Asai, et al., 2003). This pathway represents a neuroprotective route for AβPP processing (Kojro and Fahrenholz, 2005), and encouraging the α–secretase pathway may be clinically productive (Fahrenholz, 2007).
In “Signaling effect of amyloid–β42 on the processing of AβPP”, Tabaton et al present a model of Aβ function and portray it primarily as an extracellular signaling peptide that begins a cascade which regulates both β– and γ–secretase activity, thus regulating both steps of its own cleavage from the Aβ precursor protein, AβPP. Their review presents another emerging picture of the state of knowledge regarding both Aβ dysfunction and BACE1.
When summarizing the “normal” functions of Aβ, the authors stress a potential role as a signaling pathway partner to TrkA, MAPK, and JNK to the exclusion of most other potential functions. Similarities between Aβ and Notch are noted in the paper. The authors provide a more complete picture of Aβ dysfunction, highlighting its toxic and oxidative activities as individual subunits and oligomers, and its formation into amyloid plaque in AD brains. Each of these potential activities is of more than theoretical importance, since each lends itself to different therapeutic responses.
Aβ signaling and kinases
Tabaton et al emphasize a straightforward kinase pathway for Aβ function. The authors propose a model in which Aβ initiates a signaling cascade that may involve extracellular signal–related kinase (ERK), serine/threonine protein kinase, (Akt), phosphorylated c-Jun N-terminal kinase (pJNK), insulin receptor substrate (IRs), and/or G proteins, ultimately modifying the activity of transcription factors that, in turn, modify expression of the BACE1 gene. This route was proposed at least half a decade ago, specifically targeting JNK (Bogoyevitch, et al., 2004) based on the neuroprotective activity of JNK3 and association of JNK genes with diabetes and obesity, two conditions that are, themselves, associated with AD (Qiu, et al., 2007).
Aβ and metal chelators
Some functions they neglect include Aβ reducing metal charge states, such as reduction of copper (II) to copper (I) (White, et al., 1999). This suggests that Aβ may protect against metal–induced oxidative damage (Baruch-Suchodolsky and Fischer, 2009, Zou, et al., 2002). On the other hand, Aβ’s suggested protective activity against metal–induced oxidation points to a potential cause to explore for the contribution of Aβ to oxidative conditions, as it has been suggested that Aβ as antioxidant is transformed to Aβ as pro–oxidant specifically through its interaction with oxidizing metals (Kontush, 2001). This suggests exploration of chelating agents as a potential Aβ prophylactic or early therapy to administer during the stages of AD, including mild cognitive impairment (MCI).
Aβ and cholesterol transport
In addition, Aβ may control cholesterol transport (Igbavboa, et al., 2009, Yao and Papadopoulos, 2002). Addressing Aβ’s potential role in cholesterol metabolism leads to the investigation of lipid–modifying drugs.
Aβ mediated regulation of BACE1
β–secretase activity provided by BACE1 is the rate–limiting step in the production of Aβ from AβPP (Vassar, 2001). Control of secretase activity, especially β–secretase could be a fruitful path toward limiting harmful effects of Aβ. To explore the secretase route, Tabaton et al present a brief discussion of the β–secretase protein, BACE1. Notably, BACE1 activity is the rate–limiting step in formation of Aβ from AβPP. The BACE1 gene promoter has been structurally and functionally characterized, and a 91bp proximal DNA fragment appears to be the minimal constitutive promoter region (Ge, et al., 2004, Lahiri, et al., 2006, Sambamurti, et al., 2004). Among the transcription factors associated with BACE1 regulation are YY1, SP1, and MEF2 (Dosunmu, et al., 2009, Lahiri, et al., 2006, Nowak, et al., 2006).
If some feedback mechanism were to exist between Aβ and BACE1, this could provide a powerful route to investigate Aβ–related pathogenesis. The authors have recently determined that BACE1 transcription is upregulated by addition of Aβ1–42, but not by AICD (Giliberto, et al., 2009). They then speculate that the specific pathway of this upregulation is through JNK signaling, although their prior demonstration of JNK activity in BACE1 gene regulation does not show the activity of Aβ in that particular pathway (Tamagno, et al., 2009).
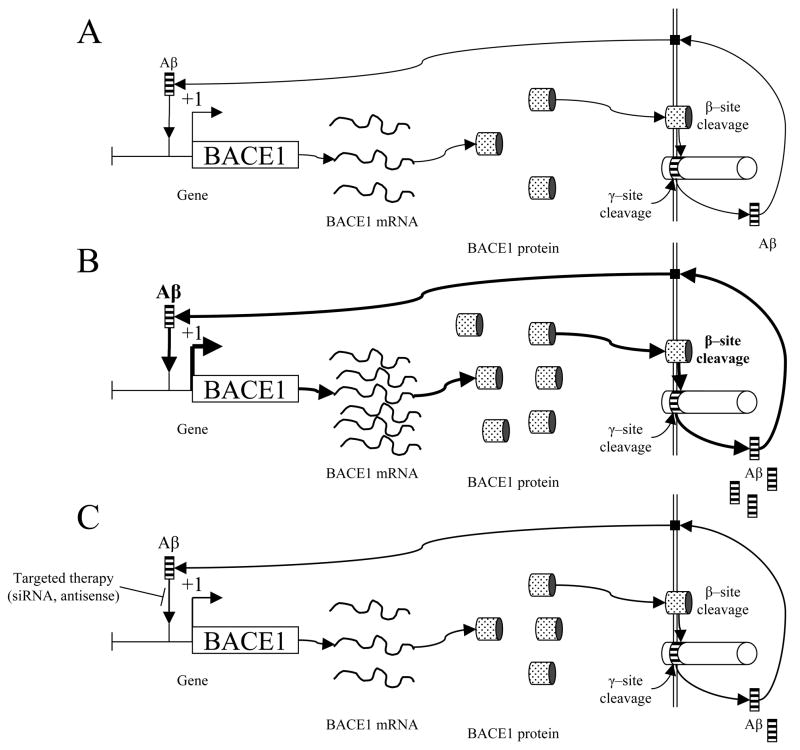
It is interesting to note that Tabaton, et al, cite a work by Ohyagi, et al, specifically the activation of the p53 promoter by intracellular Aβ (Ohyagi, et al., 2005). In addition, a p53 promoter–reporter clone containing a mutation at the Aβ binding site within the promoter produced reduced activation from intracellular Aβ (Ohyagi, et al., 2005). However, in building their model, Tabaton et al do not mention that the p53–related paper also demonstrated direct binding of DNA by Aβ, both in vitro by gel shift and in vivo by chromatin immune precipitation (ChIP) nor that altering the putative Aβ binding DNA motif altered induction of promoter activity by Aβ. Ultimately, all Aβ in the stimulation pathway proposed is either presumed to be extracellular or cytoplasmic, operating through G proteins or JNK. The earlier work demonstrated that Aβ could be induced to enter the nucleus (Ohyagi, et al., 2005), a localization that was not mentioned. Ohyagi et al’s work has been further extended to confirm inducible nuclear localization for Aβ (Bailey and Lahiri, 2009), to specify the consensus DNA motif to which Aβ binds (Maloney, et al., 2006), and to show changes in activity of the AβPP promoter following treatment of cell cultures by Aβ in solution (Lahiri, et al., 2009). Indeed, the consensus Aβ–binding sequence has recently been found in the BACE1 promoter region, and two of the putative sites have been shown to bind Aβ in vitro (Lahiri, et al., 2010). This recent evidence is suggestive of Aβ’s direct regulation of the BACE1 promoter, and, thereby, its own production, which need not be mediated through signaling intermediaries. Instead, our recent work strongly indicates that Aβ may function as a transcription factor in its own right, regulating not only BACE1, but also AβPP and potentially other genes involved in Alzheimer’s disease (Fig. 1A). Therapies based upon modifying Aβ’s activity as a transcription factor would (Fig. 1B–C) be feasible but speculative at this stage. However, oral small interfering RNA (siRNA) has proven effective in downregulating systemic inflammatory responses (Aouadi, et al., 2009), and both siRNA and antisense oligonucleotides can be successfully delivered by oral route (Akhtar, 2009).
Fig. 1. Self–regulation of Aβ cleavage from AβPP through the BACE1 gene promoter and therapeutic implications.
A) Intranuclear Aβ peptide would function as a transcription factor, upregulating BACE1 gene transcription. This would stimulate production of BACE1 mRNA as a template for BACE1 protein production. BACE1 would cleave AβPP at the β–cleavage site. When this is followed by γ–cleavage, extracellular Aβ is released. Aβ would then enter the cell through currently–uncharacterized receptor(s) that could include FPR2, insulin receptor, or NMDA receptors (Verdier, et al., 2004). Once within the cell, Aβ would then be transported into the nucleus to renew the cycle. B) Under pathogenic conditions, Aβ levels would have been stimulated to increase BACE1 transcription to the extent that normal Aβ clearance pathways, such as IDE, would fall behind. Additional Aβ would be transported into the cell, to stimulate increased BACE1 transcription, resulting in an uncontrolled positive feedback loop. C) Therapeutic blockage of Aβ–BACE1 promoter interaction by sequence specific siRNA or antisense DNA oligomers would result in reduced BACE1 gene transcription, theoretically permitting Aβ clearance mechanisms to catch up to production. Reduced production would restore a state similar to pre disease levels.
Clinical implications and drug targets of alternate Aβ functional pathways
The various exploratory routes suggested by each pathway related to Aβ production are summarized in Table 1. Tabaton et al appear to suggest that kinase modification is the most fruitful direction to choose, but this ignores four other potential avenues, each of which has varying degrees of evidentiary support. Unfortunately, the oral Akt inhibitor perifosine failed to diminish tumors as a sole oncological treatment agent (Gills and Dennis, 2009), raising questions about its potential efficacy in other pathways. Likewise, the mixed lineage kinase inhibitor CEP 1347 (mentioned as a potential agent to investigate by Bogoyevitch et al) did not delay disability in early Parkinson’s disease (Wang and Johnson, 2008).
Table 1.
Clinical implications of candidate Aβ regulatory pathways
| Pathway | Candidate Materials (examples only) | Comments |
|---|---|---|
| Kinase modification | Perifosine, Keryx (Gills and Dennis, 2009); CEP–1347, Cephalon (Wang and Johnson, 2008) |
This pathway would allow use of drugs already developed. Potential for unexpected side effects due to wide activity profiles for target kinases; Low efficacy in clinical trials |
| Chelation | PBT2, Prana (Adlard, et al., 2008, Ritchie, et al., 2004) | Parent compound Clioquinol implicated in the disorder SMOM in large doses; PBT2 lower side effects |
| Cholesterol metabolism modification | Simvastatin, Merck (Tong, et al., 2009); D–4F, Novartis (Handattu, et al., 2009); Dietary modification (Pallebage-Gamarallage, et al., 2009) |
Some agents already well–characterized for tolerability Dietary modification avoids drug therapy |
| Secretase modification | CTS–21166, CoMentis (Panza, et al., 2009); LY450139, Lilly (Bateman, et al., 2009); Etazolate, ExonHit (Marcade, et al., 2008) |
Long–term effects of secretase modification unknown; Potential for interference in similar pathways (e.g., Notch vs. γ–secretase) |
| Aβ as a transcription factor | siRNA to Aβ–binding sites in target promoters; Antisense DNA to Aβ–binding sites in target promoters |
Potential for high specificity (low side effects); Speculative; Little to no current clinical use |
On the other hand, metal chelation has appeared to bear more clinical fruit. For example, the test drug PBT2, a derivative of the chelator clioquinol, has been shown to inhibit Aβ oligomer formation, disaggregate Aβ plaque, and neutralize Aβ toxicity (Ritchie, et al., 2004). The drug has been shown to restore cognitive function to AD model mice (Adlard, et al., 2008), and it has recently finished successful phase IIb trial (Lannfelt, et al., 2008).
Cholesterol modification also appears to be promising. Agents based on this pathway would include simvastatin, which has improved cerebral function and reversed toxic effects of Aβ in mouse models (Tong, et al., 2009). The drug D–4F, which mimics ApoA–I and can be orally administered, improves cognitive function in AD model mice (Handattu, et al., 2009) and reduces brain arteriole inflammation in LDL receptor–deficient mice (Buga, et al., 2006). Even dietary modification of fat and cholesterol intake has been shown to modify levels of intracellular Aβ in rodent models (Pallebage-Gamarallage, et al., 2009). A non–negligible role for intracellular Aβ in AD has been investigated and found to be of potential therapeutic value (Ohyagi, 2008).
Several secretase–modifying drugs have also been investigated. Preclinical studies of a β–secretase inhibitor CTS 21166 have also shown promising results (Panza, et al., 2009). The γ–secretase inhibitor LY450139 dihydrate is currently undergoing phase III trial. It has been shown to reduce Aβ synthesis without altering Aβ clearance (Bateman, et al., 2009). An especially promising route to investigate would be to not only reduce β–secretase activity but encourage the α–processing pathway. The drug etazolate is currently in phase IIb trial after having demonstrated precognitive and neuroprotective properties in rodent models (Marcade, et al., 2008).
Of particular theoretical interest would be pursuing the potential role of Aβ as a transcription factor. Given the variable nature of the Aβ–binding DNA motif (Lahiri, et al., 2009), appreciable specificity for promoter targets could be designed into DNA sequence–based therapies. However, the conclusion that Aβ is a multi–functional peptide cannot be reasonably avoided. Direct alteration of Aβ levels is likely to impact cholesterol metabolism, metal–induced ROS in the nervous system, and has organism–wide implications in regulation for an as–yet weakly characterized set of genes. It is certainly a worthwhile target to explore for prevention and treatment of AD. However, its involvement in an amazing variety of pathways and activities suggests that a multi–pronged approach may prove the most effective way to safely modify Aβ’s potential pathogenic activity. In combination with the aforementioned approaches, the role of dietary and environmental factors and epigenetic regulation of BACE1 and other genes, should also be considered (Lahiri and Maloney, 2010, Lahiri, et al., 2009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2009 Alzheimer’s disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Akhtar S. Oral delivery of siRNA and antisense oligonucleotides. J Drug Target. 2009;17:491–495. doi: 10.1080/10611860903057674. [DOI] [PubMed] [Google Scholar]
- Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Hattori C, Szabo B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun. 2003;301:231–235. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- Bailey J, Lahiri DK. Nuclear localization of the amyloid beta-peptide during oxidative stress in neuronal cells. In: Lebl M, editor. Peptides: Breaking Away, Proceedings of the Twenty-First American Peptide Symposium. Prompt Scientific Publishing; Bloomington, Indiana, USA: 2009. pp. 187–188. [Google Scholar]
- Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;48:4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC, Yarasheski KE, Friedrich SW, Demattos RB, May PC, Paul SM, Holtzman DM. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Buga GM, Frank JS, Mottino GA, Hendizadeh M, Hakhamian A, Tillisch JH, Reddy ST, Navab M, Anantharamaiah GM, Ignarro LJ, Fogelman AM. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J Lipid Res. 2006;47:2148–2160. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- Dosunmu R, Wu J, Adwan L, Maloney B, Basha MR, McPherson CA, Harry GJ, Rice DC, Zawia NH, Lahiri DK. Lifespan profiles of Alzheimer’s disease-associated genes and products in monkeys and mice. J Alzheimers Dis. 2009;18:211–230. doi: 10.3233/JAD-2009-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. Abeta-degrading enzymes: modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33:1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Fahrenholz F. Alpha-secretase as a therapeutic target. Curr Alzheimer Res. 2007;4:412–417. doi: 10.2174/156720507781788837. [DOI] [PubMed] [Google Scholar]
- Ge YW, Maloney B, Sambamurti K, Lahiri DK. Functional characterization of the 5’ flanking region of the BACE gene: identification of a 91 bp fragment involved in basal level of BACE promoter expression. FASEB Journal. 2004;18:1037–1039. doi: 10.1096/fj.03-1379fje. [DOI] [PubMed] [Google Scholar]
- Giliberto L, Borghi R, Piccini A, Mangerini R, Sorbi S, Cirmena G, Garuti A, Ghetti B, Tagliavini F, Mughal MR, Mattson MP, Zhu X, Wang X, Guglielmotto M, Tamagno E, Tabaton M. Mutant presenilin 1 increases the expression and activity of BACE1. J Biol Chem. 2009;284:9027–9038. doi: 10.1074/jbc.M805685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handattu SP, Garber DW, Monroe CE, van Groen T, Kadish I, Nayyar G, Cao D, Palgunachari MN, Li L, Anantharamaiah GM. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;34:525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–338. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro E, Fahrenholz F. The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell Biochem. 2005;38:105–127. doi: 10.1007/0-387-23226-5_5. [DOI] [PubMed] [Google Scholar]
- Konietzko U, Goodger ZV, Meyer M, Kohli BM, Bosset J, Lahiri DK, Nitsch RM. Co-localization of the amyloid precursor protein and Notch intracellular domains in nuclear transcription factories. Neurobiol Aging. 2010;31:58–73. doi: 10.1016/j.neurobiolaging.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush A. Amyloid-beta: an antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic Biol Med. 2001;31:1120–1131. doi: 10.1016/s0891-5849(01)00688-8. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Farlow MR, Sambamurti K, Greig NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer’s disease. Current Drug Targets. 2003;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Ge Y-W, Maloney B, Bailey JA. Novel interaction of the Alzheimer’s amyloid beta-peptide with a specific regulatory motif present in the APOE, APP, and BACE1 gene promoters, and putative role of A beta as a transcription factor. 2010 SUBMITED. [Google Scholar]
- Lahiri DK, Ge YW, Rogers JT, Sambamurti K, Greig NH, Maloney B. Taking down the unindicted co-conspirators of amyloid beta-peptide-mediated neuronal death: shared gene regulation of BACE1 and APP genes interacting with CREB, Fe65 and YY1 transcription factors. Curr Alzheimer Res. 2006;3:475–483. doi: 10.2174/156720506779025224. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol. 2010 doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Bailey J, Ge Y-W. Role of alzheimer’s amyloid-beta peptide as a putative transcription factor. In: Lebl M, editor. Peptides: Breaking Away, Proceedings of the Twenty-First American Peptide Symposium. Prompt Scientific Publishing; Bloomington, Indiana, USA: 2009. pp. 185–186. [Google Scholar]
- Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- Maloney B, Ge Y-W, Lahiri DK. Neuroscience 2006. Atlanta, GA: 2006. Is amyloid beta peptide a transcription factor? p. 6. [Google Scholar]
- Marcade M, Bourdin J, Loiseau N, Peillon H, Rayer A, Drouin D, Schweighoffer F, Desire L. Etazolate, a neuroprotective drug linking GABA(A) receptor pharmacology to amyloid precursor protein processing. J Neurochem. 2008;106:392–404. doi: 10.1111/j.1471-4159.2008.05396.x. [DOI] [PubMed] [Google Scholar]
- Nowak K, Lange-Dohna C, Zeitschel U, Gunther A, Luscher B, Robitzki A, Perez-Polo R, Rossner S. The transcription factor Yin Yang 1 is an activator of BACE1 expression. J Neurochem. 2006;96:1696–1707. doi: 10.1111/j.1471-4159.2006.03692.x. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y. Intracellular amyloid beta-protein as a therapeutic target for treating Alzheimer’s disease. Curr Alzheimer Res. 2008;5:555–561. doi: 10.2174/156720508786898514. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira JI, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB Journal. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- Pallebage-Gamarallage MM, Galloway S, Johnsen R, Jian L, Dhaliwal S, Mamo JC. The effect of exogenous cholesterol and lipid-modulating agents on enterocytic amyloid-beta abundance. Br J Nutr. 2009;101:340–347. doi: 10.1017/S0007114508012269. [DOI] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemiale G, Capurso A, Imbimbo BP. Disease-modifying approach to the treatment of Alzheimer’s disease: from alpha-secretase activators to gamma-secretase inhibitors and modulators. Drugs Aging. 2009;26:537–555. doi: 10.2165/11315770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- Ritchie CW, Bush AI, Masters CL. Metal-protein attenuating compounds and Alzheimer’s disease. Expert Opin Investig Drugs. 2004;13:1585–1592. doi: 10.1517/13543784.13.12.1585. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB Journal. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Giliberto L, Vitali A, Borghi R, Autelli R, Danni O, Tabaton M. JNK and ERK1/2 pathways have a dual opposite effect on the expression of BACE1. Neurobiol Aging. 2009;30:1563–1573. doi: 10.1016/j.neurobiolaging.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Fernandes P, Ongali B, Brouillette J, Quirion R, Hamel E. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol Dis. 2009;35:406–414. doi: 10.1016/j.nbd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Vassar R. The beta-secretase, BACE: a prime drug target for Alzheimer’s disease. J Mol Neurosci. 2001;17:157–170. doi: 10.1385/JMN:17:2:157. [DOI] [PubMed] [Google Scholar]
- Verdier Y, Zarandi M, Penke B. Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer’s disease. J Pept Sci. 2004;10:229–248. doi: 10.1002/psc.573. [DOI] [PubMed] [Google Scholar]
- Wang LH, Johnson EM., Jr Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2008;71:462. doi: 10.1212/01.wnl.0000324506.93877.5e. author reply 462–463. [DOI] [PubMed] [Google Scholar]
- White AR, Multhaup G, Maher F, Bellingham S, Camakaris J, Zheng H, Bush AI, Beyreuther K, Masters CL, Cappai R. The Alzheimer’s disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. Journal of Neuroscience. 1999;19:9170–9179. doi: 10.1523/JNEUROSCI.19-21-09170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZX, Papadopoulos V. Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity. FASEB J. 2002;16:1677–1679. doi: 10.1096/fj.02-0285fje. [DOI] [PubMed] [Google Scholar]
- Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22:4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]