Abstract
The promise of cancer immunotherapy has not been translated into clinical successes in large part due to tumor-associated immune suppression that blocks effective antitumor immunity. Recent findings demonstrate a tumor-induced immunosuppressive mechanism whereby tumor-derived CD73 functions as an ecto-enzyme to produce extracellular adenosine that promotes tumor growth by limiting antitumor T cell immunity via adenosine receptor (AR) signaling. Results with small molecule inhibitors or monoclonal antibodies targeting CD73 in murine tumor models suggest that targeted CD73 therapy is an important alternative and realistic approach to effective control of tumor growth. In particular, it helps T cell-based therapy by enhancing the adaptive immune response machinery, which may increase the function of tumor-infiltrating T lymphocytes, and subsequently lead to improved survival in cancer patients.
Cancer immunotherapy by endogenous or adoptively transferred antitumor T cells is complementary to conventional therapies including surgery, radiotherapy, or chemotherapy. However, a number of obstacles hinder the generation of effective antitumor T cell immunity. During tumor progression, tumor cells foster a tolerant microenvironment and activate a plethora of immunosuppressive mechanisms, which may act in concert to counter effective immune responses (1). The focus of this review is to discuss the newly available experimental evidence demonstrating that CD73 on tumor cells impairs antitumor T cell responses, strongly supporting and extending the concept that extracellular adenosine and the A2A adenosine receptor (A2AR) comprise a pivotal axis in tumor immune escape (2-5). Importantly, these data point out a potential and realistic strategy of targeting CD73 for cancer treatment in addition to, or instead of, the A2AR.
Tumor CD73 controls cancer progression
CD73, known as ecto-5′-nucleotidase (ecto-5′-NT, EC 3.1.3.5) is a glycosyl-phosphatidylinositol (GPI)-linked 70-kDa cell surface enzyme found in most tissues (6, 7). CD73, originally defined as a lymphocyte differentiation antigen, is thought to function as a co-signaling molecule on T lymphocytes and as an adhesion molecule that is important for lymphocyte binding to endothelium. Recent studies have implicated CD73 in the control of a variety of physiological responses including epithelial ion and fluid transport, ischemic preconditioning, tissue injury, platelet function, hypoxia and vascular leak (6-8). However, the role of CD73 in cancer remains unclear. Previous studies reported that CD73 participates in cell–cell and cell–matrix interactions and implicated CD73 in drug resistance and tumor-promotion (9). Using flow cytometry, we have demonstrated that CD73 is widely expressed on many tumor cell lines and is up-regulated in cancerous tissues (10). In agreement, genetic data indicate that CD73 is up-regulated in various human carcinomas including those of colon, lung, pancreas and ovary. Importantly, higher expression levels of CD73 are associated with tumor neovascularization, invasiveness, and metastasis and with shorter patient survival time in breast cancer (9). Moreover, recent studies confirm that CD73 promotes invasion, migration and adhesion of human breast cancer cells (11). Up-regulated expression of CD73 has been found in highly invasive human melanoma cell lines but not in melanocytes or in primary tumor cells. The participation of CD73 per se as a proliferative factor involved in the control of glioma cell growth was described. Clinically, the strong expression of CD73 in papillary thyroid carcinomas has been suggested as a diagnostic aid in the differential diagnosis of thyroid tumors. Meanwhile, up-regulated CD73 ecto-enzymatic activity was proposed to have prognostic value for colon cancer patients. Thus, the increase in CD73 activity during tumor development might be a physiological attempt by cancer cells to provide more substrate to accelerate purine salvage pathway activity. These results suggest that CD73 is an important player in controlling tumor progression (9).
Extracellular adenosine generated by tumor CD73 accumulates in the tumor microenvironment
Elevated levels of extracellular adenosine within the mouse tumor microenvironment have recently been described (2, 12). There are several sources of extracellular adenosine that may be passively or actively released into the tumor microenvironment. Extracellular adenosine is likely produced either due to passive diffusion or active transport of intracellular adenosine or due to enzymatic hydrolysis of extracellular ATP (4, 5). Local tissue hypoxia that follows damage to endothelial cells and microcirculation and the interruption of normal blood and oxygen supply is associated with an increase in intracellular AMP, accumulation of intracellular adenosine, and subsequent transport or diffusion of intracellular adenosine into the extracellular space. Indeed, passive efflux of adenosine is observed in tissue injury, necrosis, and ischemia, and, therefore, may be a significant source of this extracellular nucleoside in solid tumors (4, 9), although this specific mechanism has yet to be clearly described. Because the amount of released adenosine depends on the extent and severity of ischemia/necrosis in tumor tissues, this mechanism may not contribute greatly to extracellular adenosine generation.
Biological actions of CD73 (ecto-5′-NT) are mainly a consequence of the regulated enzymatic phosphohydrolytic activity on extracellular nucleotides. This ecto-enzymatic cascade in tandem with CD39 (ecto-ATPase) generates adenosine from ATP that in turn activates adenosine receptors. In contrast to the intracellular generation of adenosine from cytosolic pools of adenine nucleotides catalyzed by cytosolic 5′-NT in the heart, production of extracellular adenosine by CD73 is likely the predominant means of adenosine generation in epithelial cells despite depending tightly on the availability of extracellular AMP. We have demonstrated that mouse and human epithelial tumor cells express CD39 (unpublished data) and CD73. Importantly, CD73 is significantly up-regulated in cancerous tissues accompanied by high enzymatic activity which can mediate the production of extracellular adenosine (10). Therefore, our results clearly support the concept that tumor cells themselves contribute to the elevated levels of adenosine in the tumor microenvironment through surface CD73 enzymatic activity. We assume that high levels of tumor CD73 expression are likely induced in the local tumor microenvironment. This concept is supported our demonstration of CD73 up-regulation on cultured ovarian cancer cells treated in vitro with malignant ascites, suggesting that soluble factors (presumably proinflammatory cytokines) in the tumor induce CD73 expression (10). However, reports in the literature regarding the regulation of CD73 expression by proinflammatory cytokines are conflicting. We do not know whether these inflammatory cytokines affect CD73 on cancer cells, but a hypoxia inducible factor-1α (HIF-1α)-dependent regulatory pathway for CD73 on epithelial cells has been recently recognized (13). Thus, the present evidence supports the concept that hypoxia, HIF-1α and CD73 in sequential order convert AMP to adenosine leading to elevated levels of extracellular adenosine in the tumor (4, 10).
Because the constant release of adenosine is counteracted by uptake followed by metabolism by either adenosine kinase (AK) (phosphorylation to form AMP), or by adenosine deaminase (ADA) (deamination to inosine), it is more relevant to examine the ratio of the adenosine-producing activity of CD73 to the combined adenosine-utilizing activities of ADA and AK. In humans (but not in rodents), ADA can be found on the cell surface bound to CD26 in addition to its normal cytoplasmic location. Recently, there are a number of examples showing a significant correlation between increased expression of CD73 and/or decreased expression of ADA and cancer progression (9). Moreover, HIF-1α can repress intracellular AK as well as up-regulate CD73 (4). Therefore, it appears that these four enzymatic activities (CD39/CD73, ADA and AK) collaborate to maintain high adenosine concentrations in solid tumors, but further work is needed for formal demonstration.
Extracellular adenosine generated by tumor CD73 impairs antitumor T cell responses
Adenosine potently inhibits a series of T cell responses, including antigen-induced proliferation, secretion of IL-2 and proinflammatory cytokines such as interferon-γ and TNF-α, up-regulation of CD25, induction of cytolytic effector molecules such as perforin and Fas ligand, adhesion of killer lymphocytes to tumor cells, and granule exocytosis by CTL (14). In addition, adenosine and adenosine analogues can suppress NK cell as well as LAK cell function (14). Thus, given the strong immunosuppressive properties of adenosine, and its presumed high concentration in solid tumors, it is reasonable to infer that adenosine may constitute an important part of the so-called “immunological barrier”, leading to a failure of mounting an effective antitumor immune response. Since many tumor cells express functional CD73, we examined the regulatory role of tumor CD73 in antitumor T cell immunity. We found that extracellular adenosine generated by tumor CD73 in vitro and in vivo inhibited both the activation phase and effector phase of the antitumor T cell response and promoted T cell apoptosis. Moreover, knockdown of CD73 on tumor cells by siRNA completely restored efficacy of adoptive T cell therapy and led to long-term tumor-free survival of tumor-bearing mice, suggesting that tumor CD73-mediated immune suppression through its enzymatic activity significantly contributes to cancer immune evasion (10). Consistent with our finding, another group simultaneously demonstrated that inhibition of CD73 by an anti-CD73 monoclonal antibody (mAb) triggered adaptive antitumor immunity and inhibited breast tumor growth and metastasis (15). We each concluded that CD73 can serve as a novel target for cancer treatment to improve antitumor immunity (10, 15).
Extensive mouse and human studies using adenosine receptor subtype-selective agonists and antagonists indicate that adenosine inhibits T cell activation and effector activity primarily via the A2A adenosine receptor (A2AR). We showed that blockade of the A2AR with a selective antagonist augmented the efficacy of adoptive T cell anticancer therapy (10), which is compatible with a previous ground-breaking study showing that endogenous or adoptively transferred antitumor T cells were much more resistant to inhibition in the tumor microenvironment by either genetic ablation of the A2AR or A2AR antagonist treatment (2). It has been demonstrated that A2AR engagement suppresses T cell proliferation, inflammatory cytokine secretion, and reduces surface expression of cytokine receptors by elevating the intracellular levels of cyclic (c)AMP through adenylyl cyclase stimulation (5, 16). Since A2BR (2) and A3 (17) adenosine receptors are also involved in T cell activity, further studies are needed to determine the exact contribution of A2BR and A3R to function of antitumor T cells (2).
We and others found that blockade of CD73 had no impact on primary tumor growth in T-cell-deficient mice (10, 15), suggesting that CD73 may enhance tumor growth in a T cell-dependent manner but independently of any effect on NK cells. This is further supported by the fact that depletion of NK cells using anti-asialo-GM1 antibody did not affect tumor growth in T cell-deficient mice (15). However, we cannot exclude the possibility that extracellular adenosine in the tumor modulates NK cell function because several studies have reported that adenosine and adenosine analogues suppress NK cell proliferation and killing activity (14).
Implications and future directions
One of the most critical questions is to determine whether adenosine is a mere passive product of necrosis and ischemia in solid tumors or if it is actively released during tumor progression as a result of the activity of adenosine-generating enzymes. It is now evident that extracellular adenosine produced through the activity of the ecto-enzymes (CD39 and CD73) on tumor cells can sufficiently down-regulate antitumor immunity (Figure 1). We have demonstrated that adenosine generated by tumor CD73 impairs cellular antitumor immune responses at multiple levels including T cell activation, clonal expansion of tumor-specific T cells with helper and cytolytic effector function, tumor cell killing by CTL and survival of CTL. This suggests a tumor-“autonomous” role of CD73 in tumor immune escape via generation of adenosine that is sufficient to inhibit anti-tumor T cells, and mimics the paracrine secretion of adenosine previously described for regulatory T cells (Treg) (18). Thus, targeting the enzymatic activity of tumor CD73 appears to be therapeutic for the tumor-bearing host. Indeed, it is impossible to target adenosine directly in vivo because adenosine has a short half-life (<10 seconds) and is vital to preserve normal tissue functions. Importantly, we have not noticed any signs of autoimmunity in mice treated by tumor-antigen specific T cells and tumor CD73 knockdown (10). Our data further support the recent view stated by Ohta et al. (2) that targeting the adenosine-A2AR pathway is a cancer immunotherapy strategy to prevent inhibition of antitumor T cells in the tumor microenvironment. We expect that blocking A2AR signals on T cells and targeting CD73 on tumor cells could improve therapeutic efficacy beyond that achievable with either alone. Interestingly, we also found that cancer cells express both CD39 and CD73, indicating that extracellular adenosine derived from ADP/ATP is generated most likely through the combined action of CD39 and CD73. ATP has recently been reported as an important immunological danger signal that recruits antigen-presenting cells and promotes the maturation of dendritic cells in the tumor (19). Consequently, antitumoral immune responses could be substantially improved by the inhibition of tumor CD39. Whether additional benefits would be obtained by inhibiting the expression of both CD39 and CD73 remains to be explored.
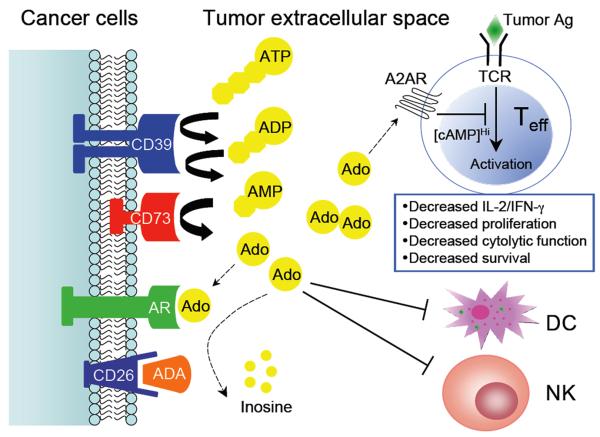
Figure 1. Schema of extracellular adenosine metabolism in tumor-induced immune suppression.
The sequential action of ecto-ATPase (CD39) and ecto-5′-nucleotidase (CD73), both of which can be induced by tumor hypoxia, to degrade ATP represents a primary pathway of extracellular adenosine generation in the tumor microenvironment. It is very likely that coordinated expression and activity of CD39/CD73 (increased) and ecto-adenosine deaminase ADA (decreased) which in human cells can be bound to the cell surface by CD26 contribute to the elevated levels of extracellular adenosine during tumor progression. The exact roles of CD39/CD73 on the non-malignant host cells (e.g. Treg) in the tumor microenvironment remain unclear. High concentrations of adenosine suppress antitumor T cell activation, survival and effector function through A2AR engagement. Meanwhile, adenosine may trigger certain adenosine receptors (AR) on cancer cells to promote tumor cell chemotaxis and metastasis. In addition, adenosine can negatively modulate differentiation and function of dendritic cells (DCs), and further inhibit natural killer (NK) cell proliferation and cytolytic function.
CD73 directed therapies have not been well-developed. The CD73 inhibitor α,β-methylene ADP (APCP) has been successfully tested in our tumor models (10). Moreover, others have documented use of APCP in various murine models (7). The drug APCP functions as an inhibitor of CD73 because it is a non-hydrolyzable structural mimic of ADP. It has the attraction of being cheap, widely available and well-tolerated in vivo. However, at levels achievable in vivo it might not fully inhibit the enzymatic activity of CD73 as its half life in vivo and biodistribution are not well characterized. Thus, tumor regression with APCP could be incomplete compared with that achieved by genetic deletion of CD73, which accomplishes nearly 100% reduction of CD73 enzyme activity for 100% of the time. Furthermore, undesirable side effects could occur when APCP is administrated intensively and frequently. In view of these considerations, it would be interesting to design and synthesize a new generation of CD73 enzyme inhibitors with the ability to inhibit enzyme activity irreversibly and with increased stability in vivo by structural optimization to achieve stronger antitumor effects. Although the anti-CD73 mAb (TY/23) is less effective in inhibiting CD73 enzymatic activity than APCP, its effects in cancer treatment have been demonstrated (15). Moreover, the effects of an anti-CD73 mAb may extend beyond inhibition of CD73 enzymatic activity. For instance, anti-CD73 mAb may directly inhibit the adhesion of tumor cells to endothelial cells, thereby inhibiting their invasiveness. It should be noted that the above observed antitumor effects of APCP or anti-CD73 mAb may or may not be attributed only to the inhibition of CD73 enzyme activity on tumor cells. It appears that the generation of extracellular adenosine by CD73 can protect tumors in both a tumor-autonomous way and by inhibiting incoming antitumor T cells by host Treg. Indeed, it was found that CD73 is over-expressed on Treg cells (20) and the CD39-CD73 tandem suppresses T cell function (18). In this regard, we have evidence that host CD39/CD73 forms an important part of the immunosuppressive apparatus of Treg and the endothelial barrier to antitumor T cell homing to tumors (submitted). Moreover, it has been recently demonstrated that human tumor-associated Treg cells highly expressing CD39 suppressed Th17 cell development through the adenosinergic pathway (21), which could be a previously unappreciated mechanism by which tumors evade the immune system (22). Therefore, the actual in vivo effects of a therapeutic approach that uses CD39/CD73 as a molecular target for cancer treatment may far exceed the expectations raised by our published experimental data.
Of note, the success of the proposed strategy is tightly dependent on the existence of ongoing antitumor T cell immunity. If antitumor T cells are not present, the ablation of CD73 likely will have a minimal effect on tumor regression, except in situations when it can interfere with CD73-mediated tumor cell adhesion and/or chemotaxis (15). Because endogenous antitumor immunity that can be induced is often insufficient and transient, targeted CD73 therapy may be most effective combined with other forms of immunotherapies, such as adoptive T cell transfer, immune-stimulating mAbs or dendritic cell (DC) vaccines. Indeed, we demonstrate that tumor-bearing mice can be cured by combinatory treatment of CD73 inhibition and T cell therapy (10). In addition, targeting CD73 may have benefits other than enhancing adaptive antitumor immunity, and could be effective even for the treatment of non-immunogenic or weakly immunogenic tumors. For instance, an important recent finding suggested that tumor CD73-generated adenosine promoted spontaneous lung metastasis of 4T1 breast tumors in the absence of T cells (15). Others have shown that knockdown of CD73 in MB-MDA-231 human breast cancer cells prevented their adhesion to extracellular matrix and inhibited their migration (11).
Finally, our identification of CD39/CD73-dependent generation of extracellular adenosine by tumor cells supports mutual dependence of CD39/CD73 and A2AR-mediated immunosuppression in the tumor microenvironment. These data raise the feasibility of potent strategies to overcome this tumor-induced immunosuppression by targeting the important axis of CD39/CD73-A2AR in the tumor. This targeted CD39/CD73-A2AR therapy directs the development of focused pharmacologic strategies to reduce or ablate the impact of adenosinergic immune suppression in cancer patients, thereby increasing the effectiveness of therapeutic cancer vaccines and other T cell-based cancer immune therapies.
Acknowledgement
I thank Drs. Tyler J. Curiel and Linda F. Thompson for their critical reading of the manuscript.
Financial assistance was provided by URC new investigator funds, CTSA grant (UL1RR025767) and the Cancer Therapy and Research Center (2P30 CA054174-17).
Due to space limitations, I regret that I could not cite many worthy references in this field from my colleagues.
References
- 1.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–20. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 4.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–52. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 5.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–82. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 6.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 7.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–60. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–87. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 9.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–73. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 10.Jin D, Fan J, Wang L, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–55. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Zhou X, Zhou T, et al. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–72. doi: 10.1007/s00432-007-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–5. [PubMed] [Google Scholar]
- 13.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int J Oncol. 2008;32:527–35. [PubMed] [Google Scholar]
- 15.Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 107:1547–52. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 17.Hoskin DW, Butler JJ, Drapeau D, Haeryfar SM, Blay J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int J Cancer. 2002;99:386–95. doi: 10.1002/ijc.10325. [DOI] [PubMed] [Google Scholar]
- 18.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aymeric L, Apetoh L, Ghiringhelli F, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–8. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 20.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 21.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]