Abstract
The effects of bacterial products on selected synovial fibroblast functions were studied. Extracts of commonly encountered microorganisms were prepared by sonic or mechanical disruption. “Purified” endotoxins were prepared from selected organisms, and in some cases were purchased commercially. Normal fibroblasts were derived from synovial connective tissue obtained from amputations or arthrotomy. The cells were grown as a monolayer on glass and were nourished by a semisynthetic nutrient medium.
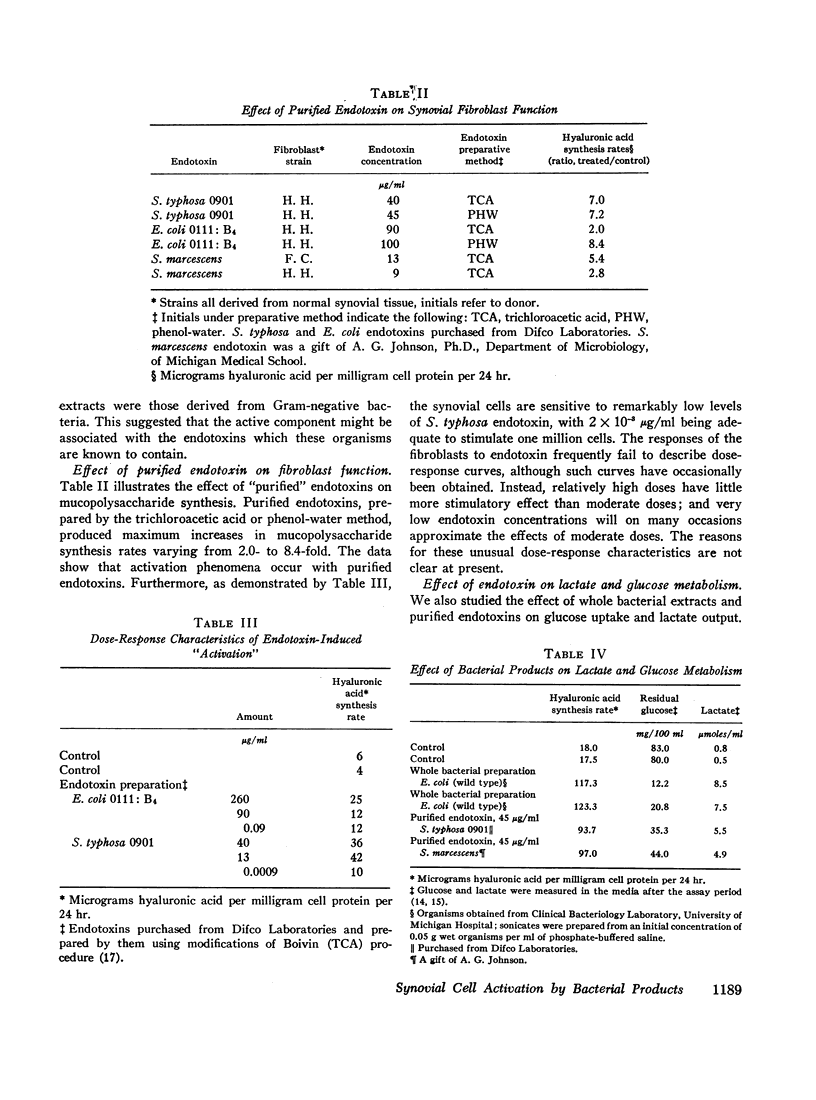
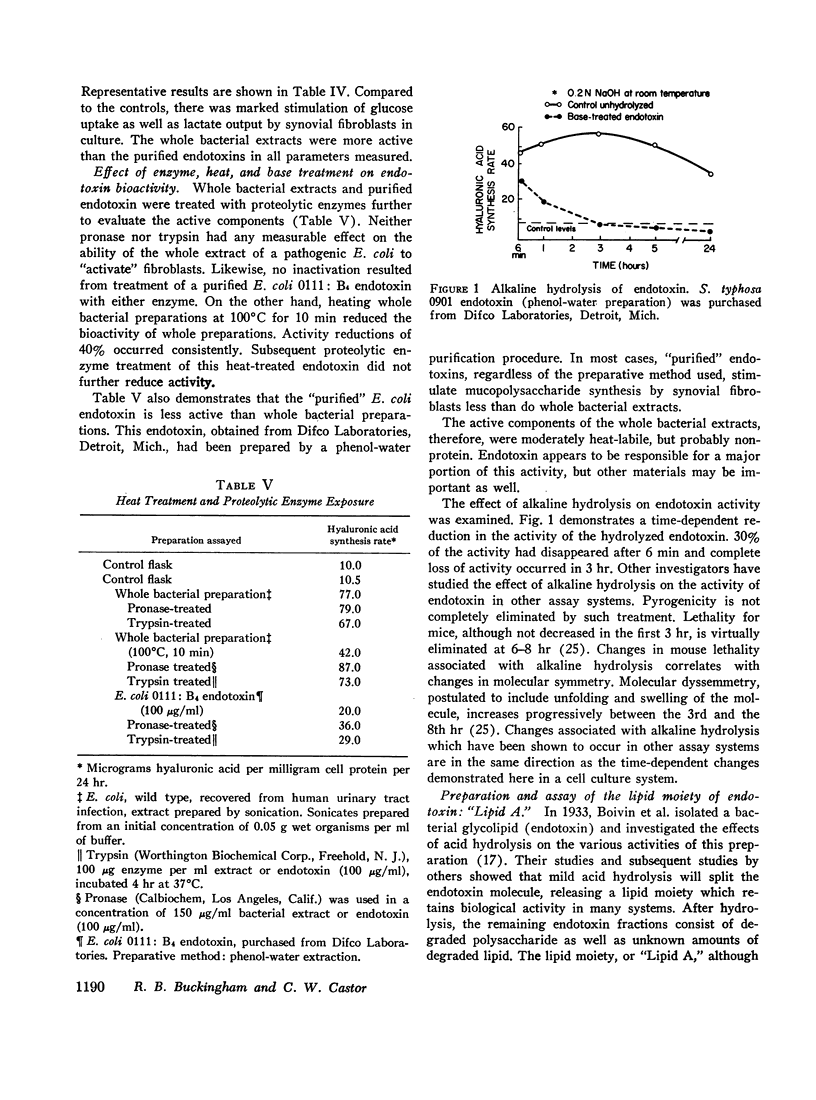
Extracts of Gram-negative bacteria, applied to fibroblast cultures, markedly increased hyaluronic acid production, glucose utilization, and lactate output. Treatment of the extracts with heat at 100°C for ½ hr decreased their effectiveness by approximately 40%. Purified Gram-negative bacterial endotoxin stimulated synovial fibroblasts to an extent comparable to that caused by heat-treated whole extracts. The lipid moiety of the endotoxin molecule appeared to account for much of the stimulatory activity of the endotoxin. Extracts of commonly encountered Gram-positive cocci, yeast, and Mycoplasma had no stimulating capabilities. Corynebacterial extracts, however, had definite stimulating potential. Endotoxin-synovial cell interaction experiments demonstrated that endotoxin was bound to fibroblasts. Reassay of the endotoxin after extraction from the cells showed that it retained its stimulatory potential.
The metabolic phenomena stimulated by bacterial products duplicate the major known actions of connective tissue-activating peptide (CTAP). The observations made in this study suggest that bacterial products may participate in a fundamental way in the activation process, and indicate a possible role for bacterial products in synovial inflammation in humans.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Ikuta K. Immunopathological studies on experimental arthritis-lesions in synovial tissue during endotoxemia. Bull Osaka Med Sch. 1968 Oct;14(2):99–109. [PubMed] [Google Scholar]
- BRAUDE A. I., JONES J. L., DOUGLAS H. The behavior of Escherichia coli endotoxin (somatic antigen) during infectious arthritis. J Immunol. 1963 Feb;90:297–311. [PubMed] [Google Scholar]
- CASTOR C. W., FRIES F. F. Composition and function of human synovial connective tissue cells measured in vitro. J Lab Clin Med. 1961 Mar;57:394–407. [PubMed] [Google Scholar]
- CASTOR C. W., PRINCE R. K. MODULATION OF THE INTRINSIC VISCOSITY OF HYALURONIC ACID FORMED BY HUMAN "FIBROBLASTS" IN VITRO: THE EFFECTS OF HYDROCORTISONE AND COLCHICINE. Biochim Biophys Acta. 1964 Jul 7;83:165–177. doi: 10.1016/0926-6526(64)90032-1. [DOI] [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. I. The nature, specificity, measurement and distribution of connective tissue activating peptide. Arthritis Rheum. 1971 Jan-Feb;14(1):41–54. doi: 10.1002/art.1780140107. [DOI] [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. II. Abnormalities of cultured rheumatoid synovial cells. Arthritis Rheum. 1971 Jan-Feb;14(1):55–66. doi: 10.1002/art.1780140108. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Dorstewitz E. L. Abnormalities of connective tissue cells cultured from patients with rheumatoid arthritis. I. Relative unresponsiveness of rheumatoid synovial cells to hydrocortisone. J Lab Clin Med. 1966 Aug;68(2):300–313. [PubMed] [Google Scholar]
- Castor C. W., Dorstewitz E. L., Rowe K., Ritchie J. C. Abnormalities of connective tissue cells cultured from patients with rheumatoid arthritis. II. Defective regulation of hyaluronate and collagen formation. J Lab Clin Med. 1971 Jan;77(1):65–75. [PubMed] [Google Scholar]
- Castor C. W., Prince R. K., Hazelton M. J. Hyaluronic acid in human synovial effusions; a sensitive indicator of altered connective tissue cell function during inflammation. Arthritis Rheum. 1966 Dec;9(6):783–794. doi: 10.1002/art.1780090606. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Wright D., Buckingham R. B. Effects of rheumatoid sera on fibroblast proliferation and hyaluronic acid synthesis. Arthritis Rheum. 1968 Oct;11(5):652–659. doi: 10.1002/art.1780110507. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Yaron M. Leukocyte-connective tissue cell interaction. II. The specificity, duration, and mechanism of interaction effects. Arthritis Rheum. 1969 Aug;12(4):374–386. doi: 10.1002/art.1780120405. [DOI] [PubMed] [Google Scholar]
- DINGLE J. T., THOMAS D. P. P. In vitro studies on human synovial membrane; a metabolic comparison of normal and rheumatoid tissue. Br J Exp Pathol. 1956 Aug;37(4):318–323. [PMC free article] [PubMed] [Google Scholar]
- Duthie J. J., Stewart S. M., Alexander W. R., Dayhoff R. E. Isolation of diphtheroid organisms from rheumatoid synovial membrane and fluid. Lancet. 1967 Jan 21;1(7482):142–143. doi: 10.1016/s0140-6736(67)91039-2. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., SWOBODA B. E., MASSEY V. KINETICS AND MECHANISM OF ACTION OF GLUCOSE OXIDASE. J Biol Chem. 1964 Nov;239:3927–3934. [PubMed] [Google Scholar]
- Hill A. G. The role of infection in the causation of rheumatoid arthritis. Proc R Soc Med. 1968 Oct;61(10):971–972. doi: 10.1177/003591576806101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth J. W., Atkins E. Synovial inflammatory response to bacterial endotoxin. Yale J Biol Med. 1965 Dec;38(3):241–256. [PMC free article] [PubMed] [Google Scholar]
- Janis R., Sandson J., Smith C., Hamerman D. Synovial cell synthesis of a substance immunologically like cartilage proteinpolysaccharide. Science. 1967 Dec 15;158(3807):1464–1467. doi: 10.1126/science.158.3807.1464. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., RYAN M., PIETRUSZKIEWICZ A. Fractionation of hyaluronic acid. The polydispersity of hyaluronic acid from the bovine vitreous body. Biochim Biophys Acta. 1960 Aug 26;42:476–485. doi: 10.1016/0006-3002(60)90826-x. [DOI] [PubMed] [Google Scholar]
- NOWOTNY A. Relation of structure to function in bacterial O antigens. II. Fractionation of lipids present in Boivin-type endotoxin of Serratia marcescens. J Bacteriol. 1963 Feb;85:427–435. doi: 10.1128/jb.85.2.427-435.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Person D. A., Rawls W. E., Sharp J. T. Replication of rubella, Newcastle disease, and vesicular stomatitis viruses in cultured rheumatoid synovial cells. Proc Soc Exp Biol Med. 1971 Nov;138(2):748–752. doi: 10.3181/00379727-138-35981. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Tripodi D., Nowotny A. Relation of structure to function in bacterial O-antigens. V. Nature of active sites in endotoxic lipopolysaccharides of Serratia marcescens. Ann N Y Acad Sci. 1966 Jun 30;133(2):604–621. doi: 10.1111/j.1749-6632.1966.tb52392.x. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- Yaron M., Castor C. W. Leukocyte-connective tissue cell interaction. I. Stimulation of hyaluronate synthesis by live and dead leukocytes. Arthritis Rheum. 1969 Aug;12(4):265–273. doi: 10.1002/art.1780120404. [DOI] [PubMed] [Google Scholar]



