Abstract
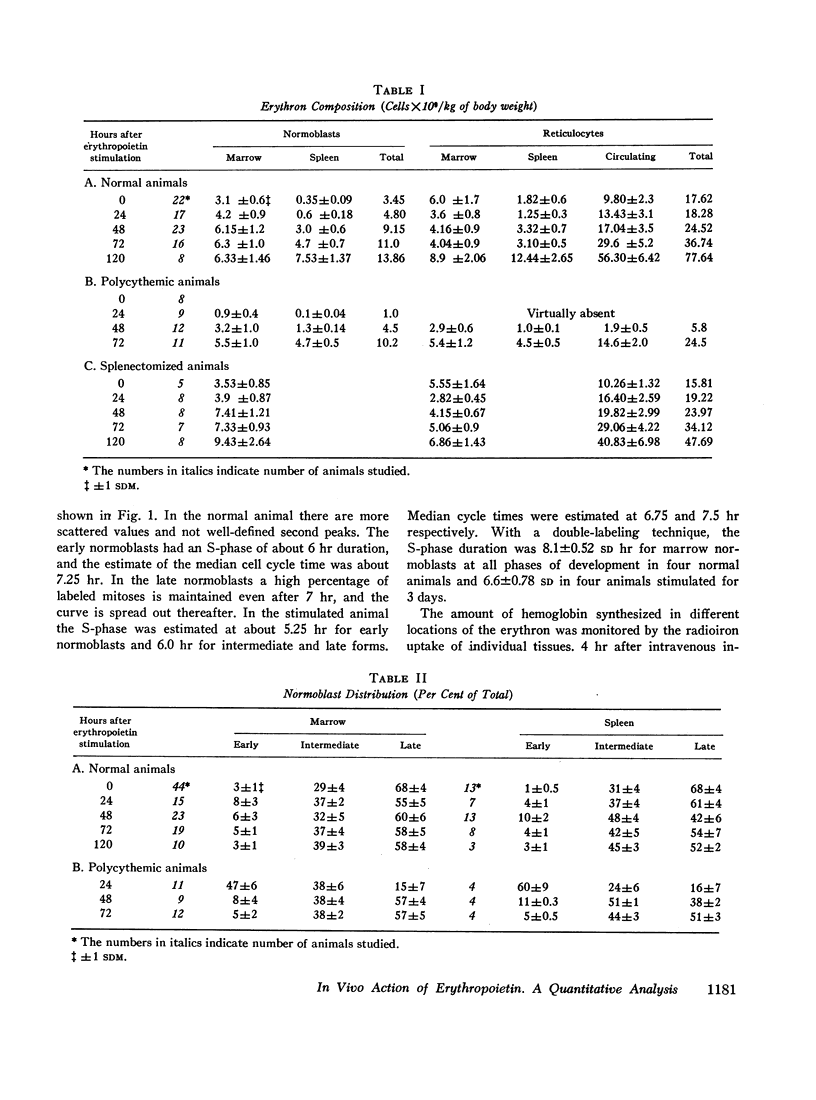
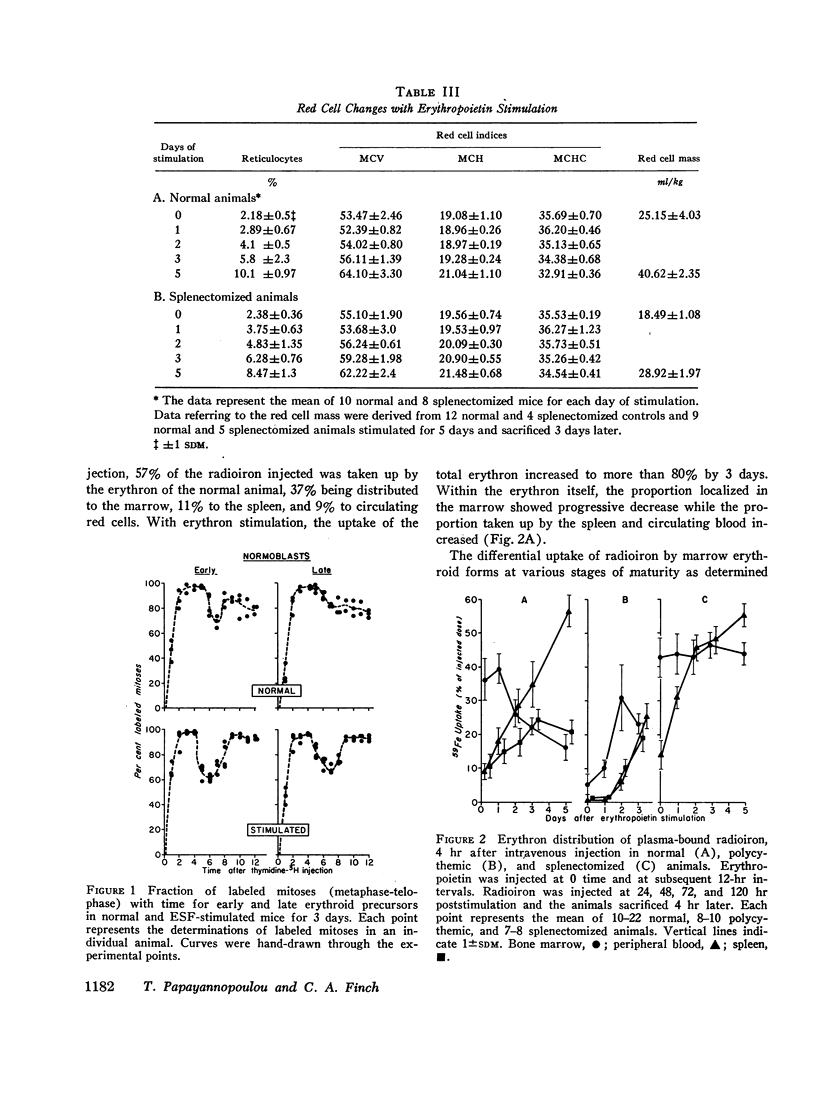
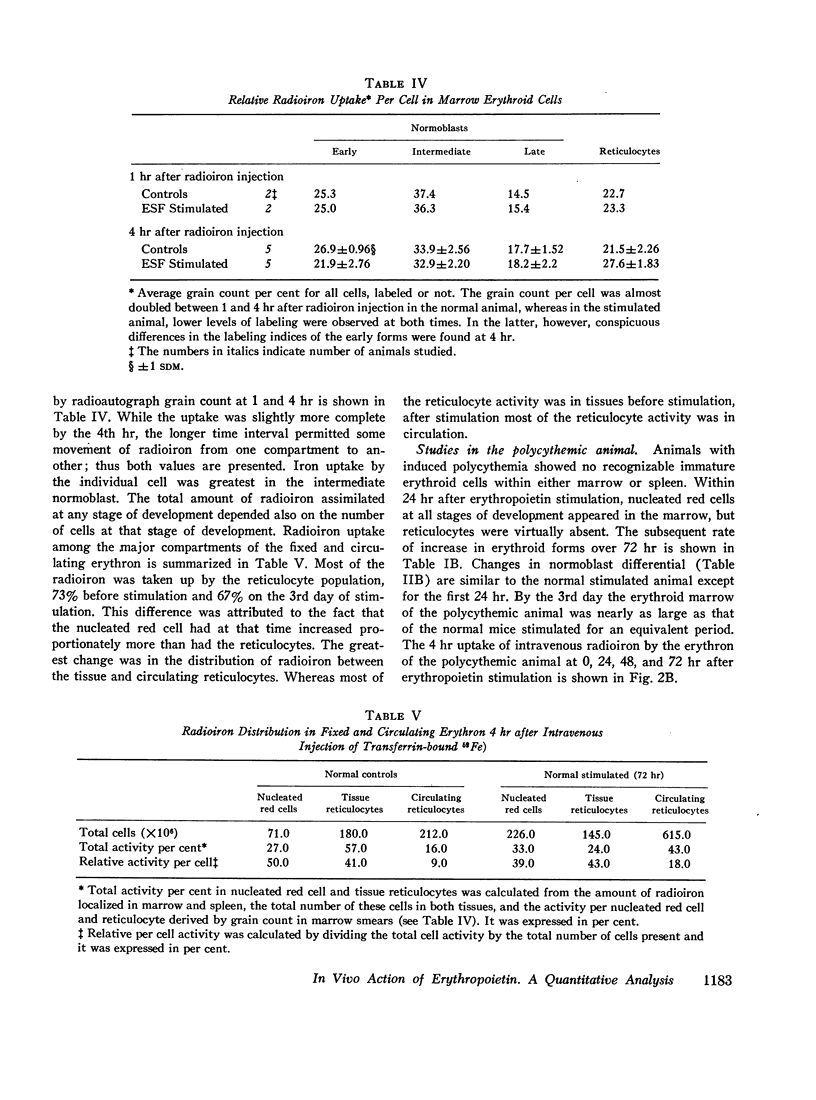
The composite response of the erythron to exogenous erythropoietin has been studied in normal, splenectomized, and polycythemic mice. After stimulation the normal animal doubled its marrow nucleated red cells by the 3rd day with little further change by the 5th. Nucleated red cells within the spleen began to increase sharply on the 2nd day and, by the 5th, exceeded those in the marrow. The total nucleated erythroid response represented a fourfold increase. Reticulocytes lagged behind the expansion of the nucleated red cell mass, but by the 5th day the original ratio was re-established. Hemoglobin synthesis was increased, but the ratio of hemoglobin synthesized in nucleated red cells and reticulocytes was basically unchanged. Early displacement of marrow reticulocytes into circulation and the production of a larger red cell also occurred. No evidence of a change in the number of erythroid mitoses was found; only a slight decrease in the average cell cycle time was demonstrated. Thus, whereas erythropoietin stimulation induced several changes in erythropoiesis, the increased number of cells entering into the maturing pool appeared to be of greatest quantitative significance.
Splenectomy reduced the proliferative response of the erythron over 5 days stimulation to three-fourths that found in the normal animal. This difference, also reflected in a proportionately lower reticulocyte response and increment in circulating red cell mass, suggests that erythropoiesis within the mouse marrow is spatially or otherwise restricted and that the spleen provided a supplemental area of erythroid expansion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs D. R., Geist A., Chervenick P. A. Contribution of the mouse spleen to post-hemorrhagic erythropoiesis. Life Sci. 1969 Jun 1;8(11):587–599. doi: 10.1016/0024-3205(69)90020-4. [DOI] [PubMed] [Google Scholar]
- DONOHUE D. M., GABRIO B. W., FINCH C. A. Quantitative measurement of hematopoietic cells of the marrow. J Clin Invest. 1958 Nov;37(11):1564–1570. doi: 10.1172/JCI103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILMANOWICZ E., GURNEY C. W. Studies on erythropoiesis. XVI. Response to a single dose of erythropoietin in polycythemic mouse. J Lab Clin Med. 1961 Jan;57:65–72. [PubMed] [Google Scholar]
- FISHER J. W., LAJTHA L. G., BUTTOO A. S., PORTEOUS D. D. DIRECT EFFECTS OF ERYTHROPOIETIN ON THE BONE MARROW OF THE ISOLATED PERFUSED HIND LIMBS OF RABBITS. Br J Haematol. 1965 May;11:342–349. doi: 10.1111/j.1365-2141.1965.tb06594.x. [DOI] [PubMed] [Google Scholar]
- FISHER J. W., ROH B. L., COUCH C., NIGHTINGALE W. O. INFLUENCE OF COBALT, SHEEP ERYTHROPOIETIN AND SEVERAL HORMONES ON ERYTHROPOIESIS IN BONE MARROWS OF ISOLATED PERFUSED HIND LIMBS OF DOGS. Blood. 1964 Jan;23:87–98. [PubMed] [Google Scholar]
- Hanna I. R., Tarbutt R. G., Lamerton L. F. Shortening of the cell-cycle time of erythroid precursors in response to anaemia. Br J Haematol. 1969 Apr;16(4):381–387. doi: 10.1111/j.1365-2141.1969.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Jacobs P., Finch C. A. Iron for erythropoiesis. Blood. 1971 Feb;37(2):220–230. [PubMed] [Google Scholar]
- Monette F. C., LoBue J., Chan P. C., Gordon A. S. DNA syndthesis time and related parameters in erythroid cell precursors of rats. Scand J Haematol. 1968;5(5):325–332. doi: 10.1111/j.1600-0609.1968.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Hara H. [Experimental studies on erythroblast kinetics under hemopoietic stimulation]. Nihon Ketsueki Gakkai Zasshi. 1968 Jun;31(3):261–269. [PubMed] [Google Scholar]
- REIFF R. H., NUTTER J. Y., DONOHUE D. M., FINCH C. A. The relative number of marrow reticulocytes. Am J Clin Pathol. 1958 Sep;30(3):199–203. doi: 10.1093/ajcp/30.3.199. [DOI] [PubMed] [Google Scholar]
- Rencricca N. J., Rizzoli V., Howard D., Duffy P., Stohlman F., Jr Stem cell migration and proliferation during severe anemia. Blood. 1970 Dec;36(6):764–771. [PubMed] [Google Scholar]
- SCHOOLEY J. C., GARCIA J. F. Immunologic studies on the mechanism of action of erythropoietin. Proc Soc Exp Biol Med. 1962 Jul;110:636–641. doi: 10.3181/00379727-110-27602. [DOI] [PubMed] [Google Scholar]
- Schooley J. C. Inhibition of erythropoietic stimulation by testosterone in polycythemic mice receiving anti-erythropoietin. Proc Soc Exp Biol Med. 1966 Jun;122(2):402–403. doi: 10.3181/00379727-122-31146. [DOI] [PubMed] [Google Scholar]
- Stohlman F., Jr, Ebbe S., Morse B., Howard D., Donovan J. Regulation of erythropoiesis. XX. Kinetics of red cell production. Ann N Y Acad Sci. 1968 Mar 29;149(1):156–172. doi: 10.1111/j.1749-6632.1968.tb15149.x. [DOI] [PubMed] [Google Scholar]
- Yoffey J. M., Jeffreys R. V., Osmond D. G., Turner M. S., Tahsin S. C., Niven P. A. Studies on hypoxia. VI. Changes in lymphocytes and transitional cells in the marrow during the intensification of primary hypoxia and rebound. Ann N Y Acad Sci. 1968 Mar 29;149(1):179–192. doi: 10.1111/j.1749-6632.1968.tb15151.x. [DOI] [PubMed] [Google Scholar]



