Abstract
Objective
The vector relationships between the Eustachian tube, Tensor veli palatini muscle and cranial base constrain the efficiency of middle ear pressure-regulation and are required parameters for computational modeling of Eustachian tube function. Here, those relationships were reconstructed from skulls and compared between children and adults.
Method
Reconstructions were made using modifications of previously described techniques for 18 child skulls aged 3–4 years and 20 adult skulls (10 female, 10 male; >18 years). Measured and calculated variables were compared between groups using a Student’s t test.
Results
Consistent with previous reports, certain variables for adult skulls exhibited sexual dimorphism. Between children and adults, significant differences were documented for measures of cranial base length and width; hard palate width; nasopharyngeal height, width and depth; Eustachian tube length; the maximum and minimum Tensor veli palatini muscle lengths; the angles of deviation of the Tensor veli palatini muscle from the Eustachian tube, and the surface area of the Tensor veli palatini muscle. There were no between-group differences in the angle of Eustachian tube decent from the cranial base, Eustachian tube deviation from the parasagittal plane or the lateral component of the Tensor veli palatine muscle-Eustachian tube angle.
Conclusions
The differences between children and adults that could account for the observed poorer Eustachian tube function in children include their shorter Eustachian tube, lesser Tensor veli palatine muscle-Eustachian tube vectors, and the lesser Tensor veli palatine muscle surface area. Other observed differences are attributable to growth and development of the craniofacial complex.
Keywords: Eustachian tube, Tensor Veli Palatini muscle, Cranial Base, Vector Relationships, Adults and Children
INTRODUCTION
Otitis media with effusion (OME) is a common disease of the pediatric age group that decreases in incidence and prevalence with increasing age [1]. Numerous past studies related the development and/or persistence of that disease to a deficiency in middle ear (ME) pressure-regulation, defined as an imbalance between the gas supplied to the ME by the Eustachian tube (ET) and the gas loss from the ME secondary to passive transmucosal diffusion of N2 from ME to local blood [2, 3]. In turn, this imbalance causes the development of subambient ME pressures that results in a conductive hearing loss and OME by hydrops ex vacuo [4, 5].
While the ET is usually closed in persons with “normal” ET function, periodic contraction of the Tensor veli palatini muscle (mTVP) opens the ET allowing for bolus gas transfers between the ME and nasopharynx (NP) which re-establishes an ambient-ME pressure equilibrium. Like the prevalence of OME, tests that evaluate the functional efficiency of the mTVP in opening the ET document relatively poor function in infants and young children which significantly improves with advancing age [6, 7]. Experiments in animals show that abolishing mTVP function causes a disease condition not different from the clinical expression of OME [5, 8].
The anatomy of the ET and its related muscles (i.e. the ET system) has been described previously [9–11]. Of importance to the present study is the vector relationship of the functional portion of the ET, the membrano-cartilagenous ET (mcET), to the CB and the vector relationship of the mTVP muscle to the mcET. There, the mcET extends from the osseous portion of the ET to terminate anteriorly as an intrusive structure, the torus tubarious, located along the posterior-lateral wall of the NP. The mcET is firmly attached posteriorly to the osseous ET orifice by fibrous bands and anteriorly to a tubercle on the posterior edge of the medial pterygoid plate. Throughout its course, the superior portion of the mcET is fitted into a bony depression in the CB, the sulcus tubaris, located between the greater wing of the sphenoid and the petrous portion of the temporal bone to which it is attached by connective tissue. The mTVP is a relatively thin, two-layered, inverted triangular-shaped muscle with a lateral fiber group taking its origin from the scaphoid fossa posteriorly and, throughout its superior border, from the sphenoid spine (lateral border of the sulcus tubaris) and, perhaps, the lateral lamina of the ET cartilage. The medial fiber group (termed the dilatator tubae) takes its origin from the posterior ½ to 1/3 of the lateral membranous wall of the mcET [12]. Both bundles descend anteriorly, inferiorly and medially to join as a tendon that rounds (with minor insertions into) the hamular process of the medial pterygoid plate to insert into the palatal aponeurosis and the transverse ridge of the hard palate [12]. Effective contraction of the medial fibers of the mTVP displaces the lateral wall of the mcET inferiorly and laterally to open the lumen of the mcET over its entire length [13].
Recently, serial histological specimens were used to reconstruct the functional-anatomy of the mcET using sophisticated computer-generated models that reproduce the functional opening of the tubal lumen by simulating mTVP force application to the mcET [14]. However, parameterization of this type of model requires information on the general form of the mcET and the areas of attachment/contact of the mTVP muscle (available from histological study and gross dissection), the mechanical properties of the various tissue elements comprising the mcET (available from the literature for similar structures and possibly measurable using specialized testing) and the mTVP (i.e. dilatator tubae) vector relationships with respect to the lateral membranous wall of the mcET which is difficult to measure with accuracy by the aforementioned techniques.
Previously, a method for reconstructing the vector relationships among the mTVP, mcET and CB from measurements on human skulls was described and applied to adult specimens [15]. The purpose of the present study was to reconstruct these vector relationships from adult and child skulls of similar ethnicity for purposes of describing the differences in those relationships associated with growth and development and to provide data for parameterizing the computer-generated, functional models of the mcET at different ages.
MATERIALS AND METHODS
Materials
The investigators visited Carolina Biological, Burlington, NC and were given permission to examine and measure all skulls available at that facility. A total of 60 specimens were present, all reportedly Caucasian and recovered from the Indian subcontinent. The skulls were aged and adult skulls (>18 years) sexed using standard anthropometric criteria [16]. The distribution of the number of skulls aged 2 through 8 and 11 years was 3, 3, 15, 2, 6, 1, 4 and 1, respectively. There were 25 adult skulls with 10 assigned as female and 15 as male. While measurements were made on all available skulls, this report focuses on those assigned to the 3–4 year age group (children) given the relatively large number of specimens in that age group and those assigned an age greater than 18 years (adults). Because of an expected sexual dimorphism for some of the measures in adult skulls [15], the unequal number of male and female skulls in the collection and the inability to determine the sex of child skulls, 10 of the 15 male skulls were chosen at random for inclusion in this report so as to avoid bias in the comparisons between adults and children. Thus, the sample sizes for the two groups were 18 child skulls aged 3–4 years and 20 adult skulls aged >18 years (10 female, 10 male).
Below, the listed ET variables refer to the mcET and unilateral measures were made on the right side of the skulls. Note that vectors have both a magnitude and direction. Figure 1 shows the CB of an adult human skull with some of the landmarks used in these reconstructions marked and labeled.
Figure 1.
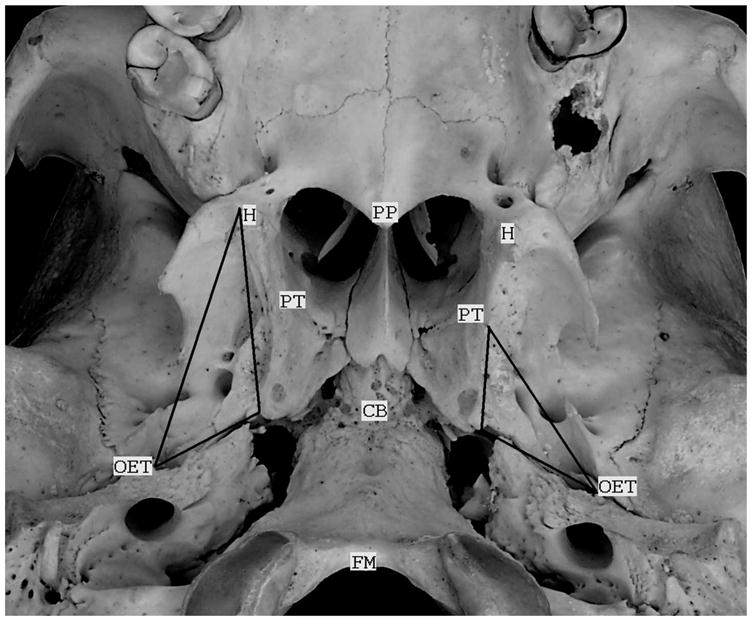
A photograph of the cranial base of an adult skull with some of the landmarks used for the reconstructions in the present study labeled. These include: the hamular processes (H), the cranial base (CB), the osseous orifices of the ET (OET), the medial pterygoid tubercles (PT), the posterior border of the hard palate (PP) and anterior margin of the foramen magnum (FM). The triangle on the left (right side of the skull) represents the borders of the mTVP muscle and that on the right represents the borders of the mcET. For both, the base of the triangle demarcates the sulcus tubaris. Note that the field of view is tilted to the right and forward in the photograph.
Measured Variables
The relevant measured dimensions for each skull included:
Variable 1: Bi-OET Distance = the linear distance between the lateral margins of the bilateral anterior orifices of the osseous portion of the ET which is a measure of CB width.
Variable 2: Bi-Hamular Distance = the linear distance between the outer margins of the bilateral hamular processes of the medial pterygoid plate which is an approximate measure of palatal width.
Variable 3: Bi-MPT Distance = the linear distance between the bilateral medial pterygoid plates at the position of the pterygoid tubercle which is a measure of NP width.
Variable 4: Inferior mcET Length = the linear distance between the osseous orifice of the ET to the tubercle of the ipsilateral medial pterygoid plate which is a measure of the length of the inferior border of the mcET.
Variable 5: Superior mcET Length = the length of the sulcus tubaris from the osseous orifice of the ET to the base of the medial pterygoid plate which is a measure of the lengths of the superior borders of the mcET and the mTVP.
Variable 6: CB-PT = the linear vertical distance from the CB to the posterior tubercle of the medial pterygoid plate which corresponds to the distance from the CB to the NP ET orifice.
Variable 7: MPT Height = the linear, perpendicular distance from a transverse plane through the hamular processes to the CB which is an approximate measure of NP height.
Variable 8: Maximum mTVP Length = the linear distance from the lateral margin of the osseous orifice of the ET to the lateral margin of the ipsilateral hamular process which corresponds to the magnitude of the mTVP-mcET vector at the osseous ET orifice.
Variable 9: MPT Base Length = the linear distance of the medial pterygoid base measured in the transverse/parasagittal plane which is an approximate measure of NP depth.
Variable 10: PP-FM Distance = the linear distance in the sagittal plane between the posterior border of the hard palate and anterior margin of the foramen magnum which is a measure of CB length.
Calculated Variables
The calculated variables for each skull were:
Variable 11: Minimum mTVP length = the linear distance from the lateral margin of the sulcus tubaris at the base of the medial pterygoid plate to the lateral margin of the ipsilateral hamular process calculated by the Pythagorean theorem as ((Variable 7)2+(Variable 9)2)0.5. This corresponds to the magnitude of the mTVP-mcET vector at the posterior base of the medial pterygoid plate.
Variable 12: OET-Hamulus = the linear distance between the anterior osseous orifice of the ET and the ipsilateral hamular process projected onto a paracoronal plane calculated as ½(Variable 1–Variable 2). This corresponds to the lateral deviation of the mcET and mTVP in the paracoronal plane.
Variable 13: Hard Palate-PT Distance = the linear vertical distance from a transverse plane passing through the hard palate to a horizontal plane passing through the posterior tubercle of the medial pterygoid plate calculated as (Variable 7–Variable 6). This is a measure of the height of the NP ET orifice relative to the hard palate.
Variable 14: CB-mcET Angle = the angle between the mcET at its osseous orifice and the tubercle of the ipsilateral medial pterygoid plate calculated as arc sine (Variable 6/Variable 4). This corresponds to the directional component of the mcET inferio-medial vector relative to the CB.
Variable 15: mcET Medial Angle = the medial angle of deviation of the mcET from a parasagittal plane calculated as arc sine (Variable 12/Variable 5). This corresponds to the directional component of the mcET medial vector relative to the osseous orifice in the transverse plane.
Variable 16: Minimum mTVP-mcET Angle = the angle between the osseous orifice of the ET and the ipsilateral hamular process calculated as arc sine (Variable 7/Variable 8). This corresponds to the directional component of the mTVP-mcET vector at the osseous ET orifice.
Variable 17: Maximum mTVP-mcET Angle = the angle between the mcET at the pterygoid base and the ipsilateral hamular process calculated as arc sine (Variable 7/Variable 11). This corresponds to the directional component of the mTVP-mcET vector at the posterior base of the medial pterygoid plate.
Variable 18: Lateral mTVP-mcET angle = the angle between the mTVP and the mcET at the osseous ET orifice projected onto the transverse plane as calculated in multiple steps. First, the projection of maximum mTVP length onto the transverse plane was calculated as Variable 18a = (cosine (Variable 16))(Variable 8). Then, the medial angle of deviation of that projection from the parasagital plane was calculated as Variable 18b = arc sine (Variable 12/Variable 18a). Finally, the medial angular deviation in the transverse plane between the mTVP and mcET was calculated as (Variable 15–Variable 18b). This variable corresponds to the lateral directional component of the mTVP-mcET vector in the transverse plane.
Variable 19: mTVP Area = the surface area of the mTVP for the triangle bounded by Variables 5, 8 and 11 as calculated in multiple steps. First, the cosine of the angle between Variable 8 and 11 was calculated using the cosine law or Variable 19a = ((Variable 8)2+(Variable 11)2−(Variable 5)2)/(2(Variable 8)(Variable 11)). Then, the height of that triangle from apex to base (at Variable 8) was calculated as Variable 19b = (sine (arc cosine (Variable 19a)))Variable 11. Finally, the area of the triangle (mTVP area) was calculated as ½(Variable 19b)(Variable 8).
Statistical Analysis
The values of these variables were compared between adult (>18 years of age) male and female skulls and between child (3–4 years of age) and adult (≥18 years of age; combined male-female) skulls using a two-tailed Student’s t test. Because of the multiple comparisons done using this test, statistical significance was assigned at alpha≤0.03.
RESULTS
The Table lists the average and standard deviations of the 10 measured and 9 calculated variables for the children and adult skulls and for male and female adult skulls as well as the 2-tailed Student’s t values and associated probability levels for the adult-child and male-female comparisons.
TABLE.
Average (Avg) and Standard Deviation (Std) of the 19 Variables for Child versus Adult and the Male versus Female Comparisons and the Absolute Values of the Student’s t Statistic (t) and Associated P-Values (P) for those Comparisons
| No. | Variable* | Child Avg | Std | Adult Avg | Std | t | P | Male Avg | Std | Female Avg | Std | t | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bi-OET Distance | 45.1 | 2.6 | 54.9 | 2.9 | 10.84 | 0.000 | 53.9 | 2.3 | 55.9 | 3.2 | 1.58 | 0.130 |
| 2 | Bi-Hamulus Distance | 24.1 | 1.4 | 32.2 | 2.4 | 12.79 | 0.000 | 31.1 | 2.4 | 33.4 | 1.7 | 2.42 | 0.030 |
| 3 | Bi-MPT Distance | 20.1 | 1.5 | 27.0 | 1.9 | 12.55 | 0.000 | 26.5 | 1.4 | 27.5 | 2.2 | 1.23 | 0.240 |
| 4 | Inferior mcET Length | 19.6 | 2.5 | 24.5 | 2.3 | 6.43 | 0.000 | 23.6 | 2.8 | 25.5 | 1.3 | 2.03 | 0.060 |
| 5 | Superior mcET Length | 14.5 | 1.8 | 16.6 | 1.2 | 4.31 | 0.000 | 16.4 | 1.5 | 16.9 | 0.8 | 1.02 | 0.320 |
| 6 | CB-PT Height | 10.3 | 1.0 | 13.3 | 1.5 | 7.21 | 0.000 | 12.8 | 1.8 | 13.7 | 1.0 | 1.39 | 0.180 |
| 7 | MPT Height | 18.3 | 1.6 | 27.2 | 2.8 | 12.06 | 0.000 | 25.2 | 2.3 | 29.3 | 1.5 | 4.62 | 0.000 |
| 8 | Maximum mTVP Length | 26.4 | 2.0 | 35.4 | 2.5 | 12.29 | 0.000 | 33.8 | 1.9 | 37.1 | 1.8 | 4.10 | 0.000 |
| 9 | MPT Base Length | 11.8 | 2.5 | 14.5 | 1.3 | 4.20 | 0.000 | 14.1 | 1.3 | 15.0 | 1.2 | 1.73 | 0.100 |
| 10 | PP-FM Distance | 35.0 | 1.9 | 41.5 | 3.1 | 6.41 | 0.000 | 40.4 | 2.7 | 42.6 | 3.2 | 0.11 | 0.113 |
| 11 | Minimum mTVP Length | 21.9 | 2.2 | 30.9 | 2.8 | 11.29 | 0.000 | 28.9 | 2.3 | 32.9 | 1.4 | 4.78 | 0.000 |
| 12 | OET-Hamulus | 10.5 | 1.0 | 11.3 | 1.2 | 2.20 | 0.030 | 11.4 | 0.9 | 11.3 | 1.5 | 0.26 | 0.800 |
| 13 | Hard Palate-PT Height | 8.0 | 1.5 | 14.0 | 2.4 | 9.09 | 0.000 | 12.4 | 2.0 | 15.6 | 1.7 | 3.87 | 0.000 |
| 14 | CB-mcET Angle | 32.5 | 6.1 | 33.0 | 5.0 | 0.32 | 0.750 | 33.4 | 5.9 | 32.7 | 4.1 | 0.27 | 0.790 |
| 15 | mcET Medial Angle | 47.1 | 5.5 | 43.6 | 7.3 | 1.66 | 0.110 | 45.0 | 7.1 | 42.3 | 7.6 | 0.85 | 0.410 |
| 16 | Minimum mTVP-mcET Angle | 44.4 | 5.7 | 50.4 | 4.6 | 3.59 | 0.000 | 48.5 | 4.9 | 52.2 | 3.6 | 1.92 | 0.070 |
| 17 | Maximum mTVP-mcET Angle | 57.4 | 5.4 | 61.8 | 2.7 | 3.27 | 0.000 | 60.8 | 2.8 | 62.8 | 2.3 | 1.79 | 0.090 |
| 18 | Lateral mTVP-mcET Angle | 12.1 | 5.9 | 13.0 | 6.0 | 0.49 | 0.630 | 13.8 | 6.0 | 12.2 | 6.3 | 0.56 | 0.580 |
| 19 | mTVP Area | 158.3 | 27.6 | 251.6 | 31.2 | 9.71 | 0.000 | 232.6 | 31.5 | 270.5 | 16.2 | 3.39 | 0.000 |
Distances and Lengths Measured in millimeters, Area in square millimeters and Angles in Degrees
For adult skulls, variables related to hard palate width, NP height, maximum and minimum mTVP lengths, height of the NP ET orifice above the plane of the hard palate and the mTVP area showed significant sexual dimorphism, with males having greater values.
For comparisons between the skulls of adult and children, all linear variables as well as the mTVP surface area and the maximum and minimum mTVP-mcET angles were significantly greater in the adult skulls. These variables can be grouped into two domains, those that affect ET function and those related to craniofacial measures. The former includes the height of the NP ET orifice above the plane of the hard palate, the inferio-medial CB-mcET vector (magnitude but not direction), the mTVP-mcET vectors (both magnitude and direction) at the osseous ET orifice and at the posterior base of the medial pterygoid plate, and the mTVP surface area. The latter includes the width of the hard palate, the width, depth and height of the NP, and the width and length of the CB. In contrast, the inferio-medial angle between the mcET and CB, the medial angle of the mcET with respect to the parasagittal plane and the lateral angle between the mTVP and mcET at the osseous ET orifice were not significantly different between adults and children.
DISCUSSION
The overall purpose of this study was to establish the differences in the CB, mcET and mTVP vector relationships between adults and children. To that end, a previously developed method for reconstruction of those relationships using skulls was used [15]. In that study, a number of landmarks including the osseous ET orifices, the sulcus tubaris, the tubercle of the medial pterygoid plates and the hamular processes were identified (see Figure) and shown to relate to the directions and magnitudes of the mcET-CB and mTVP-mcET vectors. Here, a set of variables based on those landmarks and simple mathematical functions were used to reconstruct those vector relationships for 18 child skulls aged 3–4 years and 20 adult skulls aged >18 years (20 male, 10 female). The primary assumptions of this methodology are a bilateral symmetry for the CB and an approximate orthogonal relationship between the CB and medial pterygoid plates.
For the main comparisons, the magnitude but not direction of the inferio-medial mcET-CB vector, the magnitude but not direction of the mcET vector with respect to the parasagital plane, the magnitude and direction of the mTVP-mcET vectors at the osseous ET orifice and at the posterior base of the medial pterygoid base, the mTVP surface area and the height of the NP ET orifice above the plane of the hard palate were significantly greater for adults when compared to children. In contrast, the lateral mcET-ET angle at the osseous orifice, angle of decent of the mcET from the CB and the angle of medial deviation of the ET from a parasagittal plane were not different between the two age groups. Secondary measures reflecting CB length and width, NP height, depth and width, and palatal width were significantly greater in adults when compared to the children.
Where similar variables for adult specimens were included in this and the previous study [15], the reported values were relatively consistent. While there are no published data on the vector relationships between the mTVP and mcET, cadaver dissections of adult specimens provide evidence supporting the accuracy for the values of certain measured and calculated variables in the present study. For example, the distance between the hard palate and anterior mcET orifice was reported to increase with advancing age [17] which is consistent with the data for that measure in the present study; the length of the adult mcET was reported to be approximately 24–25 mm [10, 11] as compared to 24.5 mm in the present study; the angle between the adult CB and mcET was reported to vary between 30 and 40 degrees [9, 10] as compared to 33.0 degrees for the present study, and the medial angle of the adult mcET from the parasagittal plane was reported to be approximately 45 degrees [10] as compared to 43.6 degrees for the present study.
The differences between children and adults that could account for the observed poorer ET function and consequently higher prevalence of OME in children include their lesser mTVP-ET vectors which directly relate to the efficiency of mTVP action in displacing the lateral wall of the mcET (i.e. opening the tubal lumen) and the lesser mTVP surface area which is proportional to the maximum force exerted by that muscle in opening the mcET. Of note, OME can be a complication of ME infection with NP bacterial (or viral) pathogens [18] which could be facilitated by the shorter mcET and lower anterior NP mcET orifice with respect to the hard palate in the child skulls [19]. Indeed, Todd and Martin measured the maximum lengths of the mTVP, and the lengths of the mcET and osseous ET in adult human cadavers, and reported that only mcET length directly correlated with the degree of mastoid pneumatization which is an inverse measure of past OME experience [20].
The observed differences between children and adults in the linear dimensions measured in the present study were not unexpected and are attributable to the growth of the craniofacial complex. However, it is unclear as to why the medial mcET angle from the parasagittal plane, the inferio-medial ET angle and the lateral mTVP-mcET angle in the transverse plane were not different between the two age groups. Nonetheless, the mTVP, mcET and CB vector relationships reported here for adults and children may partly explain the differences in ET function and OME prevalence between those age groups. Also, these results can be used to parameterize computer-generated models of the ET function which will allow for a more complete understanding of ET mechanics.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (P50 DC007667). The investigators thank the personnel of Carolina Biological for providing access to the specimens included in this report.
ABBREVIATIONS USED
- CB
Cranial Base
- ET
Eustachian tube
- ME
Middle Ear
- mcET
membrano-cartilagenous portion of the ET
- mTVP
Tensor veli palatini muscle
- NP
Nasopharynx
- OME
Otitis media with effusion
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REVERENCES
- 1.Bluestone CD. Pathogenesis of otitis media: role of eustachian tube. Pediatr Infect Dis J. 1996;15:281–291. doi: 10.1097/00006454-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Doyle WJ, Alper CM, Seroky JT. Trans-mucosal inert gas exchange constants for the monkey middle ear. Auris Nasus Larynx. 1999;26:5–12. doi: 10.1016/s0385-8146(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 3.Kanick SC, Doyle WJ. Barotrauma during air travel: predictions of a mathematical model. J Appl Physiol. 2005;98:1592–1602. doi: 10.1152/japplphysiol.00974.2004. [DOI] [PubMed] [Google Scholar]
- 4.Swarts JD, Alper CM, Seroky JT, Chan KH, Doyle WJ. In vivo observation with magnetic resonance imaging of middle ear effusion in response to experimental underpressures. Ann Otol Rhinol Laryngol. 1995;104:522–528. doi: 10.1177/000348949510400704. [DOI] [PubMed] [Google Scholar]
- 5.Alper CM, Tabari R, Seroky JT, Doyle WJ. Magnetic resonance imaging of the development of otitis media with effusion caused by functional obstruction of the eustachian tube. Ann Otol Rhinol Laryngol. 1997;106:422–431. doi: 10.1177/000348949710600511. [DOI] [PubMed] [Google Scholar]
- 6.Mann W, Jonas I, Munker G. Growth influence on tubal function. Acta Otolaryngol. 1979;87:451–457. doi: 10.3109/00016487909126450. [DOI] [PubMed] [Google Scholar]
- 7.Bylander A, Tjernstrom O. Changes in Eustachian tube function with age in children with normal ears. A longitudinal study. Acta Otolaryngol. 1983;96:467–477. doi: 10.3109/00016488309132733. [DOI] [PubMed] [Google Scholar]
- 8.Doyle WJ. Functional eustachian tube obstruction and otitis media in a primate model. A review. Acta Otolaryngol Suppl. 1984;414:52–57. doi: 10.3109/00016488409122882. [DOI] [PubMed] [Google Scholar]
- 9.Simkins C. Functional Anatomy of the Eustachian Tube. Arch Otolaryngol. 1943;38:476–484. [Google Scholar]
- 10.Graves G, Edwards L. The Eustachian Tube. Arch Otolaryngol. 1944;39:359–397. [Google Scholar]
- 11.Proctor B. Anatomy of the Eustachian Tube. Arch Otolaryngol. 1973;97:2–8. doi: 10.1001/archotol.1973.00780010006002. [DOI] [PubMed] [Google Scholar]
- 12.Rood SR, Doyle WJ. Morphology of tensor veli palatini, tensor tympani, and dilatator tubae muscles. Ann Otol Rhinol Laryngol. 1978;87:202–210. doi: 10.1177/000348947808700210. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone CD. Studies in otitis media: Children’s Hospital of Pittsburgh-University of Pittsburgh progress report--2004. Laryngoscope. 2004;114(11 Pt 3 Suppl 105):1–26. doi: 10.1097/01.mlg.0000148223.45374.ec. [DOI] [PubMed] [Google Scholar]
- 14.Ghadiali SN, Banks J, Swarts JD. Finite element analysis of active Eustachian tube function. J Appl Physiol. 2004;97:648–654. doi: 10.1152/japplphysiol.01250.2003. [DOI] [PubMed] [Google Scholar]
- 15.Doyle WJ. A functiono-anatomic study of Eustachian tube vector relation in four ethnic populations: An osteologic study [Dissertation] Anthropology: University of Pittsburgh; 1977. [Google Scholar]
- 16.Krogman WM. Biological timing and the dento-facial complex. ASDC J Dent Child. 1968;35:377–381. [PubMed] [Google Scholar]
- 17.Ross M. Functional anatomy of the Tensor Palati. Arch Otolaryngol. 1971;93:1–8. doi: 10.1001/archotol.1971.00770060033001. [DOI] [PubMed] [Google Scholar]
- 18.Giebink GS. Otitis media update: pathogenesis and treatment. Ann Otol Rhinol Laryngol Suppl. 1992;155:21–23. doi: 10.1177/00034894921010s105. [DOI] [PubMed] [Google Scholar]
- 19.Bluestone CD, Doyle WJ. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J Allergy Clin Immunol. 1988;81:997–1003. doi: 10.1016/0091-6749(88)90168-6. [DOI] [PubMed] [Google Scholar]
- 20.Todd NW, Martin WS. Relationship of eustachian tube bony landmarks and temporal bone pneumatization. Ann Otol Rhinol Laryngol. 1988;97(3 Pt 1):277–280. doi: 10.1177/000348948809700313. [DOI] [PubMed] [Google Scholar]


