Abstract
Although transgenic and knockout mice have helped delineate the mechanisms of action of diverse noxious compounds, it is still difficult to determine unequivocally the subpopulations of primary afferent nociceptor that these molecules engage. As most noxious stimuli lead to tissue and/or nerve injury, here we used induction of activating transcription factor 3 (ATF3), a reliable marker of nerve injury, to assess the populations of primary afferent fibers that are activated after peripheral administration of noxious chemical stimuli. In wild-type mice, hindpaw injections of capsaicin, formalin, mustard oil or menthol induce expression of ATF3 in distinct subpopulations of sensory neurons. Interestingly, even though these noxious chemicals are thought to act through subtypes of transient receptor potential (TRP) channels, all compounds also induced ATF3 in neurons that appear not to express the expected TRP channel subtypes. On the other hand, capsaicin failed to induce ATF3 in mice lacking TRPV1, indicating that TRPV1 is required for both the direct and indirect induction of ATF3 in sensory neurons. By contrast, only low doses of formalin or mustard oil failed to induce ATF3 in TRPA1 null mice, indicating that injections of high doses (>0.5%) of formalin or mustard oil recruit both TRPA1 and non-TRPA1 expressing primary afferent fibers. Finally, peripheral injection of menthol, a TRPM8 receptor agonist, induced ATF3 in a wide variety of sensory neurons, but in a TRPM8-independent manner. We conclude that purportedly selective agonists can activate a heterogeneous population of sensory neurons, which ultimately could contribute to the behavioral responses evoked.
INTRODUCTION
Our appreciation of the complexity of primary afferent nociceptors and their contribution to the transmission of pain messages has increased tremendously in recent years, in large part because of the cloning and characterization of the receptors and channels that are activated by different noxious stimuli [5], [12], [44] and [58]. For example, temperature-sensitive afferents, which express the transient receptor potential TRPV1, not only respond to noxious heat and capsaicin, but are also modulated by the acidity of the chemical milieu of inflammation [61]. TRPA1, by contrast [34] and [61] has been implicated in the production of pain by other chemical mediators (notably formalin) and, at least in some studies, noxious cold [2], [3], [4], [7], [23], [30], [46] and [49]. More recently, TRPA1 has been implicated in mechanotransduction in cutaneous sensory neurons [38] and [55]. Finally, afferents that express TRPM8 not only respond to menthol but are also activated by cold stimuli [8] and [19].
Despite our expanding knowledge of the molecular targets of these “pain-producing” stimuli, there is still a lack of agreement as to the expression of these targets in primary afferents. For example, it was originally reported that TRPA1 is restricted to a subset of TRPV1 afferents [30], [34], [49] and [57], the majority of which are of the peptidergic subclass [21]. However, electrophysiological studies found that high doses of formalin unquestionably activate both myelinated and unmyelinated afferents [27], [33], [53] and [54], suggesting that TRPA1 has a wider distribution than does TRPV1. In fact, Kwan and colleagues [38] recently reported that dorsal root ganglion (DRG) neurons of all sizes express TRPA1. Similarly, cold sensitivity can be generated in primary afferents that lack TRPM8 or TRPA1 channels [8] and [48], suggesting that other afferents and or/molecular targets come into play (See also [50]). In fact, a recent report found TRPM8 expression in both myelinated and unmyelinated afferents [34].
An alternative approach to localization of these channels by immunohistochemistry or in situ hybridization is to assay for the functional consequences of their activation. For example, Mizushima and colleagues [47] reported that a noxious cold stimulus induces p38 in TRPA1 but not in TRPM8-expressing small DRG neurons. Given that peripheral administration of a noxious stimulus is often associated with tissue and/or nerve injury, here we took a different approach to the problem. Specifically, we monitored induction of activating transcription factor 3 (ATF3), a sensitive cellular marker of nerve injury [62], to reveal the primary afferent fibers that are engaged by diverse chemical, noxious stimuli. We show that peripheral injection of different chemical stimuli reliably induces a dose- and time-dependent expression of ATF3 in subpopulations of DRG neurons. In some experiments the pattern of ATF3 expression was consistent with the anatomical localization of a particular target; in other instances, e.g. capsaicin induction of ATF3 in non-TRPV1 expressing afferents, this was not the case. We conclude that purportedly selective agonists activate a heterogeneous population of sensory neurons, which ultimately could contribute to the behavioral response elicited by these diverse noxious stimuli.
MATERIAL AND METHODS
Animals and treatments
All experiments were reviewed and approved by the Institutional Care and Animal Use Committee at the University of California San Francisco. TRPV1-, TRPA1- and TRPM8-null mice were kindly provided by David Julius (UCSF). MrgprdDTR mice were kindly provided by David Anderson (CalTech).
Into the plantar side of the left hindpaw of lightly restrained, awake animals, we injected 20μl of a solution containing either saline (0.9% sodium chloride), complete Freund’s adjuvant (CFA, Sigma, 50% diluted in saline), carrageenan (CARR, Sigma, 2.5% diluted in saline), dimethyl sulfoxyde (DMSO 25% diluted in saline), formalin (0.5%, 2% or 5% diluted in phosphate buffer 0.1M; Formaldehyde, Fisher Scientific), mustard oil (0.75% or 10% diluted in oil; allyl isothiocyanate, Sigma), icilin (0.1, 1, 10 or 50 mM diluted in 25% DMSO; Tocris) or menthol (4, 40 or 80 mM diluted in 25% DMSO; Tocris). Mice were euthanized at different times after the injection (1, 2 or 7 days).
For capsaicin (8-methyl-N-vanillyl-6nonenamide, Sigma) and resiniferatoxin (RTX, Sigma) injections, mice were first anesthetized with an intraperitoneal injection of ketamine (60 mg/kg)/ xylazine (8.0 mg/kg). For capsaicin, mice received 3, 20, 200 or 400 μg of capsaicin diluted in 10% ethanol, 10% Tween-80, 0.9% sodium chloride [ETS], in the plantar surface of the left paw. Some mice received an injection of capsaicin every 3 days. Three days after the last injection, the mice were euthanized. For RTX injections, mice received increasing doses of RTX (intraperitoneally), once a day: 30 μg/kg (day 1), 70 μg/kg (day 2), 100 μg/kg (day 3), 200 μg/kg (day 4) and 200 μg/kg (day 14). Two weeks after the last injection, some of the RTX-injected mice received an injection of 0.5% formalin into the intraplantar surface of the left paw and were euthanized 2 days later. We used 3 animals per group, unless otherwise indicated.
Immunohistochemistry
Antibodies: rabbit anti-ATF3 (1:1000, Santa Cruz Biotechnologies), mouse anti-CGRP (1:500, Sigma), goat anti-diphtheria toxin (DTR; 1:500, R&D Systems), mouse anti-N52 (1:10,000, Sigma), guinea-pig anti-Substance P (1:10,000; generous gift from J.Maggio), guinea-pig anti-TRPV1 (1:1000, gift of D, Julius) and biotynylated IB4 (1:500, Vector Laboratories). The antibodies used in this study produce expression patterns that are very comparable to those reported in a host of studies. Most importantly, neither the TRPV1 nor the SP antibodies show positive staining in TRPV1 and preprotachykinin A mutant mice, respectively. Furthermore, we only find ATF3 labeling in animals in which a particular injury paradigm was used. Finally, we never observed DTR immunoreactivity in mice that do not express the DT receptor.
We anesthetized the mice with pentobarbital (100 mg/kg) and then perfused them transcardially with 10 ml saline (0.9% NaCl) followed by 30 ml of 3.7% formaldehyde in phosphate buffer (PB) 0.1M, at room temperature (RT). Dorsal root ganglia were dissected out, post-fixed in the same solution for 3h and cryoprotected in 30% sucrose-phosphate-buffered saline (PBS) overnight at 4°C. Fourteen μm cryostat sections were preincubated for 30 min at RT, in PBS containing 0.5% Triton X-100 and 10% normal goat serum (NPBST) and then immunostained overnight at RT in the same buffer containing the antibody. For double labeling experiments, primary antibodies were incubated simultaneously. After washing 3 times in NPBST, sections were incubated for 1h with Alexa-conjugated (488 and/or 546) secondary antibodies (1:700; Invitrogen), rinsed 3 times in NPBST, mounted in fluoromount-G and coverslipped. Sections were viewed with a Nikon Eclipse fluorescence microscope and images were acquired with a Spot Camera and processed with Adobe Photoshop, version 6.0.
Cell counting
Serial sections of entire L4-5 DRGs from 3 animals were cut (14μm) and placed on Superfrost microslides (Fisherbrand). To ensure that all neurons were sampled, we mounted, immunostained and eventually counted patterns in every 4th section of the ganglia. With this approach, at least 6-7 sections per DRG per animal were counted, which depending on the marker studied, corresponds to 600 to 1000 neurons sampled per DRG. The percentage of ATF3-labeled neurons was calculated by dividing the number of ATF3+ neurons by the total number of neurons counted × 100. The percentage of double-labeled neurons (marker vs ATF3) was calculated by dividing the number of double-labeled neurons by the number of single ATF3-labeled neuron × 100. Values are given as mean ± standard deviation (STD). Statistical significance was assessed with ANOVA and Student’s t-Test. A p-value of ≤0.05 was considered significant and is indicated with an asterisk (*).
RESULTS
ATF3 is a marker of nerve injury
Sciatic nerve transection (axotomy) induces strong expression of ATF3 in large numbers of sensory neurons (figure 1A; ~73% of total neurons). By contrast, inflammation induced by Complete Freund’s Adjuvant (CFA, figure 1B, 48h post-injection) does not. This indicates that nerve damage, not merely increased activity of sensory fibers, is the critical trigger of ATF3 expression in stimulated sensory neurons. Importantly control injections of saline (figure 1D), DMSO (Figure 1E) or ETS (figure 1F) did not induce ATF3, which indicates that neither needle insertion nor injection of the vehicles used to dilute the drugs, produced measurable damage to the peripheral terminals of DRG neurons. Injections of carrageenan (CARR, figure 1C, 48h post-injection), another inflammatory agent, doubled the number of ATF3+ DRG neurons compared to controls (needle injection), two days after injection. This corresponded to a very slight increase (from ~1 to ~2%, Table 1), albeit a statistically significant one. Having established these baseline parameters, we next asked whether ATF3 was induced after administration of different noxious, chemical stimuli including capsaicin, formalin, mustard oil and menthol (Table 1).
Figure 1. Nerve damage, not merely activation of peripheral afferents, is required for ATF3 induction in DRG neurons.
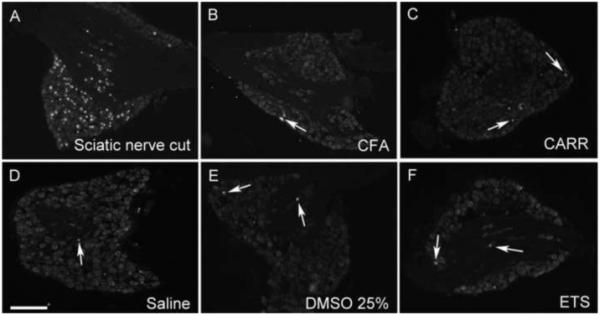
(A) Nerve injury (axotomy) induces expression of ATF3 (red) in the nuclei of large numbers of DRG neurons. By contrast, neuronal activation associated with the inflammation induced by Complete Freund’s Adjuvant (CFA, 48h post-injection; B) does not. Carrageenan (CARR, 48h post-injection; C) induced a very small increase in the percentage of ATF3+ neurons compared to control animals. Neither saline (D), dimethyl sulfoxyde (DMSO 25%; E) nor the vehicle for capsaicin (ethanol/triton/saline; ETS, F) induces ATF3 expression above that produced by needle injection alone. Arrows point to ATF3+ neurons. Calibration bar 200μm.
Table 1. ATF3 induction in sensory neurons of wild-type (WT) and different TRP null (KO) mice, after administration of various noxious, chemical stimuli.
| % ATF3/neuron counted (WT) |
% ATF3/neuron counted (KO) |
|
|---|---|---|
| Needle | 1.2 ± 0.4 | - |
| CFA | 1.5 ± 1.3 | - |
| CARR | 2.6 ± 0.8*** | - |
| DMSO 25% | 1.1 ± 0.3 | - |
| Axotomy | 72.8 ± 1.7 | - |
| CAP 3μg | 1.6 ± 0.2 | - |
| CAP 200μg | 12.1 ± 2.1*** | 1.5 ± 0.3††† (TRPV1) |
| Form 0.5% | 16.4 ± 2.6*** | 2.2 ± 0.9††† (TRPA1) |
| Form 2.0% | 30.8 ± 1.8*** | 27.8 ± 2.7 (TRPA1) |
| Form 5.0% | 50.8 ± 5.3*** | 45.9 ± 5.4 (TRPA1) |
| MO 10% Top | 8.5 ± 1.5*** | 1.5 ± 0.6††† (TRPA1) |
| MO 0.75% inj | 8.9 ± 2.2*** | 2.3 ± 0.5††† (TRPA1) |
| MO 10% lnj | 30.4 ± 8.8*** | 22 ± 9.3 (TRPA1) |
| Ment 4mM | 1.2 ± 0.4 | - |
| Ment 40 mM | 9 5 ± 2.5*** | 10.5 ± 2.0 (TRPM8) |
| Ment 80 mM | 8.6 ± 1.3*** | 11.1 ± 1.9††† TRPM8 |
CARR: carrageenan; CAP: capsaicin; CFA: complete Freund’s adjuvant; DMSO: dimethyl sulfoxyde; Form: formalin; MO: mustard oil; Ment: menthol. Values are given as mean ± standard deviation; (−) not determined
p<0.001 relative to control/vehicle injected mice
p<0.001 relative to WT mice that received the same compound.
Nerve-injury induced by capsaicin is TRPV1-mediated
Capsaicin, the pungent ingredient in hot chili peppers, elicits pain (burning sensation) and inflammation by activating small nociceptive primary afferents that express the TRPV1 channel [13] and [14]. This initial excitation is followed by a period in which TRPV1+ neurons become unresponsive to further noxious stimuli. In fact, high topical-applied doses (5-10%) of capsaicin provide long-lasting analgesia in part through a desensitization process and also by inducing a transient degeneration of TRPV1+ peripheral terminals [16] and [29]. Consistent with the latter results, figure 2 illustrates that hindpaw injection of a high dose of capsaicin induces ATF3 (i.e. nerve terminal injury) in a large number of DRG neurons. All ATF3 positive neurons are located ipsilateral to the injection side. Importantly, however, at doses routinely used to assess pain behavior (3.0 μg), capsaicin failed to induce ATF3 expression in DRG neurons (Table 1). The magnitude of ATF3-induction was dose-dependent (figure 2A and 2D), with the number of ATF3-positive neurons increasing with higher doses of capsaicin (Table1) or with repeated injections of the same dose (data not shown). ATF3 expression in the contralateral DRG or in trigeminal neurons did not differ significantly from that in control groups, regardless of dose (data not shown).
Figure 2. Capsaicin injures both TRPV1 and non-TRPV1 expressing DRG neurons.
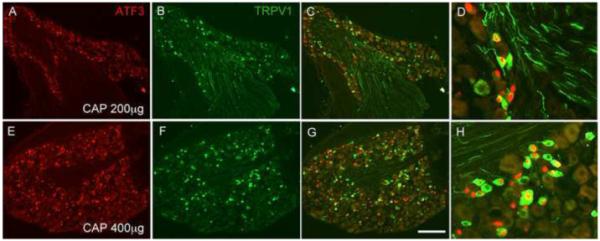
The effect of capsaicin (CAP) injection on ATF3 expression (red) is dose-dependent, in both TRPV1 (green) and non-TRPV1 expressing sensory neurons. Panels D and H show higher magnifications of C and G, respectively. Calibration bar 200μm.
Because administration of the 200μg dose of capsaicin (twice, 3 days apart) is tolerated by the mice, but still induces ATF3 in a large number of neurons, we used this dose to determine the neurochemical identity of the capsaicin-induced ATF3+ sensory neurons. Figure 3C-F shows that capsaicin injection induces ATF3 primarily in small, unmyelinated DRG neurons (only ~4% N52-positive, a marker of neurons with myelinated axons, figure 3C and Table 2), consistent with the predominant expression of TRPV1 in small diameter DRG neurons. Surprisingly however, no more than 50% of ATF3+ neurons immunostained for TRPV1, regardless of dose or even after repeated injections (Figures 2C and 2F and Table 2). This result indicates either that capsaicin can influence (i.e. damage) sensory neurons that do not express TRPV1 (presumably secondary to an action on the TRPV1+ afferents) or that a large proportion of DRG neurons expresses levels of TRPV1 that are too low to be detected by immunohistochemistry (see Discussion). Note however that ATF3 was not induced in all TRPV1-positive neurons (~60%, see Table 2), even at the highest dose used, which is expected given that not all capsaicin-sensitive DRG neurons innervate the hindpaws.
Figure 3. Capsaicin-induced ATF3 expression in unmyelinated afferents of both the peptidergic and nonpeptidergic class is TRPV1-dependent.
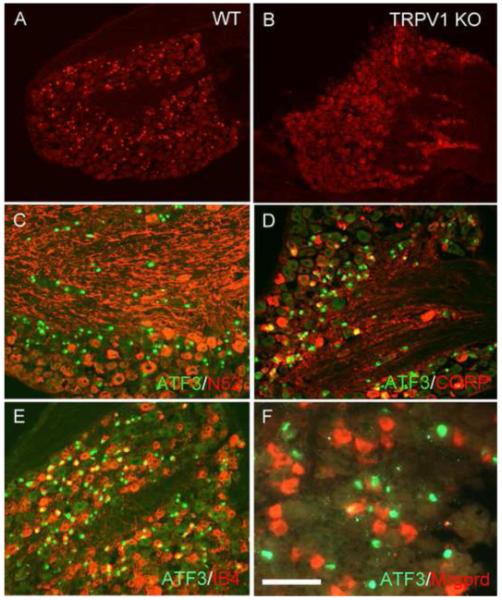
(A-B) In wild-type animals (A) capsaicin induces ATF3 expression (red) in large numbers of DRG neurons. By contrast, in TRPV1 null mice, the same dose of capsaicin fails to induce ATF3, indicating that the capsaicin-induced ATF3 expression is TRPV1-dependent. (C-F) In wild-type mice, capsaicin induces ATF3 expression (green) almost exclusively in unmyelinated afferents (only ~4% are N52+, a marker of neurons with myelinated axons, red in C). The capsaicin-induced ATF3 expression occurs in both the peptidergic/CGRP-immunoreactive, (red in D) and nonpeptidergic (bind IB4, red in E) subsets of unmyelinated DRG neurons. But the ATF3 expression only occurred in a very small percentage of Mrgprd subset of IB4 afferents (<5%) red in F). Calibration bar A-B 200μm; C-E 100μm and F 75μm.
Table 2. Distribution of ATF3 expression in subpopulations of sensory neurons after administration of various noxious, chemical stimuli.
| TRPV1/ATF3 | ATF3/TRPV1 | IB4/ATF3 | ATF3/IB4 | Mrgprd/ATF3 | ATF3/Mrgprd | N52/ATF3 | ATF3/N52 | |
|---|---|---|---|---|---|---|---|---|
| CAP 200μg | 48.0 ± 5.2 | 60.0 ± 16.4 | 31.0 ± 2.0 | 33.0 ± 2.7 | 3.6 ± 0.2 | 1.9 ± 0.1 | 4.0 ± 1.2 | 4.6 ± 0.9 |
| Form 0.5% | 32.6 ± 1.4 | 16.0 ± 3.6 | 74.5 ± 2.2 | 47.9 ± 9.4 | 44.0 ± 6.3 | 24.3 ± 3.7 | 12.9 ± 0.6 | 4.5 ± 0.9 |
| Form 2.0% | 29.4 ± 2.3 | 19.7 ± 3.3 | 50.7 ± 7.8 | 43.3 ± 4.0 | (−) | (−) | 40.9 ± 3.4 | 17.0 ± 3.5 |
| Form 5.0% | 23.1 ± 2.1 | 27.3 ± 0.6 | 48.6 ± 6.5 | 62.5 ± 3.9 | (−) | (−) | 35.5 ± 5.7 | 34.7 ± 3.0 |
For capsaicin (CAP) the analysis was performed 3 days post-injection; for formalin (Form) the analysis was at 2 days. Values are given as mean ± standard deviation; (−) not determined.
Double labeling experiments further showed that injured neurons (ATF3+) included those of the peptidergic (~40% CGRP-positive; figure 3D) and non-peptidergic (~31% bind the isolectin IB-4; figure 3E and Table 2) classes of unmyelinated afferents. To determine if the capsaicin-responsive nonpeptidergic afferents also expressed the Mrgprd receptor, a marker of nonpeptidergic cutaneous sensory neurons [67], we injected capsaicin into the paw of a reporter mouse that expresses green fluorescent protein and the human diphtheria toxin receptor (DTR) in the Mrgprd locus [15]. As shown Figure 3F, among ATF3+ DRG neurons, very few co-stained for DTR (~4%, see also Table 2), indicating that the great majority of nonpeptidergic cutaneous sensory neurons that express DTR (i.e. are Mrgprd receptor positive) do not respond to capsaicin.
Because a rather high dose of capsaicin was required to induce ATF3 and because of the induction of ATF3 in DRG neurons that do not express TRPV1, we next asked whether the capsaicin-induced nerve damage was a non-specific phenomenon, rather than a consequence of TRPV1 activation. To this end we injected capsaicin in TRPV1 null mice. Figures 3A-B illustrate that these high doses of capsaicin failed to induce ATF3 in the mutant mice (see also Table 1), indicating that TRPV1 is indeed required for ATF3 induction following peripheral injections of capsaicin. Interestingly, the induction of ATF3 in non-TRPV1 neurons was also prevented in the TRPV1 mutant mice. This result indicates that capsaicin-induced ATF3 expression in the non-TRPV1 sensory neurons must have been indirect, through activation of neighboring, TRPV1-expressing DRG neurons, either in the periphery or at the level of the DRG.
Formalin-induced nerve-injury at low doses is TRPA1-mediated
Recent reports found that TRPA1 is the channel through which formalin binds and exerts its pronociceptive effects [39] and [46]. That conclusion, however, was based on the use of relatively low doses of formalin, much lower than is typically used in the formalin test, which is presumed to model postoperative pain. On the other hand, Puig and Sorkin [54] reported evoked activity in a wide variety of afferents after administration of high doses of formalin. If the two reports are compatible, then TRPA1 must have a much broader distribution than has been described, or high doses of formalin must have a non-TRPA1-dependent mechanism of action. As we are particularly interested in identifying the populations of sensory neurons that are influenced by doses of formalin used in routine pain tests, we assayed for formalin-induced ATF3 expression using a range of doses, in both WT mice and in mice in which TRPA1 is deleted.
Injection of a low dose of formalin (0.5%) indeed induced ATF3 expression in a small population of DRG neurons (Table 1, figure 4A). One day after the injection, ATF3 was induced in both myelinated and unmyelinated afferents. However, most formalin-responsive neurons were unmyelinated (only ~13% N52+, figure 4B and Table 2), consistent with the TRPA1 channel being expressed in a subset of TRPV1 afferents. Surprisingly however, the vast majority of unmyelinated ATF3+ neurons were nonpeptidergic (~75% bind the isolectin B4, figure 4C and ~44% were DTR+, i.e. expressed the Mrgprd receptor, Table 2) and only a third expressed TRPV1 (figure 4D and Table 2).
Figure 4. Formalin injures both TRPV1 and non-TRPV1 expressing unmyelinated afferents.
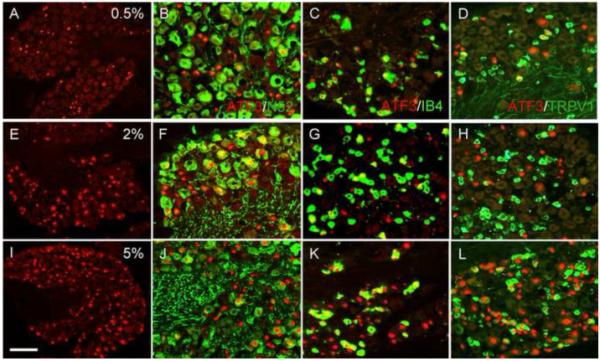
(A-L) Formalin induces ATF3 expression (red) in large numbers of DRG neurons in a dose-dependent manner, including both myelinated afferents (N52+, green in B, F and J) and unmyelinated afferents that bind IB4 (green in C, G and K). (D, H and L). As for capsaicin, formalin induces ATF3 (red) in both TRPV1 (green) and non-TRPV1 expressing sensory neurons. Calibration bar A, E and I 200μm; B-D, F-H and J-L 75μm.
The induction of ATF3 was both dose and time-dependent. Thus, increasing the dose of formalin into the range used in the formalin test of pain behavior (2% and 5%) produced much greater ATF3 expression, and in a very diverse population of sensory neurons, which included both myelinated and unmyelinated afferents (figures 4E-L and Table 2). Furthermore, ATF3 was still detected in a large number of neurons one week after the injection (longest time studied; data not shown).
Consistent with some of these effects arising from interaction of formalin with TRPA1, we found that the ATF3 expression induced by formalin at low doses was significantly reduced in TRPA1-null mice (by ~85%; Table 1). In fact, ATF3 induction in TRPA1 mutants after 0.5% formalin was not significantly different from the ATF3-induction in WT mice after an injection of saline (figure 5D), indicating that in the absence of TRPA1, low doses of formalin do not produce nerve damage. In contrast, we found that higher doses of formalin elicited the same ATF3 response in WT and TRPA1 mutant mice (figures 5B-C and 5E-F, respectively and Table 1). These results indicate that in WT mice, high doses of formalin (>0.5%) recruit both TRPA1 and non-TRPA1 expressing DRG neurons.
Figure 5. TRPA1 is required for the induction of ATF3 expression by low doses of formalin.
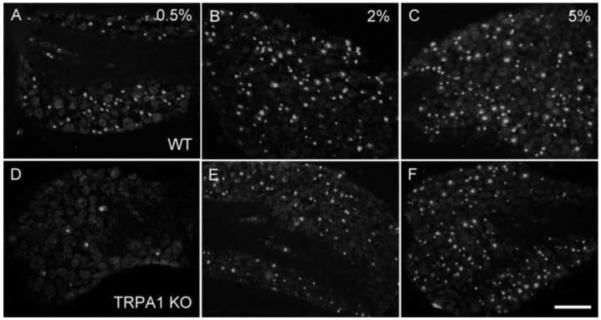
(A-C) In wild-type mice, intraplantar injection of formalin induces expression of ATF3 (red) in large numbers of DRG neurons in a dose-dependent manner. (D-F) The expression induced by the low dose (D), but not high doses of formalin (E, F), was blocked in TRPA1 null mice. Calibration bar 200μm.
A subpopulation of formalin-responsive neurons does not express TRPV1
To determine whether the formalin-induced ATF3 expression in non-TRPV1 afferents was directly or indirectly generated (i.e., following activation of TRPV1+ neurons), we injected 0.5% formalin into the paw of animals previously treated with the potent TRPV1 neurotoxin, resiniferatoxin (RTX). RTX presumably induces its cytotoxicity by allowing for toxic levels of calcium to enter the terminals via the TRPV1 ion channel [51]. Figure 6A illustrates that DRG neurons from mice injected with increasing doses of RTX, as expected, lacked immunostaining for TRPV1 (figure 6A) or substance P (SP, figure 6B), a marker of the peptidergic sensory neurons. In contrast, binding for IB4 (figure 6C) and immunostaining for N52 (data not shown) were still present, indicating that RTX treatment only destroyed the peptidergic subpopulation of afferents, namely those that express TRPV1. On the other hand, two weeks after the last RTX injection, we did note that a very small number of DRG neurons expressed ATF3 (5.2%±0.6 ATF3+ neurons; figure 6D), corresponding we suggest to dying neurons. Surprisingly, we found that an injection of 0.5% formalin in RTX-treated mice was still able to increase the number of ATF3+ neurons (~2 fold; p<0.05; 9.6%±1.5 ATF3+ neurons, ipsilateral to the injection side). The great majority of these neurons (~63%) were nonpeptidergic (figure 6E), consistent with the dramatic loss of TRPV1 expression in the RTX-treated mice. We also detected ATF3 in a small population of neurons with myelinated axons (~10%; figure 6F). Thus, even in the absence of TRPV1-expressing neurons, low doses of formalin can still induce ATF3 in DRG neurons. Given that 0.5% formalin fails to induce ATF3 in TRPA1 mutant mice, our results point to the presence of a nonpeptidergic population of afferents that expresses TRPA1, but not TRPV1.
Figure 6. TRPA1 is not restricted to TRPV1 expressing DRG neurons.
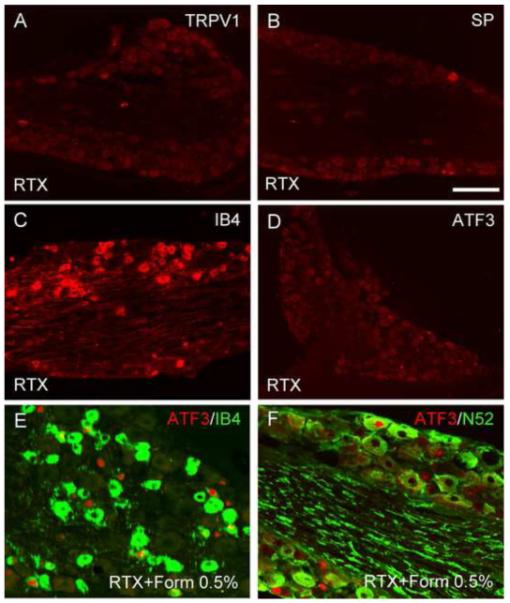
(A-D) TRPV1 expression is lost in resiniferatoxin (RTX)-treated mice (red in A) as is immunoreactivity for substance P (SP, a marker of peptidergic neurons, red in B). In contrast, IB4 binding (red in C) is unchanged, indicating that the latter neurons are TRPV1-negative. Compared to the contralateral side (D), 0.5% formalin induces ATF3 in a significantly higher number of DRG neurons, most of which are nonpeptidergic (~63% bind IB4; green in E). A smaller proportion (~10%) has myelinated axons (N52+; green in F). Given that 0.5% formalin fails to induce ATF3 in TRPA1 mutant mice, these results point to the presence of a nonpeptidergic population of afferents that expresses TRPA1, but not TRPV1. Calibration bar A-C 200μm; E-F 75μm.
ATF3 induced by mustard oil requires TRPA1
Mustard oil (MO) is an irritant chemical compound found in horseradish and wasabi that causes a sharp burning sensation in humans [36] and [42] and is widely used to assess pain behavior in animals. Topical application of MO produces neurogenic inflammation [24] and [26] and a concurrent heat and mechanical hyperalgesia [35], presumably via a centrally mediated sensitization process. Several studies have shown that these effects are TRPA1-mediated [3], [6], [30], [37] and [39]. We thus asked whether mustard oil, similarly to formalin, induces ATF3 in a population of DRG neurons beyond those that express TRPV1.
As shown in figure 7, doses of MO routinely used in pain testing (10% topical/MOt; 0.75% intraplantar/MOi) induce significant levels of ATF3 in sensory neurons. Thus, topical application of 10% MO evoked the same number of ATF3+ neurons as an intraplantar injection of 0.75% MO (Table 1). Not surprisingly, injection of 10% MO induced ATF3 in an even greater number of DRG neurons (~3 times greater than the 10% MOt), indicating that the MO-induced ATF3 is both dose and route-dependent. We next looked at the neurochemistry of the afferents engaged by MO. As shown Table 3, the phenotype of the afferents activated by a particular dose of MO was quantitatively the same regardless of the route of administration. Thus, after MO, ~40% of ATF3+ afferents were nonpeptidergic (bound IB4, figures 7J-L) and ~30% had myelinated axons (N52+; Figures 7G-I). Although we did not assay for TRPV1 expression in these studies, we assume that the bulk of the remaining ATF3 expression was in neurons that expressed both TRPA1 and TRPV1.
Figure 7. ATF3 expression induced by mustard oil requires TRPA1.
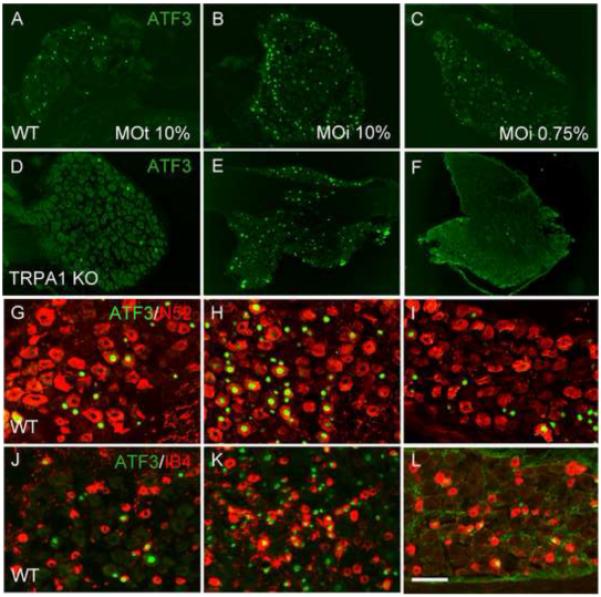
(A-C) In wild-type mice, both topical application (MOt; A) and intraplantar (MOi; B-C) injection of mustard oil induces ATF3 expression (green) in large numbers of DRG neurons. (D-F) These effects are eliminated in TRPA1 null mice (D and F). By contrast, the ATF3 expression induced by an intraplantar injection of 10% MO persisted in the TRPA1 mutant mice (E). (G-L) Mustard oil induces ATF3 (green) in both myelinated (N52+; red in G-I) and unmyelinated nonpeptidergic (bind IB4, red in J-L) afferents. Calibration bar A-F 200μm; G-L 75μm.
Table 3. Distribution of ATF3 expression in subpopulations of sensory neurons after administration of various noxious, chemical stimuli.
| IB4/ATF3 | ATF3/IB4 | N52/ATF3 | ATF3/N52 | |
|---|---|---|---|---|
| MO 10% Top | 41.0 ± 2.7 | 13.3 ± 4.7 | 34.0 ± 4.3 | 5.8 ± 0.7 |
| MO 0.75% Inj | 75.9 ± 5.8 | 14.0 ± 2.8 | 13.9 ± 4.1 | 3.4 ± 0.8 |
| MO 10% Inj | 46.2 ± 4.2 | 37.4 ± 11.1 | 37.2 ± 1.3 | 24.2 ± 9.7 |
| Ment 40mM | 27.7 ± 11.5 | 7.4 ± 4.1 | 57.3 ± 5.4 | 9.5 ± 2.4 |
| Ment 80mM | 25.0 ± 6.5 | 7.3 ± 0.1 | 54.3 ± 7.5 | 10.8 ± 1.9 |
MO: mustard oil; Ment: menthol. Values are given as mean ± standard deviation.
To confirm that the MO-induced ATF3 was secondary to an action on TRPA1, we administered the same doses of MO to TRPA1 null mice. As shown in figure 7, neither 10% MOt nor 0.75% MOi induced ATF3 in the TRPA1 null mice (figure 7D and 7F, respectively and Table 1), indicating that the MO-induced ATF3 response at these doses is TRPA1-dependent, regardless of the route of administration. Importantly however, intraplantar injections of higher doses of MO (10%) in TRPA1-deficient mice still induced ATF3 expression, in large numbers of DRG neurons (figure 7E), which was not significantly different from WT controls (Table 1). The latter results indicate that injection of high doses of MO also activate non-TRPA1 afferents. Apparently there is a “threshold” dose of MO for which ATF3 induction is no longer TRPA1-dependent. These results indicate that, as for formalin, MO elicits peripheral nerve damage of TRPA1-expressing nociceptors at the same doses at which the MO induces frank pain behaviors. However, depending on the dose and the route of administration, MO, like formalin engages primary afferents that do not express TRPA1.
Menthol-induced nerve injury is TRPM8 independent
Finally, we examined the effects of a hindpaw injection of menthol, an agonist of the cold-sensitive TRPM8 channel [8], [17], [19], [45] and [52]. At doses routinely used to assess pain behavior in rodents (4.0 mM), menthol failed to induce ATF3 in DRG neurons (Table 1). At higher doses however, there was a significant induction of ATF3 (figure 8), indicating that menthol can provoke damage to peripheral terminals of DRG neurons. This effect, however, is not dose-dependent (Table 1). In fact, we recorded the same number of ATF3+ neurons in mice injected with either 40 or 80 mM of menthol (10 and 20 mg/kg respectively). Interestingly, about 50% of the ATF3+ DRG neurons have myelinated axons (N52+, figure 8E-F and Table 3) and about 25% are nonpeptidergic (bind IB4; figure 8G-H and Table 3). Unexpectedly, however, we found that the number and the phenotype of ATF3+ DRG neurons did not differ in TRPM8-null mice (figure 8C-D and Table 3).
Figure 8. Menthol-induced ATF3 expression is TRPM8-independent.
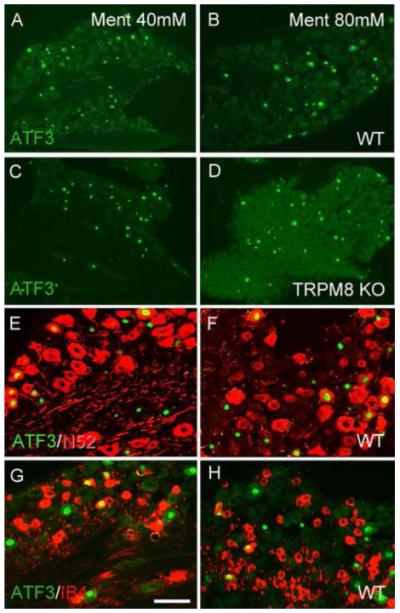
(A-D) Menthol (Ment) induces ATF3 expression (green) in large numbers of DRG neurons, in both wild-type (A-B) and TRPM8 null mice (C-D), indicating that TRPM8 is not required for the full induction of ATF3 by menthol. (E-H) Menthol induces ATF3 (green) in both myelinated (N52+; red in E-F) and unmyelinated nonpeptidergic (bind IB4, red in G-H) afferents. Calibration bar A-D 200μm; E-H 75μm.
Given that some studies have suggested that menthol modulates other TRP channels [31] and [40] we also injected menthol into the paw of TRPA1 null mice. We found that an injection of 40mM menthol induces ATF3 in a large number of DRG neurons in TRPA1 deficient mice, comparable to what we recorded in their WT littermates (8.6 ± 3.1 and 9.1 ± 2.6 %ATF3/neuron counted, respectively). Taken together, our results indicate that the menthol-induced nerve injury is neither TRPM8-nor TRPA1-mediated.
Finally, we examined the effects of an intraplantar injection of icilin, a more potent TRPM8 agonist. Similar to what we observed for menthol, icilin only induced ATF3 in DRG neurons at very high doses (> 50 mM). Icilin was without effect at doses that are routinely used to assess pain behavior (~1.0 mM; data not shown).
DISCUSSION
In the present studies, we adopted a simple paradigm (ATF3 induction) to determine the identity of the primary afferent fibers that are recruited after hindpaw injection of various noxious chemical stimuli. In agreement with von Banchet et al [56], we found that profound inflammation produced by CFA did not induce ATF3 expression in DRG neurons. We conclude that frank nerve injury, not merely the increased afferent barrage induced by the injury, is the critical trigger for induction of ATF3. This further suggests that when ATF3 is observed in a model of inflammation (e.g. mono-iodoacetate-induced osteoarthritis), it is likely that there is concurrent nerve damage [28]. Based on the requirement of nerve injury to induce ATF3 expression, we conclude that its induction by capsaicin, formalin, MO, menthol and icilin is secondary to the peripheral nerve terminal damage produced by these agents.
Capsaicin-responsive afferents
Capsaicin failed to induce ATF3 in TRPV1 null mice, indicating that capsaicin-induced nerve damage requires TRPV1. Given this requirement, it is significant that capsaicin-induced ATF3 expression in wild-type animals was not limited to DRG neurons that express TRPV1. Given the very low percentage of IB4-binding neurons that co-stain for TRPV1 in the mouse, our finding that a rather large proportion of nonpeptidergic neurons responded to capsaicin was unexpected [11] and [66]. Note, however, that Dirajlal and colleagues [20] showed in vitro that proton exposure doubled the proportion of IB4-binding DRG neurons (~54%) that respond to capsaicin. This suggests that under certain conditions, about half of the nonpeptidergic neurons have the ability to respond to capsaicin and thus, likely express TRPV1.
There are several possible (and non-mutually exclusive) explanations for the ATF3 expression in non-TRPV1 afferents: first, the method used to identify TRPV1-expressing neurons may not be sufficiently sensitive to detect low, yet functional, levels of TRPV1. On the other hand, because the ratio of TRPV1+ to TRPV1-neurons that expressed ATF3 was constant at all doses tested (~48%), it appears that mechanisms other than very low levels of TRPV1 expression in the IB4 population account for the ATF3 induction in these neurons, (as the relative percentage should increase with dose).
A second possibility is that the ATF3 induction in non-TRPV1 neurons occurred indirectly, secondary to activation of the TRPV1 expressing afferents. Such an interaction could occur either at the peripheral terminals of the afferents, or within the DRG itself. Conceivably mediators released from the terminals (or cell bodies) of the injured TRPV1 afferents are sufficiently abundant and neurotoxic to trigger ATF3 expression in the non-TRPV1 afferents. There, in fact, is evidence for such intraganglionic, “cross-communication” among DRG neurons [18]. This phenomenon, referred as “cross-excitation”, in fact, increases in the setting of injury [1] and [64]. Regardless of the proportion of nonpeptidergic neurons that are directly or indirectly activated by capsaicin, it is nevertheless striking that only a very small number expressed the Mrgprd receptor. That result is consistent with in situ studies showing that the Mrgprd and TRPV1 cell populations are mutually exclusive in the mouse DRG [21] and with electrophysiological studies that demonstrated that cutaneous sensory neurons expressing the Mrgprd receptor are capsaicin-insensitive in vitro [22].
It follows that if an indirect mechanism is operating, then it must be a highly organized and topographic one, involving receptors for the released chemicals that predominate on the cell bodies or terminals of non-TRPV1 neurons with unmyelinated axons. This feature is essential in order to explain the strikingly selective induction of ATF3 in unmyelinated neurons (only ~4% were N52+) after capsaicin injection.
Formalin- and mustard oil (MO)-responsive afferents
We found that the ATF3 induction after formalin or MO is greatly reduced in mice lacking TRPA1, which is consistent with the reduced behavioral response of these mice to both agents [6], [41] and [46] and with the report that TRPA1 (and TRPA1-expressing afferents) mediate a component of the behavioral response to formalin and MO [30]. However, failure of formalin to induced ATF3 in the TRPA1 mutant mice was only true for low doses of formalin (≤0.5%) or MO. Higher doses induced equivalent ATF3 expression in WT and TRPA1 mutant mice. Clearly, the higher doses of formalin and MO can recruit both TRPA1- and non TRPA1-expressing afferents. This conclusion is supported by the demonstration that high doses (>0.5%) of formalin activate both myelinated and unmyelinated afferents [27], [33], [53] and [54]. The fact that the pain-related behaviors produced by intraplantar injections of low doses of formalin (0.5%) or MO (0.75%) in TRPA1 mutant mice are greatly reduced, but not completely eliminated [58], is also consistent with our present findings. Given its mode of action (cross-linking of proteins), we presume that at high doses, formalin induces ATF3 in a non-specific manner.
We conclude that low doses of formalin or MO exert their effects via TRPA1-expressing afferents. Unexpectedly, however, we found that formalin- and MO-induced ATF3 expression predominated in nonpeptidergic (~75%) neurons, even in the absence of TRPV1-expressing afferents. In fact, in RTX-treated mice, which lack TRPV1-expressing neurons, ~24% of IB4-binding afferents still responded to 0.5% formalin, a dose that fails to induce ATF3 in TRPA1 null mice. These results are unexpected as TRPA1 is supposedly restricted to a subset of TRPV1 afferents [30], [34], [49] and [57]. Given that capsaicin, MO and formalin all induce ATF3 in a large number of nonpeptidergic DRG neurons, we conclude that 1) capsaicin induces ATF3 in both TRPV1 and non-TRPV1 DRG neurons (including the nonpeptidergic, TRPV1-subset) in a TRPV1-dependent manner 2) the nonpeptidergic, capsaicin-responsive afferents likely express TRPA1 and 3) TRPA1 must be expressed in a population of nonpeptidergic DRG neurons that does not express TRPV1. Of course, we cannot exclude the possibility that the nonpeptidergic class of primary afferent neurons is more susceptible to injury than are other classes, which would increase the likelihood of the nonpeptidergic afferents expressing ATF3 after capsaicin, MO or formalin. Finally, we cannot rule out the possibility of an indirect activation of non-TRPA1 neurons, i.e., after activation of TRPA1+ neurons, a mechanism comparable to what we proposed above for capsaicin.
Menthol- and icilin-responsive afferents
We found no evidence that the menthol-induced ATF3 is associated with the activation of a specific neuronal population. Thus, menthol injection induced ATF3 in all classes of sensory neuron, and in a dose- and TRPM8-independent manner. Thus even though TRPM8 is expressed in both myelinated and unmyelinated fibers, there is no TRPM8 mediated ATF3 induction by menthol in these afferents. Note that the DMSO vehicle did not by itself induce ATF3 expression, indicating that the ATF3 induction required menthol. These results are reminiscent of the report that TRPM8-independent pathways mediate menthol-induced Ca2+ transport in cultured cell systems [43] and menthol induced cell death of prostate cancer cells [32]. Conceivably therefore, the menthol-induced ATF3 response results from triggering of a general stress response in the DRG neuron, rather than from frank nerve injury. Whether the icilin-induced ATF3 expression that occurs at high doses (50x the doses that are routinely used to assess pain behavior) involves the same mechanisms through which high dose menthol induces ATF3 expression remains to be determined.
Behavioral implications
Our results underscore the fact that even reportedly selective agonists activate a heterogeneous population of sensory neurons. For example, we found that an injection of formalin provokes direct damage of TRPA1+ peripheral terminals, but the specificity is lost with increasing doses. What then is the basis for the pain produced by the high doses of formalin typically used in pain testing (2-5%)? Based on our results and those of Puig and Sorkin [54], we suggest that formalin-induced pain behavior involves a complex pattern of activity generated by injury to a heterogeneous population of neurons, including unmyelinated and myelinated afferents, which in turn engage different CNS pathways [9] and [10]. The magnitude of central sensitization (and conceivably the populations of spinal cord neurons in which central sensitization occurs) is thus likely to be very different whether one uses a low or a high dose of formalin.
Most traditional pain tests only assess the acute effects (first 2h) of a frankly noxious chemical stimulus. They generally do not assess the long-term consequences that may be triggered by the stimulus. In the case of the formalin test, there are, in fact, profound changes that occur well beyond the traditional one-hour post injection period of analysis [25], [59], [60], [63] and [65]. The fact that ATF3 continues to be expressed one week after the injection of formalin raises the possibility that the downstream consequences of ATF3 expression contribute to the long-term behavioral effects of these injuries.
Conclusion
Regardless of the functional significance of the enhanced ATF3 expression, our results indicate that many of the noxious chemical compounds that are routinely used in studies of pain processing unquestionably elicit behavioral responses through activation of receptor/channel-specific populations of primary afferents. However, several of these compounds also exert non-specific effects, secondary to nerve terminal damage.
Acknowledgements
This work was supported by NIH grants NS14627 and 48499. We are particularly grateful to Dr. David Julius (UCSF) for providing the TRPV1-, TRPA1- and TRPM8-null mice and Dr. David Anderson (CalTech) for providing the MrgprdDTR mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There was no conflict of interest during this study.
Many noxious compounds used in pain testing elicit behavioral responses through selective activation of primary afferents and through non-specific effects, secondary to nerve terminal damage.
REFERENCES
- [1].Amir R, Devor M. Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience. 2000;95:189–195. doi: 10.1016/s0306-4522(99)00388-7. [DOI] [PubMed] [Google Scholar]
- [2].Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- [4].Bang S, Hwang SW. Polymodal ligand sensitivity of TRPA1 and its modes of interactions. J Gen Physiol. 2009;133:257–262. doi: 10.1085/jgp.200810138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- [7].Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci (USA) 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- [9].Braz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163:1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [11].Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [12].Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- [13].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- [14].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [15].Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci (USA) 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chung K, Klein CM, Coggeshall RE. The receptive part of the primary afferent axon is most vulnerable to systemic capsaicin in adult rats. Brain Res. 1990;511:222–226. doi: 10.1016/0006-8993(90)90165-8. [DOI] [PubMed] [Google Scholar]
- [17].Colburn RW, Lubin ML, Stone DJ, Jr., Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- [18].Devor M, Wall PD PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990;64:1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- [19].Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- [20].Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)-positive and - negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–524. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- [21].Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- [22].Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–1589. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci. 2008;28:7863–7875. doi: 10.1523/JNEUROSCI.1696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fiorentino PM, Cairns BE, Hu JW. Development of inflammation after application of mustard oil or glutamate to the rat temporomandibular joint. Arch Oral Biol. 1999;44:27–32. doi: 10.1016/s0003-9969(98)00095-8. [DOI] [PubMed] [Google Scholar]
- [25].Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- [26].Handwerker HO, Anton F, Kocher L, Reeh PW. Nociceptor functions in intact skin and in neurogenic or non-neurogenic inflammation. Acta Physiol Hung. 1987;69:333–342. [PubMed] [Google Scholar]
- [27].Heapy CJ, Jamieson A, Russel NJW. Afferent C-fiber and A-delta activity in models of inflammation. Br J Pharmacol. 1987;90:164P. [Google Scholar]
- [28].Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterization. Pain. 2007;128:272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- [29].Jancso G, Kiraly E, Joo F, Such G, Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci Lett. 1985;59:209–214. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- [30].Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- [31].Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim SH, Nam JH, Park EJ, Kim BJ, Kim SJ, So I, Jeon JH. Menthol regulates TRPM8-independent processes in PC-3 prostate cancer cells. Biochim Biophys Acta. 2009;1792:33–38. doi: 10.1016/j.bbadis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- [33].Klemm F, Carli G, Reeh PW. Peripheral neural correlates of the formalin test in the rat. Pflugers Arch. 1989;414:414(S42). [Google Scholar]
- [34].Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- [35].Koltzenburg M, Lundberg LE, Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- [36].Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117:579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- [37].Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- [38].Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- [40].Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [41].Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Magerl W, Geldner G, Handwerker HO. Pain and vascular reflexes in man elicited by prolonged noxious mechano-stimulation. Pain. 1990;43:219–225. doi: 10.1016/0304-3959(90)91075-T. [DOI] [PubMed] [Google Scholar]
- [43].Mahieu F, Owsianik G, Verbert L, Janssens A, De Smedt H, Nilius B, Voets T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J Biol Chem. 2007;282:3325–3336. doi: 10.1074/jbc.M605213200. [DOI] [PubMed] [Google Scholar]
- [44].McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- [45].McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- [46].McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci (USA) 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mizushima T, Obata K, Katsura H, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Mashimo T, Noguchi K. Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience. 2006;140:1337–1348. doi: 10.1016/j.neuroscience.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [48].Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [49].Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Namer B, Kleggetveit IP, Handwerker H, Schmelz M, Jorum E. Role of TRPM8 and TRPA1 for cold allodynia in patients with cold injury. Pain. 2008;139:63–72. doi: 10.1016/j.pain.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [51].Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- [52].Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- [53].Porro CA, Cavazzuti M. Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog Neurobiol. 1993;41:565–607. doi: 10.1016/0301-0082(93)90044-s. [DOI] [PubMed] [Google Scholar]
- [54].Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- [55].Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Segond von Banchet G, Boettger MK, Fischer N, Gajda M, Brauer R, Schaible HG. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain. 2009;145:151–159. doi: 10.1016/j.pain.2009.06.002. [DOI] [PubMed] [Google Scholar]
- [57].Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- [58].Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res Rev. 2009;60:2–23. doi: 10.1016/j.brainresrev.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- [60].Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- [61].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- [62].Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- [63].Vierck CJ, Yezierski RP, Light AR. Long-lasting hyperalgesia and sympathetic dysregulation after formalin injection into the rat hind paw. Neuroscience. 2008;153:501–506. doi: 10.1016/j.neuroscience.2008.02.027. [DOI] [PubMed] [Google Scholar]
- [64].Xu GY, Zhao ZQ. Cross-inhibition of mechanoreceptive inputs in dorsal root ganglia of peripheral inflammatory cats. Brain Res. 2003;970:188–194. doi: 10.1016/s0006-8993(03)02342-4. [DOI] [PubMed] [Google Scholar]
- [65].Zeitz KP, Giese KP, Silva AJ, Basbaum AI. The contribution of autophosphorylated alpha-calcium-calmodulin kinase II to injury-induced persistent pain. Neuroscience. 2004;128:889–898. doi: 10.1016/j.neuroscience.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [66].Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]


