Abstract
NF-κB is well established as a key component of the inflammatory response. However, the precise mechanisms through which NF-κB activation contributes to inflammatory disease states remain poorly defined. To test the role of NF-κB in inflammation, we created a knock-in mouse that expresses a constitutively active form of NF-κB p65 dimers. These mice are born at normal Mendelian ratios, but display a progressive, systemic hyperinflammatory condition that results in severe runting and, typically, death 8–20 d after birth. Examination of homozygous knock-in mice demonstrates significant increases in proinflammatory cytokines and chemokines. Remarkably, crossing this strain with mice lacking TNF receptor 1 (TNFR1) leads to a complete rescue of the hyperinflammatory phenotype. However, upon aging, these rescued mice begin to display chronic keratitis accompanied by increased corneal expression of TNFα, IL-1β, and MMP-9, similar to that seen in human keratoconjunctivitis sicca (KCS) or “dry eyes.” Therefore, our results show that, while constitutively active NF-κB can trigger systemic inflammation, it does so indirectly, through increased TNF production. However, certain inflammatory disease states, such as keratitis or KCS, a condition that is seen in Sjogren's syndrome, are dependent on NF-κB, but are independent of TNFR1 signaling.
Keywords: NF-κB; gene expression, inflammation; TNF-α; keratoconjunctivitis
As human life expectancy has increased, so too has the need to understand and treat diseases that predominantly affect the elderly. Such diseases include cancer, Alzheimer's, osteoporosis, atherosclerosis, and diabetes. In all cases, aberrant chronic inflammation is believed to underlie the development and progression of such diseases (Weiner and Selkoe 2002; Hansson et al. 2006; Libby 2006, 2007; von Herrath et al. 2007; Mantovani et al. 2008; Tilg et al. 2008). Inflammation is a condition that results from hyperactivation of physiological pathways that normally play important roles in innate immune responses. Thus, key physiological effectors of inflammation, such as cytokines and chemokines, are synthesized in increased amounts, and some of the most effective new therapies for inflammatory diseases have involved strategies blocking the action of proinflammatory cytokines such as TNFα (Kalden 2002; Reimold 2002). Hence, understanding the molecules and pathways that affect the production of proinflammatory mediators is likely to be crucial for the development of effective new therapies for such diseases.
The transcription factor NF-κB regulates the expression of a wide range of genes, including various proinflammatory cytokines, cell adhesion proteins, and several anti-apoptotic molecules that together play pivotal roles in almost all aspects of immune and inflammatory responses (Hayden and Ghosh 2008). In resting cells, NF-κB associates with members of the inhibitory family of IκB proteins, resulting in retention of these complexes in the cytoplasm. Following appropriate stimulation, IκB proteins are phosphorylated on two specific NH2-terminal serine residues by one of the catalytic subunits of the IκB kinase (IKK). Phosphorylated IκBs are subsequently ubiquitinated and degraded by the proteasome, leaving NF-κB free to translocate to the nucleus, where it binds to cognate enhancer/promoter elements in its cohort of target genes (Hayden and Ghosh 2008). However, we and others have shown that, besides the regulated degradation of IκBs, phosphorylation of nuclear NF-κB p65 is also an obligatory step for efficient transcription of NF-κB-dependent genes (Zhong et al. 1997; Chen and Greene 2004; Vermeulen et al. 2006; Hayden and Ghosh 2008). Several different protein kinases and putative sites of phosphorylation on p65 have been identified, and, in general, it is believed that these phosphorylation events occur concomitantly with IKK-mediated phosphorylation of IκB proteins (Zhong et al. 1997; Duran et al. 2003; Vermeulen et al. 2003; Chen et al. 2005). We suggest that this additional step in the NF-κB activation pathway helps ensure that only NF-κB that enters the nucleus from the cytoplasm in response to appropriate inducing signals is able to trigger gene expression (Zhong et al. 1998, 2002).
We recently tested the biological importance of one of the putative sites of phosphorylation on p65, Ser 276, by generating a knock-in mouse expressing a serine-to-alanine mutation (Dong et al. 2008). Cells from these mice show a variable dependence on Ser 276 for gene expression, where expression of some target genes is nearly completely abolished, while other NF-κB target genes are affected moderately, if at all (Dong et al. 2008). Phosphorylation of p65 is believed to promote transcription by recruiting the histone acetyltransferase coactivators CBP/p300, and, consistent, with this hypothesis, the mutant S276A fails to recruit CBP/p300 (Zhong et al. 1998, 2002). However, the ability to induce the expression of a subset of target genes relatively normally suggests that recruitment of histone acetyltransferases is not obligatory for expression of all NF-κB target genes. On the other hand, the genetic studies demonstrated the critical role for phosphorylation of Ser 276 in the activation of NF-κB (Dong et al. 2008).
To determine whether changing the Ser 276 to a phosphomimetic amino acid could mimic phosphorylation, and to examine the biological consequences of such a modification, we knocked in a mutant form of p65, where Ser 276 was changed to aspartic acid (S276D), into the genome. Mice bearing the p65 S276D mutation are born at normal Mendelian ratios, but soon begin to display a progressive, systemic hyperinflammatory condition that results in severe runting and, typically, death 8–20 d after birth. We demonstrated that a significant number of NF-κB target genes are up-regulated in these mice, thereby explaining the hyperinflammatory phenotype. Remarkably, crossing the knock-in strain with mice lacking TNF receptor 1 (TNFR1) leads to a complete rescue of the systemic inflammatory phenotype, illustrating the importance of TNF signaling in the development of inflammation, but not in certain local inflammatory conditions—including one that resembles human keratoconjunctivitis sicca (KCS) or “dry eye,”—that appear to be dependent on NF-κB, but independent of TNF signaling. Therefore, the p65 S276D knock-in mice provide a unique model system, demonstrating the distinct roles of NF-κB in systemic inflammation and certain localized inflammatory diseases.
Results
Generation of p65 S276D mice
To create knock-in mice expressing the p65 S276D phosphomimetic protein, we used homologous recombination to introduce the mutant allele (Supplemental Fig. 1). An EagI restriction enzyme site was coupled to the mutation for ease of screening through PCR genotyping (Fig. 1A). The presence of the mutant allele was confirmed by sequencing the cDNA product of the p65 gene (Fig. 1B) to ensure that the S276D mutation was the only change that had been introduced into the p65 allele in the knock-in mice. Since residue 275 in p65 is a proline (P), and the residue 276 in the mutant allele is aspartic acid (D), this p65 S276D knock-in was designated as the “PD” allele (p65PD). To assess expression of the p65PD protein, we derived mouse embryonic fibroblasts (MEFs) from wild-type and homozygote littermates using 12.5-d-post-coitum (dpc) embryos. The homozygous knock-in fibroblasts were readily obtained and cultured. The level of p65 protein was nearly identical in wild-type and p65PD MEFs, indicating that the mutation did not significantly affect p65 stability (Fig. 1C). Immunoprecipitation of the p65 protein demonstrated that the mutant behaved identically to the wild-type protein in forming NF-κB:IκBα/β complexes (Fig. 1D). p65PD homozygous pups were born at normal Mendelian ratios, and were indistinguishable from their littermates at birth. After birth, p65PD mice exhibited progressive growth retardation, beginning at postnatal day 4∼5 (P4∼P5), and leading to dramatically runted animals by P8∼P10 (Fig. 1E).
Figure 1.
Generation and phenotypic characterization of p65 S276D (PD) knock-in mice. (A) PCR analysis of mouse tail DNA. Restriction enzyme digestion with EagI was performed to detect the PD mutation on p65. (B) Sequencing of p65 cDNA obtained by RT–PCR from wild-type and PD/PD homozygous mice. (C) Immunoblot analysis of p65 expression levels in wild-type and PD homozygous MEFs generated from embryos at 12.5 dpc. (D) Interaction between p65 and IκBα or IκBβ detected by immunoprecipitation in MEFs. (E) Gross appearance of wild-type and PD/PD homozygous knock-in P10 mice.
p65 S276D mice display a progressive, systemic hyperinflammation
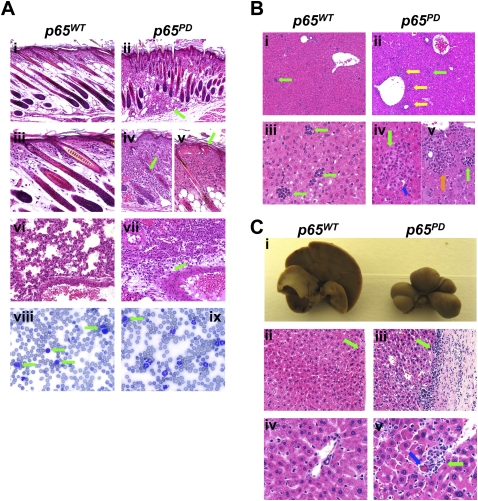
In addition to growth retardation, profound systemic inflammation occurred in these mice, and they typically died 8–20 d after birth. Gross pathological analysis suggested that all of the major organ systems were affected. The skin developed predominantly suppurative dermatitis with dermal fibrosis and multifocal epidermal necrosis (Fig. 2A, panels i–v). There was marked superficial and deep dermal inflammation in the skin of p65PD mice (Fig. 2A, panels ii,iv, green arrow) accompanied by epidermal hyperplasia and shortened distorted hair follicles (Fig. 2A, panels ii,iv). Inflammation was severe in all skin sections examined, and extended into the hypodermis and muscle (Fig. 2A, panels ii,iv). The lungs of the p65PD mice also exhibited multifocal suppurative and histiocytic interstitial and perivascular pneumonia (Fig. 2A, panel vii). While, in wild-type mice, lymphocytes predominated on peripheral blood smear, in p65PD mice, lymphocytes (Fig. 2A, panel vii, green arrow) were outnumbered by immature and mature neutrophils (Fig. 2A, panels vii,ix). The liver of p65PD mice was notable for its hyperceullarity (Fig. 2B), reflecting both perivascular (Fig. 2B, panel ii, yellow arrow) and intrasinusoidal (Fig. 2B, panel ii, green arrow) cellular infiltrates (Fig. 2B, panel ii). A histiocytic inflammatory infiltrate (Fig. 2B, panel iv, green arrow) accompanied by dying hepatocytes (Fig. 2B, panel iv, blue arrow) was also seen in the PD/PD liver (Fig. 2B, panel iv). Also, extramedullary hematopoeisis (EMH) with a predominance of myeloid (Fig. 2B, panel v, orange arrow) and megakaryocytic (Fig. 2B, panel v, green arrow) forms was much more abundant in the p65PD liver (Fig. 2B, panel v).
Figure 2.
Characterization of hyperinflammatory phenotype in PD mice. (A) Hematoxylin and eosin (H&E) staining of skin (panels i–v) and lung (panels vi,vii), and peripheral blood smear analysis (panels viii,ix) of wild-type and PD/PD littermates at P10. Bars: panels i,ii, 50 μm; panels iii–vii, 20 μm; panels viii,ix, 10 μm. (B) H&E staining of wild-type and PD/PD livers at P10. Bars: panels i,ii, 50 μm; panels iii–v, 20 μm. (C) Gross appearance and H&E staining of wild-type and PD/PD livers at 3.5 mo. Bars: panels ii,iii, 50 μm; panlesl iv,v, 20 μm.
Although the majority of p65PD mice died within 20 d after birth, a few individuals survived to 3.5 mo. In the aged p65PD mice, the liver was shrunken and distorted by bridging fibrosis extending from the capsule through the parenchyma (Fig. 2C). Multifocal capsular fibrosis and lymphocytic inflammation that extended into underlying parenchyma were also seen (Fig. 2C, panel iii, green arrow). Aggregates of predominantly histiocytic granulomas (Fig. 2C, panel v, green arrow) were present in the p65PD liver (Fig. 2C, panel v) but not in the wild-type liver (Fig. 2C, iv). These were often accompanied by dying hepatocytes (Fig. 2C, panel v, blue arrow). Other organ systems—including the brain, heart, spleen, colon, and bone—were also significantly affected in the p65PD mice. Taken together, these findings provide evidence that the S276D mutation in p65 is sufficient to alter the biological function of NF-κB in vivo.
Hyperactivation of NF-κB by p65PD
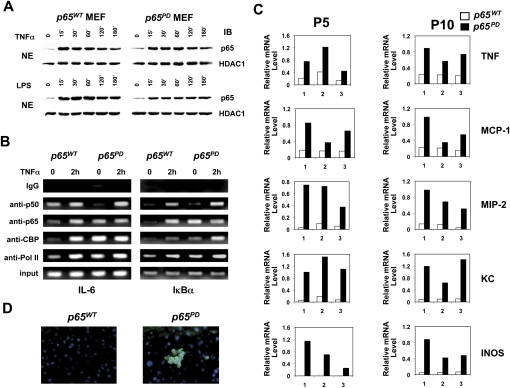
The hyperinflammatory phenotype of the p65PD mice suggested that the PD mutation was leading to hyperactivation of NF-κB. However, the levels of p65 protein in the nucleus in resting wild-type and PD/PD MEFs were similar, and stimulation with TNFα or lipopolysaccharide (LPS) led to normal kinetics of nuclear translocation of p65 in p65PD cells (Fig. 3A). Our previous studies had suggested that phosphorylation of Ser 276 is critical for recruitment of CBP/p300 to κB-binding sites on DNA both in vitro and in vivo (Zhong et al. 2002; Dong et al. 2008). CBP (and its close homolog, p300) acts as a co-activator by promoting histone acetylation near target promoters, thereby facilitating gene transcription. To study the behavior of the PD mutant protein on the promoters of NF-κB target genes, we tested the IL-6 and IκBα promoters using chromatin immunoprecipitation (ChIP) assays. Interestingly, we found that, in resting cells, p65 from PD mice bound to the promoters of both IL-6 and IκBα, while wild-type p65 did not (Fig. 3B). Meanwhile, the p65 PD protein, which can likely bind to DNA as a homodimer like phosphorylated p65 (Zhong et al. 1998), recruited CBP and RNA polymerase II (Pol II) to DNA (Fig. 3B). Upon stimulation, both wild-type p65 and the PD mutant bound to these two promoters with p50, likely as p50:p65 heterodimers, and both recruited CBP and Pol II to DNA (Fig. 3B). This observation offered an explanation for the hyperinflammatory phenotype observed in the PD mice.
Figure 3.
Hyperactivation of NF-κB induced by PD mutation. (A) Translocation of p65 following TNFα (10 ng/mL) or LPS (10 μg/mL) stimulation in MEFs. (B) ChIP assay was performed with untreated or TNFα-treated (2 h) MEFs using the indicated antibodies. Precipitated IL-6 κB site DNA and IκBα κB site DNA were assayed by semiquantitative PCR. (C) Differential expression of NF-κB-regulated genes in wild-type and PD/PD livers at P5 and P10. Total RNA isolated from three pairs of wild-type and PD/PD littermates was quantified by real-time RT–PCR, and was normalized to the level of GAPDH. (D) Immunostaining of TNF in wild-type and PD/PD livers at P10. Liver cross-sections were immunostained with FITC-conjugated antibody recognizing TNF. Nuclei were stained by DAPI.
To further address the biological consequences of expressing the PD mutant protein, we studied a number of typical NF-κB target genes involved in inflammation using real-time RT–PCR analysis. Liver samples were chosen for this study due to the dramatic inflammatory condition observed in the liver of the PD mice (Fig. 3C). The expression levels of TNFα, MCP-1, MIP-2, KC, and INOS were significantly increased by the PD mutant at both P5 and P10. Also, using immunostaining, an increase in TNFα was detected in the liver sections generated from p65PD mice (Fig. 3D). These data confirmed that the PD mutant protein hyperactivated NF-κB and resulted in up-regulated expression of NF-κB target genes.
PD knock-in mice can be rescued by TNFR1 knockout
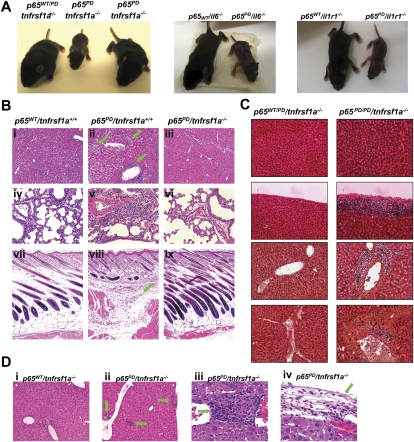
To investigate the role of TNF signaling in the pathogenesis of the hyperinflammation observed, we crossed the PD mice with mice deficient in TNFR1, IL1R, or IL-6. Remarkably, the phenotype presented in the young p65PD mice was fully rescued by knocking out TNFR1. p65PD mice lacking TNFR1 (p65PD/tnfrsf1a−/−) did not develop any macroscopic abnormalities, compared with the littermate controls (Fig. 4A). However, p65PD mice lacking IL1R or the IL6 gene maintained the hyperinflammatory phenotype, strongly suggesting that TNF was the key cytokine responsible for the observed inflammation (Fig. 4A). To confirm the effect of knocking out TNFR1 on rescuing the p65PD phenotype, histological analysis was performed on P10 littermates (Fig. 4B). p65PD/tnfrsf1a+/+ mice demonstrated perivascular and interstitial hepatic inflammatory infiltrates (Fig. 4B, panel ii, green arrow), while ablation of the TNFR1 gene restored wild-type hepatic morphology (Fig. 4B, panels i,iii). Inflammatory lung lesions seen in p65PD/tnfrsf1a+/+ mice were also eliminated by ablation of TNFR1 (Fig. 4B, panels iv–vi). Similarly, superficial and deep dermatitis (Fig. 4B, panels vii–ix, green arrow) and abnormal follicular length (Fig. 4B, panels vii–ix, indicated by vertical green line) were rescued in p65PD mice after ablation of TNFR1 (Fig. 4B, panels vii–ix). These results demonstrate that TNFR1 signaling is critical for the systemic hyperinflammatory phenotype in the PD knock-in mice.
Figure 4.
Phenotype of PD homozygous knock-in mice rescued by knocking out TNFR1. (A) Phenotype of PD/PD mice can be rescued by crossing with TNFR1 knockouts, but not IL6 or IL1R knockouts. Gross appearance of P10 littermates is presented. (B) H&E staining of major organs to confirm the rescue phenomenon resulted from knocking out TNFR1. Organs from P10 littermates were analyzed. Bars: panels i–iii,vii–ix, 50 μm; panels iv–vi, 20 μm. (C) H&E staining of liver in aged wild-type and PD/PD mice that lack TNFR1. Bars: panels i–iii, 50 μm; panels iv–vi, 20 μm. (D) H&E staining of the liver in one age-matched wild-type and three PD/PD TNFR1−/− mice that died at 6 mo of age.
Although TNFR1 knockout-rescued p65PD mice did not show any obvious defects at an early age, we found that a great number of these rescued mice (∼50%–60%) died sometime between 1 and 6 mo of age. The liver from the rescued p65PD mice was then studied as a model system to investigate differences between the p65PD mice and their wild-type p65 littermates on the TNFR1-deficient background. No hepatic inflammation was observed in p65wt mice lacking TNFR1, whereas, in p65PD mice lacking TNFR1, slowly progressive hepatic inflammation was seen (Fig. 4C). Inflammatory foci characterized by increasing numbers of interstitial (Fig. 4D, panel ii) and perivascular (Fig. 4D, panel iii) infiltrates were present in PD but not wild-type TNFR1-deficient mice, and were composed predominantly of macrophages with fewer neutrophils. Capsular fibrosis and inflammation were also seen in some p65PD/tnfrsf1a−/− mice (Fig. 4D, panel iv, green arrow). The overall distribution of lesions appeared to be vasculocentric, and, in some cases, were found to affect most of the vascular circumference. In those p65PD/tnfrsf1a−/− mice that died at only a few months of age, the liver showed a dramatic inflammatory phenotype. Although the ratio of liver weight to body weight was only slightly increased in aged p65PD/tnfrsf1a−/− mice (Supplemental Fig. 3A), the number of inflammatory foci was significantly increased compared with p65wt/tnfrsf1a−/− littermates (Supplemental Fig. 3B). This observation suggests that the systemic inflammatory phenotype characterizing the p65PD mice, exemplified by the hepatic pathologies described, is dependent on TNFR1 signaling. Thus, ablation of TNFR1 ameliorated the majority of hepatic inflammation. However, low-grade local inflammation was present, demonstrating that progressive localized inflammatory processes can be mediated by p65 PD independent of TNFR1.
A subset of genes are hyperactivated by p65PD, causing TNFR1-independent keratitis
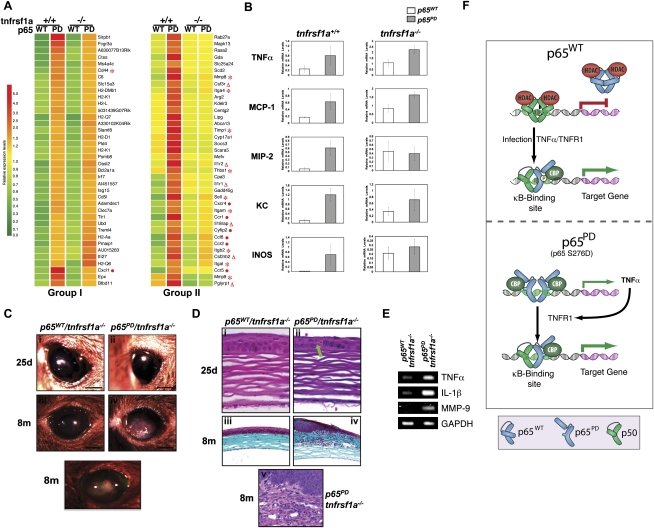
To further demonstrate the effects of p65 PD on NF-κB transactivation, we performed a genome-wide Affymetrix microarray expression analysis using RNA isolated from wild-type, p65PD, p65wt/tnfrsf1a−/−, and p65PD/tnfrsf1a−/− livers from P5 mice. The representative genes were hierarchically clustered and displayed (Fig. 5A). Genes in group I were significantly induced in the PD liver, irrespective of the tnfrsf1a genotype; that is, genes up-regulated by p65PD independent of TNFR1 signaling (Fig. 5A). Genes in group II were significantly induced in the p65PD liver but not the p65PD/tnfrsf1a−/− liver, and, therefore, their expression was dependent on signaling through TNFR1 (Fig. 5A). The majority of the genes that were up-regulated by the p65 PD mutant fell into group II. In Figure 5A, genes encoding extracellular matrix and adhesion molecules involved in inflammation are indicated by a red asterisk; genes encoding cytokines and receptors are indicated by a red triangle; and genes encoding chemokines and receptors are indicated by a red dot. Real-time RT–PCR analysis also demonstrated that the up-regulation of TNFα and MCP-1 by the p65 PD mutant was TNFR1-independent, while the up-regulation of MIP-2, KC, and INOS was TNFR1-dependent (Fig. 5B). These results provided a clear molecular explanation for the hyperinflammatory phenotype in PD mice, and the rescue of this phenotype upon TNFR1 deletion. Our data therefore strongly support the model that expression of the PD mutant protein creates a constitutively active form of NF-κB (consisting of p65 homodimers) that has the ability to up-regulate TNFα, which then acts in a paracrine manner through TNFR1 to trigger systemic hyperinflammation (Fig. 5F).
Figure 5.
Regulation of gene expression and KC by p65 PD. (A) Comparison of gene expression in wild-type versus PD/PD livers obtained from P5 mice, either with or without TNFR1, using Affymetrix microarrays. Representative genes in two groups of genes were hierarchically clustered and displayed. Each row represents a single gene, and each column represents an experimental sample. The ratio of the abundance of transcripts of each gene to the median abundance of the gene's transcript among all the samples is represented by the color in the matrix, as indicated. Relative expression levels are shown at the bottom. (B) Total RNA isolated from the livers of three pairs of wild-type p65 and PD/PD littermates, either with or without TNFR1, at P5 was quantified by real-time RT–PCR, and was normalized to the level of GAPDH. The average mRNA level of three mice in each group is presented. Ocular lesions accompanying inflammation developed in the eye of the PD homozygous knock-in mice rescued by knocking out TNFR1. (C) Gross appearance of cornea in weanling (25d) and adult (8m) mice. Bar: panels i–v, 1 mm. (D) Corneal histology in weanling (25d; H&E staining in panels i,ii) and adult (8m; masson's trichrome staining in panels iii,iv; H&E staining in panel v) mice. Bars: panels i,ii, 20 μm; panels iii,iv, 100 μm; panel v, 50 μm. (E) Corneal expression of TNFα, IL-1β, and MMP-9 at 25 d, determined by semiquantitative RT–PCR. (F) A model of the mechanism through which p65 S276D mutant protein hyperactivates gene expression. Expression of the PD mutant protein creates a constitutively active form of NF-κB (consisting of p65 homodimers) that up-regulates TNFα, which then acts in a paracrine manner through TNFR1 to trigger systemic hyperinflammation.
In addition to the death observed in the p65PD/tnfrsf1a−/−-rescued adult mice, ocular lesions occurred in almost all mice, whether they died early or late. No such ocular lesions were seen in any littermate wild-type or TNFR1 knockout mice, indicating that these lesions are induced by the PD mutant protein, not the loss of TNFR1.
In the absence of TNFR1, ocular features were comparable in p65PD and wild-type mice at 25 d of age. Eyelid development and opening, as well as corneal appearance, were normal in both strains (Fig. 5D, panels i,ii). As the mice aged, however, a vascularized and proliferative corneal lesion developed in the central region of the cornea in p65PD/tnfrsf1a−/− mice (Fig. 5D, panel iv), but not in p65WT/tnfrsf1a−/− mice (Fig. 5D, panel iii). Corneal proliferation could be seen in p65PD/tnfrsf1a−/− mice, as evidenced by whitish vascularized tissue occupying the central cornea (Fig. 5D, panel iv). This lesion developed over variable periods of time in individual mice, but was never present before 2 mo, and was always present after 6 mo of age. Fluorescein staining failed to identify ulceration, indicating that the corneal epithelium was intact (Fig. 5D, panel v). Schirmer tear test results in tnfrsf1a−/− and p65PD/tnfrsf1a−/− mice at 25 d were comparable (Supplemental Fig. 4), as was the histology of lacrimal glands and conjunctival mucosa (data not shown). These results suggested that an alteration in the amount of tear flow was unlikely to be the cause of the corneal epithelial defect in p65PD/tnfrsf1a−/− mice. In addition, a similar spectrum of commensal bacterial species (such as Streptococcus viridans and Pasteurella pneumotropica) (data not shown) was cultured from p65wt/tnfrsf1a−/− and p65PD/tnfrsf1a−/− eyes, thus ruling out the possibility that the PD mutant predisposes to bacterial keratitis. When the expression of NF-κB-regulated proinflammatory mediators—including TNFα, IL-1β, and MMP-9—were studied by semiquantitative RT–PCR, it was found that the corneal epithelium in p65PD/tnfrsf1a−/− mice showed significantly increased expression of these genes (Fig. 5E). The increased expression of these genes can therefore help explain the ocular lesions induced by the PD mutant protein. Histological analysis demonstrated that, at 25 d, the corneal epithelium was slightly thinner and keratinocytes were less orderly in p65PD/tnfrsf1a−/− mice (Fig. 5E, panel ii), compared with p65wt/tnfrsf1a−/− mice (Fig. 5E, panel i). Occasional dying keratinocytes were present in p65PD/tnfrsf1a−/− mice (Fig. 5E, panel ii, green arrow). However, the corneal stroma appeared normal in p65PD/tnfrsf1a−/− mice at 25 d (Fig. 5E, panel ii). By 6 mo, in p65PD/tnfrsf1a−/− mice, central corneal lesions were fully developed. Severe corneal epithelial thickening was observed, occurring abruptly at the junction of the central and peripheral cornea, and occupying the central third of the cornea (Fig. 5E, panel iv). There was readily noticeable neovascularization and inflammation of superficial to mid-layers of the corneal stroma (Fig. 5E, panels iv,v). Inflammatory infiltrate within the corneal stroma consisted of a mixed population of inflammatory cells, composed predominantly of neutrophils and macrophages (Fig. 5E, panels iv,v). This infiltrating population was similar to that seen in the liver of aged p65PD/tnfrsf1a−/− mice.
Discussion
The studies presented in this study describe the development and characterization of a novel mouse model of constitutively activated NF-κB. Similar attempts to obtain constitutive NF-κB activation by knocking in active forms of IKKβ have been unsuccessful, most likely because the very efficient activation of NF-κB in those models leads to embryonic lethality. In contrast, the knock-in of the p65 PD allele, which mimics a key phosphorylation event in the regulation of p65, leads to a more selective activation of NF-κB-dependent genes. This difference is probably because the active NF-κB moiety in this model is p65 homodimers present at low levels in the nucleus of unstimulated cells. Such p65 homodimers in wild-type cells are bystanders, as they are unable to bind to DNA; however, the S276D mutation, by mimicking the effect of phosphorylating this site, allows these p65PD homodimers to bind to DNA, and also to recruit CBP/p300 (Fig. 5F). Phosphorylation or replacement of S276 with a phosphomimetic amino acid likely augments DNA binding of p65 homodimers by both unmasking the DNA-binding interface on p65 and augmenting the interaction of p65 with CBP/p300 (Zhong et al. 1998, 2002). While the former allows the PD protein to bind to certain κB sites, the latter is likely to stabilize the binding by maintaining open chromatin structure. The result is the up-regulation of a subset of NF-κB-regulated genes, and the induction of an inflammatory phenotype in vivo.
One implication of our findings that we are currently exploring is that up-regulation of NF-κB-dependent genes may be triggered by signaling pathways that activate kinases capable of phosphorylating p65 at S276, bypassing traditional IKK-dependent NF-κB signaling pathways. Interestingly, the kinases that phosphorylate p65 S276, including MSK1/2 and PKAc, are regulated independently of NF-κB signaling pathways. For example, epidermal growth factor signaling, which activates MSK1, has been shown to induce p65-dependent gene expression independently of IκBα degradation or p65 nuclear translocation (Deak et al. 1998; Anest et al. 2004). The p65 PD model may thus represent a novel, IKK-independent, mechanism of NF-κB activation. This “atypical” NF-κB pathway may contribute to the biological activity of NF-κB, given the numerous pathways that can signal to kinases implicated in p65 phosphorylation. However, as the p65 homodimers probably do not bind efficiently to most κB-sites, which are designed to bind to p50:p65 heterodimers, only a small subset of genes are induced directly by the p65 PD homodimer complexes. Nevertheless, despite the relatively select group of genes that can apparently be targeted by phosphorylated p65 dimers, mice expressing the phosphomimetic p65 allele exhibit a robust systemic and lethal inflammatory phenotype.
One of the genes that is directly regulated by the PD is TNFα, and it is the aberrant, increased synthesis of TNFα that triggers a cascade of events that results in the hyperinflammatory phenotype seen in the knock-in mice. Consistent with the key role for TNFα at the top of this cascade, crossing the p65PD mice with mice lacking TNFR1 completely abolishes all signs of systemic inflammation. Thus, surprisingly, it is the response of the TNFα gene to p65 PD homodimers that drives the phenotype of the p65PD mouse.
Despite the lack of obvious inflammation in the rescued mice, consistent with both biochemical and microarray studies, a significant number of NF-κB-dependent genes are up-regulated continuously in the p65PD/tnfrsf1a−/− mice. However, these genes do not include many of the genes traditionally thought to be NF-κB-regulated inducible genes, including most cytokine, chemokine, and anti-apoptosis genes (Hayden and Ghosh 2004, 2008). It is therefore not surprising that these mice fail to display enhanced susceptibility to the development of tumors when treated with tumor promoters such as DEN or DSS (data not shown). These results therefore suggest that tumor promotion is selectively due to NF-κB complexes that are induced in response to signals that proceed through cytoplasmic signaling pathways that involve the IKK complex.
While the p65PD/tnfrsf1a−/− mice do not display any profound, visible abnormality, the continuous up-regulation of a subset of NF-κB-regulated genes significantly affects the life span of these animals. As a result, a fraction of these mice die within 6 mo of age, and it is possible that this premature lethality is a manifestation of an accelerated aging process. Recent studies have shown that NF-κB plays an important role in aging (Adler et al. 2007), and therefore it is possible that the p65PD/tnfrsf1a−/− mice die due to aging-related events. We are currently studying these mice more carefully to better understand the events that lead to their early death. Histological analyses demonstrate a more limited, localized, and variable inflammatory condition in the livers of p65PD/tnfrsf1a−/− mice. Therefore, aberrant NF-κB activation manifests systemic inflammation chiefly through the induction of TNFα, while NF-κB activation can induce localized inflammation and tissue damage in the absence of TNFR1.
Besides early lethality, the most consistent shared property manifested by the knock-ins is the development of eye pathology. Keratitis of p65PD/tnfrsf1a−/− mice is indicative of chronic corneal irritation. Gene expression data showing increased levels of TNFα, IL-1β, and MMP-9 confirm the presence of corneal inflammation at P25, prior to the development of lesions, suggesting that TNFR1-independent corneal inflammation is induced by the PD mutation. A similar gene expression profile is seen in human KCS (Pflugfelder 2004). However, the etiological basis of the lesions in p65PD mice and human dry eye is clearly different. The human disease is secondary to decreased tear production as a result of either pathologic processes that affect tear production, as in the case of Sjogren's syndrome, or environmental factors that affect tears, such as dry atmosphere (Pflugfelder 2004; Fox 2007). On the other hand, in the p65PD/tnfrsf1a−/− mice, the trigger is genetic and independent of decreased lacrimal function. Therefore, we propose that the cornea possesses innate proinflammatory and anti-inflammatory mechanisms, and that these can be deranged by a number of stimuli, including activation of NF-κB, and by the expression of the PD mutant, leading to an inflammatory disease condition that mimics the pathology observed in KCS.
A similar complement of proinflammatory factors has also been implicated in other forms of KC, including atopic KC. Therefore activation of the proinflammatory NF-κB pathway may play an important role in the pathogenesis of KCS and other forms of KC, and this pathway may represent an important aspect of inflammatory homeostasis in the cornea. Thus, our data suggest that inflammatory conditions may contribute to the formation of KC both secondary to and independent of the lacrimal gland damage and decreased tear production that is observed in Sjogren's syndrome and other KCS diseases. Furthermore, these results provide new insights into the mechanism of action of anti-inflammatory compounds in the treatment of KCS, and suggest that more targeted therapeutics may be a viable alternative for the treatment of this common disease.
Materials and methods
Generation of PD knock-in mice
An EagI restriction enzyme site was coupled to PD mutation for genotyping using PCR analysis. The PD knock-in allele was generated by homologous recombination in TC1 embryonic stem cells. Targeted clones were selected, and homologous recombination was confirmed by Southern blot analysis. Positive clones were injected into C57BL/6 blastocysts and produced germline chimeras. Chimeras were mated with C57BL/6 female mice to yield heterozygous mice. Heterozygous male mice were bred with female splice mice to excise the floxed neo cassette. The offspring were screened for the targeted allele without the neo cassette and absence of the cre transgene. Heterozygous mice were interbred and maintained.
Immunoblots, immunoprecipitation, and ChIP
For immunoblotting, whole-cell lysates or nuclear extracts were resolved in SDS-PAGE gels, transferred to PVDF membranes, and incubated with the following antibodies: anti-p65 (Biomol); anti-IκBα and anti-IκBβ (Santa Cruz Biotechnology); anti-HDAC1 (Sigma); anti-GAPDH (Fitzgerald). Immunoprecipitation was performed as described previously (Marienfeld et al. 2003). ChIP assay was performed essentially according to the manufacturer's instructions (Upstate Biotechnologies) using anti-p65 antibody (Biomol); anti-p50, anti-CBP, and anti-Pol II antibodies (Santa Cruz Biotechnology); or mouse IgG (Upstate Biotechnologies). The extracted DNA was used for semiquantitative PCR to amplify the DNA fragments of interest using HotStarTaq DNA polymerase (Qiagen). Primer sequences are available on request.
Quantitative real-time RT–PCR analysis
Total RNA was isolated from mouse liver samples using TRIzol reagent (Invitrogen), purified with RNeasy mini kit (Qiagen), and reverse-transcribed with QuantiTect reverse transcription system (Qiagen) to produce cDNA. cDNA was used as a template for quantitative real-time PCR analysis, performed using Brilliant SYBR Green QPCR Master Mix (Stratagene) with Stratagene Mx3000P QPCR system following instructions. Primer sequences are available on request.
Histology and immunostaining
Tissues were fixed in 10% neutral-buffered formalin solution, and paraffin-embedded tissue sections (5 μm) were stained with hematoxylin and eosin or masson's trichrome using standard method. Cryostat sections (5 μm) containing the liver were prepared, fixed with acetone, and immunostained with a FITC-conjugated TNF-specific antibody (BD Biosciences). Sections were counterstained with DAPI to visualize nuclei.
Gene expression profiling
Total RNA was isolated from mouse liver samples using TRIzol reagent (Invitrogen), and was purified with RNeasy mini kit (Qiagen). RNA was analyzed on Affymetrix GeneChip Mouse Genome 430 2.0 Array at W.M. Keck Affymetrix GeneChip Core Facility (Yale University). Data analysis was performed and a gene expression heat map was generated using GeneSpring GX 7.3 Expression Analysis software (Agilent Technologies) at W.M. Keck Biostatistics Resource (Yale University).
Acknowledgments
We thank Dr. Aiping Lin at the Yale W.M. Keck Biostatistics Resource Affymetrix microarray analysis. The research in this study was supported by grants from the National Institutes of Health (R37-AI033443 and R01-AI066109).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1958410.
Supplemental material is available at http://www.genesdev.org.
References
- Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY 2007. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev 21: 3244–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anest V, Cogswell PC, Baldwin AS Jr 2004. IκB kinase α and p65/RelA contribute to optimal epidermal growth factor-induced c-fos gene expression independent of IκBα degradation. J Biol Chem 279: 31183–31189 [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC 2004. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol 5: 392–401 [DOI] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC 2005. NF-κB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25: 7966–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J 17: 4426–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Jimi E, Zhong H, Hayden MS, Ghosh S 2008. Repression of gene expression by unphosphorylated NF-κB p65 through epigenetic mechanisms. Genes Dev 22: 1159–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Diaz-Meco MT, Moscat J 2003. Essential role of RelA Ser311 phosphorylation by ζPKC in NF-κB transcriptional activation. EMBO J 22: 3910–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PC 2007. Autoimmune diseases and Sjogren's syndrome: An autoimmune exocrinopathy. Ann N Y Acad Sci 1098: 15–21 [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson AK, Soderberg-Naucler C 2006. Inflammation and atherosclerosis. Annu Rev Pathol 1: 297–329 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S 2004. Signaling to NF-κB. Genes Dev 18: 2195–2224 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S 2008. Shared principles in NF-κB signaling. Cell 132: 344–362 [DOI] [PubMed] [Google Scholar]
- Kalden JR 2002. Emerging role of anti-tumor necrosis factor therapy in rheumatic diseases. Arthritis Res 4: S34–S40 doi: 10.1186/ar552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P 2006. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83: 456S–460S [DOI] [PubMed] [Google Scholar]
- Libby P 2007. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr Rev 65: S140–S146 doi: 10.1111/j.1753-4887.2007.tb00352.x [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F 2008. Cancer-related inflammation. Nature 454: 436–444 [DOI] [PubMed] [Google Scholar]
- Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M 2003. RelB forms transcriptionally inactive complexes with RelA/p65. J Biol Chem 278: 19852–19860 [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC 2004. Antiinflammatory therapy for dry eye. Am J Ophthalmol 137: 337–342 [DOI] [PubMed] [Google Scholar]
- Reimold AM 2002. TNFα as therapeutic target: New drugs, more applications. Curr Drug Targets Inflamm Allergy 1: 377–392 [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR, Kaser A, Pines A, Dotan I 2008. Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut 57: 684–694 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G 2003. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22: 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, Vanden Berghe W, Haegeman G 2006. Regulation of NF-κB transcriptional activity. Cancer Treat Res 130: 89–102 [PubMed] [Google Scholar]
- von Herrath M, Sanda S, Herold K 2007. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 7: 988–994 [DOI] [PubMed] [Google Scholar]
- Weiner HL, Selkoe DJ 2002. Inflammation and therapeutic vaccination in CNS diseases. Nature 420: 879–884 [DOI] [PubMed] [Google Scholar]
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S 1997. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424 [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1: 661–671 [DOI] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S 2002. The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol Cell 9: 625–636 [DOI] [PubMed] [Google Scholar]