Abstract
Differentiated neurons balance the need to maintain a stable identity with their flexible responses to dynamic environmental inputs. Here we characterize these opposing influences on gene expression in Caenorhabditis elegans olfactory neurons. Using transcriptional reporters that are expressed differentially in two olfactory neurons, AWCON and AWCOFF, we identify mutations that affect the long-term maintenance of appropriate chemoreceptor expression. A newly identified gene from this screen, the conserved transcription factor hmbx-1, stabilizes AWC gene expression in adult animals through dosage-sensitive interactions with its transcriptional targets. The late action of hmbx-1 complements the early role of the transcriptional repressor gene nsy-7: Both repress expression of multiple AWCOFF genes in AWCON neurons, but they act at different developmental stages. Environmental signals are superimposed onto this stable cell identity through at least two different transcriptional pathways that regulate individual chemoreceptor genes: a cGMP pathway regulated by sensory activity, and a daf-7 (TGF-β)/daf-3 (SMAD repressor) pathway regulated by specific components of the density-dependent C. elegans dauer pheromone.
Keywords: Chemoreceptor, olfactory receptor; homeostasis; neuronal development; olfactory neuron
Neurons and other long-lived cells are subject to ongoing modification throughout life, but use transcriptional strategies to maintain stable cell fates (Shirasaki and Pfaff 2002; Ringrose and Paro 2004). In some cells, transcription factors required for the establishment of cell fate remain active throughout life. In the mammalian immune system, the transcription factor Pax5 is required both for initial commitment to the B-cell lineage and for continued expression of B-cell identity: Deletion of Pax5 from committed pro-B cells resulted in their reversion to a multipotential state (Nutt et al. 1999; Mikkola et al. 2002). In other cases, dedicated transcriptional regulators maintain cell identity. For example, the Caenorhabditis elegans aristaless homolog alr-1 is required to maintain, but not establish, functions of sensory glia (Tucker et al. 2005). In addition to cell-specific transcription factors, general chromatin remodeling factors can stabilize cell fates. The Polycomb group (PcG) genes in Drosophila maintain a precise Hox gene expression pattern after the disappearance of early developmental regulators (Busturia and Morata 1988; Cao et al. 2002; Muller and Kassis 2006), and the histone acetyltransferase system regulated by BET-1 and MYST maintains cell type-specific gene expression in C. elegans neuronal lineages (Shibata et al. 2010).
Sensory neurons are faced with the special challenge of maintaining a stable state while responding to a changing environment. In the senses of taste and smell, heterogeneous populations of sensory neurons express different chemoreceptor genes to detect different environmental chemicals (Buck and Axel 1991; Troemel et al. 1995; Vosshall et al. 1999; Clyne et al. 2000; Etchberger et al. 2007). The expression of chemoreceptor genes is initiated by innate developmental programs. For example, in C. elegans, chemoreceptor expression in the two ASE taste neurons is initiated by a general transcriptional regulator for ASE, che-1, and refined by a double negative feedback loop that distinguishes right and left ASE fates (Chang et al. 2003, 2004; Johnston and Hobert 2003; Johnston et al. 2005; Etchberger et al. 2007, 2009). che-1 maintains its own expression in the ASE chemosensory neurons, and also acts on target genes throughout life (Etchberger et al. 2009); this combination of initiation and differentiation roles defines che-1 as a “terminal selector gene” (Hobert 2008). Analogous genetic studies of other chemosensory cell types—including the AWA, AWB, and AWC olfactory neurons—have generated a sophisticated understanding of the transcription factors that initiate unique neuronal identities (Sengupta et al. 1994; Sagasti et al. 1999; Sarafi-Reinach and Sengupta 2000; Sarafi-Reinach et al. 2001; Colosimo et al. 2003; Lanjuin et al. 2003; Nokes et al. 2009; Kim et al. 2010).
Superimposed on stable chemosensory neuron fates are environmental factors that modify gene expression. The C. elegans dauer pheromone, a mixture of compounds containing the sugar ascarylose, represses the expression of chemoreceptor genes in ASH and ASI neurons by regulating intercellular signaling through a TGF-β signaling pathway (Peckol et al. 2001; Nolan et al. 2002; Kim et al. 2009). A salt-inducible kinase affects chemoreceptor expression in AWB olfactory neurons by regulating the transcription factor MEF2 (Lanjuin and Sengupta 2002; van der Linden et al. 2007, 2008). The relationship between these environmental regulators and stable cell fates raises intriguing questions about the relative roles of fixed and variable aspects of neuronal function.
The two C. elegans AWC olfactory neurons provide a system in which the acquisition and maintenance of cell fates are distinct. The Otx transcription factor CEH-36 and the HMX/NLX homeodomain protein MLS-2 initiate a general AWC identity, which is subsequently maintained by ceh-36 (Lanjuin et al. 2003; Kim et al. 2010). In addition to promoting the expression of an AWC-specific transcriptional program, ceh-36 maintains its own expression, suggesting that ceh-36 is the terminal selector gene in AWC (Kim et al. 2010). Later in embryogenesis, a stochastic cell fate decision causes the right and left AWC olfactory neurons to take on asymmetric fates, such that one AWC becomes AWCON, defined as a neuron that expresses the G protein-coupled receptor (GPCR) str-2 and senses the odor butanone, and the other AWC becomes AWCOFF, which expresses the GPCR srsx-3 and senses the odor 2,3-pentanedione (Troemel et al. 1999; Wes and Bargmann 2001). The decision to become AWCON or AWCOFF is made through a signaling pathway that generates the initial asymmetry of chemoreceptor gene expression and also drives asymmetric expression of the transcription factor NSY-7 in AWCON (Troemel et al. 1999; Lesch et al. 2009). After hatching, the initial signaling pathway becomes inactive, and NSY-7 maintains appropriate chemoreceptor expression in AWCON. In addition, a cGMP pathway regulated by olfactory signal transduction maintains post-embryonic expression of both str-2 and srsx-3 using two receptor-type guanylate cyclases (encoded by odr-1 and daf-11), a cyclic-nucleotide gated cation channel (encoded by tax-2 and tax-4), and a cGMP-responsive protein kinase (encoded by egl-4) (Troemel et al. 1999; Lesch et al. 2009).
To better understand the factors responsible for the stability of AWCON and AWCOFF fates, we performed a screen for mutants that failed to maintain expression of one or both of the asymmetric AWC markers str-2 and srsx-3. Here, we describe the mutants isolated from this screen. We identify the transcription factor hmbx-1, a homolog of mammalian HMBOX1, as a regulator of AWC receptor gene expression that acts primarily in adult animals. Using newly identified GPCR genes expressed in AWC neurons, we show that the maintenance of asymmetric receptor gene expression involves at least three repressor pathways: nsy-7, an AWCON-specific cell identity gene; hmbx-1, a dosage-sensitive repressor of AWCOFF genes in AWCON neurons; and daf-3, a pheromone-regulated repressor that affects chemoreceptors on a gene-by-gene basis.
Results
Genetic pathways required for maintenance of GPCR expression in AWC
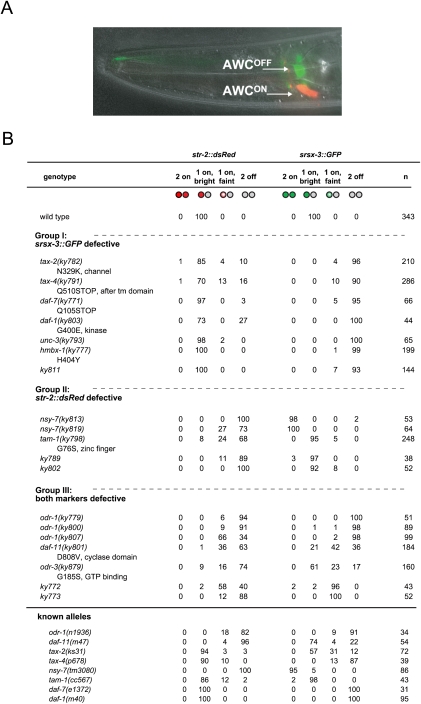
We sought mutants that expressed the AWCON-specific receptor str-2 and the AWCOFF-specific receptor srsx-3 appropriately as early larvae, but failed to maintain expression of one or both of these receptors in adulthood. A strain with an integrated transgene containing str-2∷dsRed2 and srsx-3∷GFP reporters was mutagenized, and the adult F2 progeny were examined for defects in expression of str-2, srsx-3, or both genes (Fig. 1A). After verifying the adult phenotype in subsequent generations, expression of str-2 and srsx-3 was evaluated in early larvae, 14 h after hatching (L1 stage), and mutants with a wild-type phenotype at this stage were retained. The screen yielded 19 mutants with defects in the maintenance of AWC markers (Fig. 1B). Genetic mapping and complementation testing indicated that 13 mutations fell in previously characterized genes and pathways, although only a subset had been known to affect AWC gene expression. These genes are described briefly below.
Figure 1.
Expression phenotypes of mutants defective for str-2∷dsRed and srsx-3∷GFP maintenance. (A) DIC image of the head of an adult worm overlaid with a fluorescence image of the str-2∷dsRed2 (AWCON) and srsx-3∷GFP (AWCOFF) reporters. (B) Mutants from the screen. (Group I) srsx-3 expression affected more strongly than str-2 expression. (Group II) str-2 expression affected more strongly than srsx-3 expression. (Group III) Both markers affected. Other alleles of genes identified in the screen are shown for reference. Bright and faint fluorescence were scored qualitatively; in other figures, these two categories are combined into a single “1 AWC” class.
Olfactory transduction
Six of the new mutations affect the olfactory cGMP transduction pathway that maintains str-2 and srsx-3 expression, including three alleles of odr-1, one allele of daf-11, one allele of tax-2, and one allele of tax-4 (Fig. 1B; Troemel et al. 1999; Lesch et al. 2009).
Previous studies have revealed both cell-autonomous and nonautonomous effects of sensory signaling proteins on AWC gene expression (Lans and Jansen 2006). Moreover, tax-4 promotes expression of daf-7 (Coburn et al. 1998), which acts in ASI neurons to promote srsx-3 expression in AWC neurons (see below). To ask where tax-4 acts to regulate srsx-3 expression, we expressed the tax-4 cDNA in AWC or ASI in a tax-4(ky791) mutant background. AWC-selective expression of TAX-4 rescued the srsx-3 expression defect of tax-4(ky791) mutants, but ASI expression did not (Supplemental Fig. S1). Therefore, tax-4 acts in AWC to promote srsx-3 expression.
The screen also yielded a dominant mutation in the Gα subunit odr-3, odr-3(ky879), which encodes a G → S missense mutation at position 185. The affected glycine is a conserved residue in the region that changes conformation upon GTP binding; stabilization of the GTP-bound conformation should result in a constitutively active protein (Rens-Domiano and Hamm 1995). The nature of the odr-3(ky879) allele suggests that increased olfactory G protein activity disrupts maintenance of GPCR expression, perhaps by reducing cGMP levels (Chalasani et al. 2007). Loss-of-function mutations in odr-3 have little effect on str-2 expression, although str-2 expression is reduced when odr-3(lf) alleles are combined with mutations in other Gα subunits (Lans and Jansen 2006).
Transcriptional regulation
Two alleles of nsy-7 isolated in the screen had defective maintenance of str-2 expression, accompanied by bilateral srsx-3 expression—the same phenotype observed in previously characterized nsy-7 alleles (Lesch et al. 2009).
Another mutation affected the tam-1 gene, which encodes a transcriptional regulator that inhibits silencing of repetitive transgenes (Hsieh et al. 1999). A null allele of tam-1 also affects str-2 expression, but had a weaker defect than the new missense allele from the screen (Fig. 1B). The stronger phenotype of the missense allele could result from an altered function of the mutant protein, or from modifying effects of background mutations. tam-1 was not known previously to affect gene expression in AWC, but diminished expression of the AWC reporter genes is consistent with the general reduction in transgene expression reported in tam-1 mutants.
DAF-7/TGFβ signaling
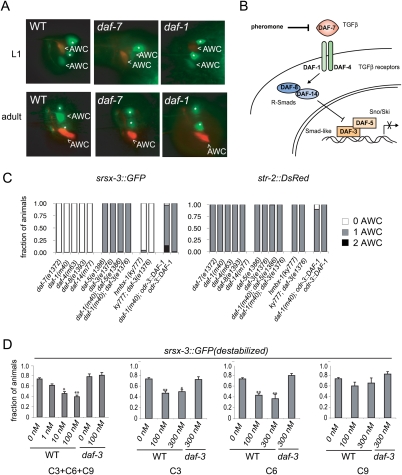
Several mutations from this screen primarily affected srsx-3 and not str-2 expression. These included an allele of daf-7 (the TGFβ ligand that regulates the dauer developmental decision and chemoreceptor gene expression in ASH and ASI neurons) and an allele of daf-1 (a TGFβ type I receptor) (Figs. 1B, 2A,B; Georgi et al. 1990; Ren et al. 1996; Schackwitz et al. 1996; Peckol et al. 2001; Nolan et al. 2002). A mutation in unc-3, a transcription factor required for expression of daf-7 in the ASI neurons, was also isolated in the screen, and had a similar phenotype (Prasad et al. 1998; Kim et al. 2005). Existing daf-4 (the type II TGFβ receptor), daf-8 (a Smad-like protein), and daf-14 (a Smad-like protein) mutants displayed similar adult phenotypes to daf-7 and daf-1 (Fig. 2C). These results indicate that TGFβ signaling maintains srsx-3 expression in AWCOFF.
Figure 2.
TGFβ and dauer pheromone signals regulate srsx-3 expression. (A) Images of L1 larvae and adults in wild-type, daf-7(e1372), and daf-1(m40) animals. AWC neurons are labeled, and asterisks mark AWB neurons. (B) Schematic illustration of the daf-7 TGFβ pathway. (C) srsx-3 and str-2 expression phenotypes for daf-7/TGF-β pathway mutants and rescue of daf-1(m40) under the AWC-selective promoter odr-3. Also shown is the phenotype of the hmbx-1(ky777); daf-3(e1376) double mutant. n > 25 for all genotypes. (D) Regulation of srsx-3 by a mixture of C3, C6, and C9 ascarosides (left) and by each ascaroside alone (two middle and right). Graphs show the fraction of adults expressing a destabilized GFP in AWC under the control of the srsx-3 promoter. (*) P < 0.01; (**) P < 0.001 compared with no pheromone control (one-way ANOVA with Dunnett's post-test).
daf-3, which encodes a co-Smad that binds DNA, and daf-5, which encodes a proline-rich transcriptional regulator, act downstream from and antagonistically to daf-1 to promote dauer formation (Patterson et al. 1997; da Graca et al. 2004). Expression of str-2 and srsx-3 reporters was normal in daf-3 and daf-5 single mutants, and in daf-3; daf-1 or daf-5; daf-1 double mutants, recapitulating the regulatory relationships seen in dauer formation (Fig. 2B,C). Expression of DAF-1 in AWC rescued the srsx-3 expression defect of daf-1(m40) mutants, suggesting that TGF-β signals directly to AWC to maintain receptor gene expression (Fig. 2C).
daf-7 expression is inhibited by the dauer pheromone, a mixture of structurally related chemicals termed ascarosides (Jeong et al. 2005; Butcher et al. 2007, 2008). To ask whether srsx-3 expression responded acutely to pheromones, we exposed adult worms to the ascarosides C3, C6, and C9 for 4 h, and monitored expression of srsx-3 using a destabilized GFP protein that has a half-life of ∼1 h in C. elegans (Gaudet and Mango 2002; Frand et al. 2005). An equal mixture of C3, C6, and C9 suppressed srsx-3 expression in a dose-dependent manner (Fig. 2D). The ascarosides C3 and C6 each suppressed srsx-3 expression about as well as the mixture, while C9 was less effective. All effects of ascarosides were blocked in daf-3 mutants, suggesting that C3 and C6 pheromones regulate srsx-3 expression through the TGFβ pathway (Fig. 2D).
A missense allele of the transcription factor hmbx-1 suppresses srsx-3 expression
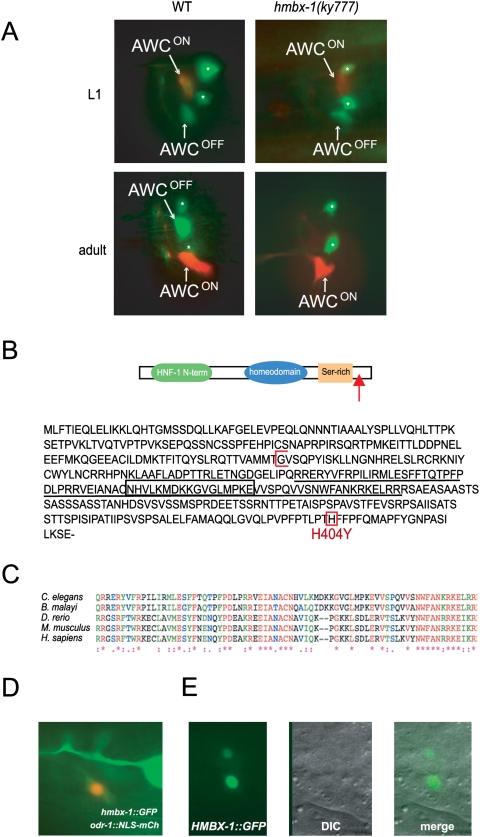
In the mutant ky777, srsx-3 expression in AWCOFF was lost after L1, but str-2 expression in AWCON was retained (Figs. 1, 3A). Although this phenotype resembled that of daf-7/TGFβ mutants, ky777 mutants were normal for dauer formation, and the srsx-3 expression defect in these mutants was not suppressed by daf-3 (Fig. 2C). Therefore, ky777 appeared to act separately from the TGFβ pathway. ky777 was mapped to an interval of ∼440 kb on the left arm of chromosome I, but no rescue was observed after injection of fosmids or PCR products covering the interval. Therefore, a Solexa-Illumina whole-genome sequencing approach was used to identify mutations in the interval. Unique alignments of ky777 sequence reads to the C. elegans genome accounted for 84% of sequence in the interval, with an average coverage depth of 7.9×. The gigaBayes program identified 19 high-probability point mutations and three single-base indels between ky777 and the reference genome. Two missense mutations and one silent mutation were present in coding exons, one mutation was in a 3′ untranslated region (UTR), and one mutation was in a 5′UTR. PCR and conventional sequencing determined that one of the coding mutations and both UTR mutations were present both in the mutant strain and in the original, unmutagenized strain, but that the remaining coding mutation was present in the ky777 mutant but not in the original strain. This mutation represents a C → T transition in the predicted gene F54A5.1 that results in a missense H → Y mutation at the C-terminal end of the protein (Fig. 3B, red box), and was considered the most likely candidate for ky777. Transgenic introduction of a wild-type genomic copy of F54A5.1 partially restored srsx-3 expression in ky777 (Fig. 4C; see below), supporting further analysis of this gene.
Figure 3.
ky777 is an allele of the homeodomain transcription factor hmbx-1. (A) Fluorescence images of early larval and adult wild-type and ky777 mutant animals showing late loss of srsx-3∷GFP from AWCOFF. AWC neurons are labeled, and asterisks mark AWB neurons. (B, top) Domain structure and amino acid sequence of HMBX-1. Red arrow indicates the location of the ky777 mutation in the domain structure diagram. (Bottom) The ky777 mutation is boxed in red, the beginning of the tm1274 deletion is indicated with a red bracket, the homeodomain is underlined, and a 17-amino-acid insertion in the homeodomain is boxed in black. (C) Conservation of the HMBX-1 homeodomain. (D) Expression of hmbx-1(promoter)∷GFP in AWC. AWC is marked by a nuclear-localized mCherry reporter under the control of the odr-1 promoter. Also visible are parts of the anterior process and cell body of the FLP neuron. (E) Nuclear localization of HMBX-1∷GFP fusion protein in AWC. GFP is restricted to nuclei, visualized by DIC microscopy.
Figure 4.
ky777 is an altered-function allele of hmbx-1. (A) srsx-3 and str-2 expression phenotypes of wild-type, hmbx-1(tm1274), and hmbx-1(ky777) homozygotes, and ky777/+ and ky777/tm1274 heterozygotes. n > 50 for all genotypes. (B) RNAi against hmbx-1 in wild-type and ky777 backgrounds. All strains contained the RNAi-sensitizing eri-1(mg366) and lin-15B(n744) mutations. (***) Different at P < 0.001 (Fisher's exact test). n > 40 for all conditions. (C) Moderate overexpression of hmbx-1 under endogenous regulatory elements. Expression of str-2 and srsx-3 in wild-type, ky777, or tm1274 mutant animals expressing a genomic fragment covering wild-type or ky777 mutant genomic coding sequence with 7 kb of upstream sequence. (***) Different from transgene-negative control at P < 0.001 (χ2 test). n > 30 for all conditions. (D) Overexpression of hmbx-1 from a strong AWC promoter. Expression of str-2 and srsx-3 in wild-type, hmbx-1(tm1274), and hmbx-1(ky777) animals overexpressing a wild-type HMBX-1 cDNA under the odr-3 promoter. (***) Different from transgene-negative control at P < 0.001 (χ2 test). n > 60 for all genotypes.
F54A5.1 encodes a predicted conserved homeodomain transcription factor that contains an HNF-1 N-terminal-like domain and a serine-rich region at its C terminus; it is closely related to the mammalian gene HMBOX1 (Fig. 3C). The homeodomain of F54A5.1 and its homologs includes an unusual 17-amino-acid insertion not present in other homeodomains (Fig. 3B, black box); the entire protein is highly conserved in multiple species, including zebrafish, mice, and humans (Fig. 3C). Because of this high degree of conservation with HMBOX1 genes, we named F54A5.1 hmbx-1.
A transgene in which GFP was expressed under the control of 7 kb of the hmbx-1 upstream region drove expression in both AWCs; in the chemosensory neurons ASI, AFD, ASH, and URX; in the mechanosensory neurons ALM, PLM, PVD, and FLP; in a few additional head and tail neurons; and in the seam cells of the hypodermis (Fig. 3D; data not shown). The expression of hmbx-1 in the AWCs supports the hypothesis that it regulates srsx-3 expression in these neurons. An HMBX-1 cDNA tagged with GFP localized to the nucleus of AWC, consistent with its predicted function as a transcription factor (Fig. 3E). A GFP-tagged HMBX-1(H404Y) protein corresponding to the ky777 missense mutant also localized to the nucleus.
ky777 is an altered-function allele of hmbx-1
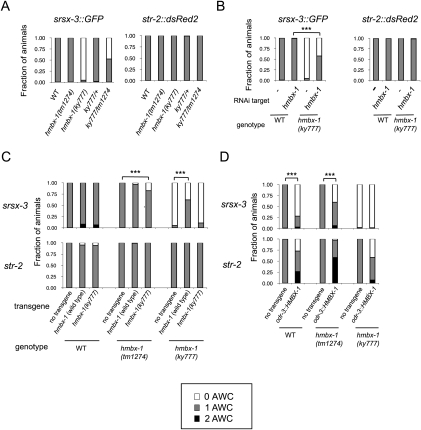
A deletion allele of hmbx-1, tm1274, was kindly provided by the National BioResource Project in Japan. The deletion eliminates the HMBX-1 homeodomain, and results in a frameshift and early stop codon, and is therefore likely to be a null allele. The hmbx-1(tm1274) mutant was healthy and fertile, and had mild defects in chemotaxis to odors sensed by AWC neurons (Supplemental Fig. S2). Surprisingly, srsx-3 expression was normal in the hmbx-1(tm1274) mutants (Fig. 4A). The different phenotypes of the hmbx-1(ky777) and hmbx-1(tm1274) mutants indicate that ky777 is not a null allele of hmbx-1.
A set of genetic experiments suggested that the ky777 allele results in altered, dosage-sensitive activity of the hmbx-1 gene. hmbx-1(ky777)/+ animals were largely normal, indicating that ky777 is recessive to the wild-type allele (Fig. 4A). hmbx-1(ky777)/hmbx-1(tm1274null) animals had an intermediate phenotype compared with either starting strain, a result suggesting that tm1274 eliminates the wild-type gene activity that suppresses ky777, and supporting the hypothesis that the two mutations affect the same gene (Fig. 4A). Reducing hmbx-1 expression using RNAi in wild-type animals had little effect on srsx-3 expression, but RNAi against hmbx-1 in hmbx-1(ky777) mutants restored srsx-3 expression to many animals (Fig. 4B). These results suggest that RNAi is reducing an altered hmbx-1 activity to generate an hmbx-1-null (wild-type-like) phenotype.
A moderate increase in hmbx-1 activity was attained by injecting wild-type hmbx-1 and hmbx-1(ky777) genomic DNAs into wild-type, hmbx-1(ky777), and hmbx-1(tm1274) animals. In a wild-type background, neither the wild-type nor the mutant hmbx-1 gene had a significant effect on srsx-3 expression, in agreement with the observation that hmbx-1(ky777) is a recessive allele (Fig. 4C). In a null background, expression of the hmbx-1(ky777) mutant DNA, but not wild-type hmbx-1, partly repressed srsx-3 expression, confirming that hmbx-1(ky777) represses srsx-3 under conditions in which the wild-type hmbx-1 gene does not. Finally, in a hmbx-1(ky777) mutant background, wild-type hmbx-1 partly restored srsx-3 expression, indicating that it antagonized hmbx-1(ky777) (Fig. 4C).
The results described above indicate that wild-type hmbx-1 antagonizes hmbx-1(ky777), and are consistent with two additional possibilities: (1) On its own, the hmbx-1(ky777) allele has a high or unregulated level of hmbx-1 activity, or (2) the hmbx-1(ky777) allele has an abnormal activity unrelated to normal hmbx-1 function. To distinguish between these alternatives, wild-type hmbx-1 cDNA was expressed at high levels in the AWC neurons using the odr-3 promoter, and the effects were examined in wild-type, hmbx-1(tm1274), and hmbx-1(ky777) animals. High-copy odr-3∷hmbx-1 transgenes repressed srsx-3 in wild-type and tm1274 backgrounds, like the recessive ky777 mutant (Fig. 4D). These results suggest that hmbx-1(ky777) mutants resemble animals with increased hmbx-1 repressor activity in AWC. However, a few complications suggest that the effects of hmbx-1 overexpression may not be entirely straightforward. First, str-2 expression was sometimes misregulated in odr-3∷hmbx-1 animals, but not in hmbx-1(ky777) animals (Fig. 4D). Second, expression of hmbx-1 under the osm-3 promoter, which drives expression in 26 chemosensory neurons but not in AWC (Tabish et al. 1995), resulted in ectopic expression of srsx-3 in AWCON in some animals (Supplemental Fig. S3). hmbx-1 may therefore have cell-nonautonomous as well as cell-autonomous effects on srsx-3.
Bearing these potential complications in mind, the genetic results suggest that hmbx-1(ky777) has unregulated or increased hmbx-1 activity that inappropriately represses srsx-3 activity in AWCOFF. A GFP-tagged HMBX-1(ky777) protein was expressed at similar levels to a tagged wild-type protein, suggesting that there were no major effects on protein stability. It is possible that the mutation affects an autoregulatory activity of hmbx-1, as expression of an hmbx-1∷GFP transcriptional reporter was reduced in the AWC neurons of hmbx-1(ky777) mutants (Supplemental Fig. S3).
Single-copy srsx-3∷GFP transgenes are regulated by hmbx-1 and its predicted binding site
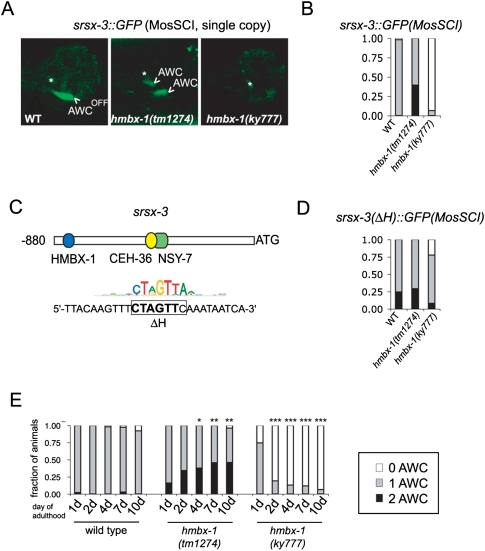
During the gene dosage studies, we were struck by the variation in the mutant phenotype, depending on whether the copy number of hmbx-1 was low (endogenous hmbx-1), intermediate (genomic hmbx-1 DNA injection), or high (odr-3∷hmbx-1 cDNA injection). In all of these experiments, str-2∷dsRed2 and srsx-3∷GFP reporter genes were present at high-copy number in the kyIs408 transgene, which could distort their interactions with a dosage-sensitive hmbx-1 transcription factor. Therefore, to provide a more natural context for examining gene regulation effects, we generated single-copy srsx-3∷GFP transgene reporters at a defined site on chromosome II using the Mos Single-Copy Insertion (MosSCI) technique (Frokjaer-Jensen et al. 2008).
In wild-type animals, the single-copy srsx-3∷GFP reporter recapitulated the expression pattern of a high-copy srsx-3∷GFP array, albeit with a weaker GFP signal (Fig. 5A). Young larvae expressed GFP in both AWC neurons, but expression was restricted to a single AWCOFF neuron in adults (Fig. 5A; data not shown). Expression of the single-copy srsx-3∷GFP transgene was lost in hmbx-1(ky777) mutants, and genetic interactions with hmbx-1(ky777) mutations were similar to those with a high-copy srsx-3∷GFP array (Fig. 5A,B; Supplemental Fig. S4). Remarkably, the single-copy srsx-3∷GFP reporter uncovered an opposite phenotype for the hmbx-1(tm1274)-null allele: The srsx-3∷GFP reporter was misexpressed in both AWCs in a fraction of hmbx-1(tm1274) adults (Fig. 5A,B). Thus, in an hmbx-1-null mutant, srsx-3 is derepressed in AWCON, whereas, in the hmbx-1(ky777) mutant, srsx-3 is inappropriately repressed in AWCOFF. These straightforward results with single-copy transgenes support and extend the conclusions from high-copy arrays. They suggest that hmbx-1 represses srsx-3 expression in AWCON neurons in the adult stage, that its effect is partly redundant with other repressors, as it is only partially penetrant, and that hmbx-1(ky777) is a recessive gain-of-function allele of hmbx-1 that inappropriately represses srsx-3 in AWCOFF neurons.
Figure 5.
Regulation of single-copy srsx-3∷GFP lines by hmbx-1. (A) Confocal images of the single-copy srsx-3∷GFP reporter in wild-type, hmbx-1(tm1274), and hmbx-1(ky777) animals. (Arrowheads) AWC; (asterisks) AWB. (B) Expression of singly integrated srsx-3∷GFP in wild-type and mutant adults. n > 40 for all conditions. (C, top) A diagram of the srsx-3 promoter with the positions of predicted transcription factor-binding sites. (Bottom) Binding site for HMBOX1, the mouse homolog of HMBX-1, shown above the sequence in the srsx-3 promoter that was deleted in the srsx-3(ΔH)∷GFP reporter. (D) Phenotypes of wild-type, hmbx-1(tm1274), and hmbx-1(ky777) animals expressing the singly integrated srsx-3(ΔH)∷GFP reporter. n > 40 for all genotypes. (E) Expression of singly integrated srsx-3∷GFP in young (1-d-old; 12 h after the L4 stage) and older (2-, 4-, 7-, or 10-d-old) adults in wild-type and mutant backgrounds. For hmbx-1(tm1274), one asterisk (*) indicates difference from young (1-d-old) adults at P < 0.05 and two asteriaks (**) indicate difference from young adults at P < 0.01 (Fisher's exact test). For hmbx-1(ky777), three asterisks (***) indicate difference from young adults at P < 0.001.
The binding site of the mouse homolog of HMBX-1, HMBOX1, has been identified using an in vitro binding assay (Berger et al. 2008). A similar site is present in the srsx-3 promoter, suggesting a potential site for regulation by HMBX-1 (Fig. 5C). The significance of this site was examined by deleting it from the srsx-3 promoter [srsx-3(ΔH)] and introducing a single-copy insertion of the srsx-3(ΔH)∷GFP sequence into the same MosSCI site used for the wild-type srsx-3∷GFP reporter. Unlike the wild-type reporter, the mutated reporter was expressed in both AWCs in a fraction of wild-type animals, suggesting that the sequence normally represses srsx-3 in AWCON (Fig. 5D). The expression of single-copy srsx-3 reporters with and without the predicted HMBX-1-binding site was then compared in wild-type, hmbx-1(tm1274), and hmbx-1(ky777) backgrounds. The single-copy srsx-3 reporter lacking the binding site behaved identically in wild-type and hmbx-1(tm1274) mutants (Fig. 5D), as predicted if HMBX-1 regulates srsx-3 expression by binding to this site. Deletion of the predicted binding site partly suppressed the effects of the hmbx-1(ky777) mutation, suggesting that the altered-function protein also interacts with this site (Fig. 5D). Thus, the use of single-copy srsx-3∷GFP reporters identified a loss-of-function phenotype for hmbx-1, and a likely site for HMBX-1 binding and regulation in vivo.
In agreement with the idea that hmbx-1 is involved in long-term maintenance of AWC gene expression, the effects of both hmbx-1(tm1274) and hmbx-1(ky777) mutants were greater in older adults than in young adults (Fig. 5E). For example, only 16% of young hmbx-1(tm1274) adults (12 h after the L4 stage) expressed srsx-3∷GFP in both AWC neurons, but this fraction reached ∼45% by 1 wk of adulthood.
Multiple repressors interact to regulate asymmetric AWC-specific genes
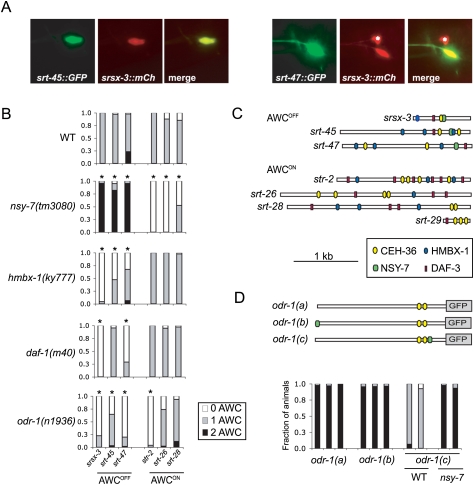
Because str-2 is the only known AWCON-specific gene and srsx-3 is the only known AWCOFF-specific gene, it was not clear whether transcription factors such as hmbx-1, nsy-7, and daf-3 regulate individual receptor genes or the entire asymmetric AWC identity. Therefore, we sought and characterized additional markers that distinguished the AWCON and AWCOFF neurons. The C. elegans gene expression project at the University of British Columbia has reported expression of several dozen predicted chemoreceptor genes in head sensory neurons (Dupuy et al. 2007); by examining 20 of these strains, we found that the srt-26 and srt-28 reporter genes were expressed strongly in a single AWC neuron, and the srt-29 gene was expressed weakly in a single AWC. These three genes were coexpressed with str-2 but not srsx-3, indicating that their expression was specific to AWCON.
AWCOFF-specific reporters were sought using the binding site for the transcription factor NSY-7, which was defined previously by direct DNA-binding experiments (Lesch et al. 2009). Fourteen predicted chemoreceptor genes in the C. elegans genome contain the CCTTAAC NSY-7-binding sequence within 300 base pairs (bp) of the coding start site. Fluorescent reporters for these 14 genes were generated by fusing 2 kb upstream of the start site to GFP; two of these 14 genes, srt-45 and srt-47, were expressed strongly in a single AWC neuron and weakly in an additional pair of head neurons. In both cases, expression was present in AWCOFF but not AWCON, based on coexpression with the srsx-3∷mCherry reporter (Fig. 6A). It is interesting that five genes of the srt family are expressed in AWC neurons, but four other tested srt genes were expressed in different neurons, so it is not a universal pattern (data not shown).
Figure 6.
The NSY-7-binding site predicts AWCOFF-specific expression, and is sufficient for repression in AWCON. (A) srt-45 and srt-47 are coexpressed with the AWCOFF reporter srsx-3∷mCherry, which also labels AWB (asterisks). (B) Expression of the AWCOFF markers (srsx-3, srt-45, and srt-47) and the AWCON markers (str-2, srt-26, and srt-28) in wild-type and mutant backgrounds. n > 30 for all conditions; (*) difference from wild-type at P < 0.01 (Fisher's exact test). (C) Schematic of AWCON and AWCOFF promoters showing predicted binding sites for the transcription factors CEH-36, HMBX-1, NSY-7, and DAF-3. (D, top) Schematic of odr-1∷GFP constructs inserted as single copies. [odr-1(a)] Wild-type 2.4-kb odr-1 promoter fragment; [odr-1(b)] NSY-7-binding site added to the 5′ end of the odr-1 promoter; [odr-1(c)] NSY-7-binding site added 200 bp upstream of the ATG in the odr-1 promoter. For odr-1(c), the NSY-7-binding site is inserted close to, but does not disrupt, two CEH-36-binding sites involved in driving odr-1 expression (Kim et al. 2010). (Bottom) Expression of integrated single-copy odr-1 plasmids in wild-type and nsy-7(tm3080) backgrounds. Multiple bars within the same condition represent independently integrated lines. n > 25 for all genotypes.
The newly identified AWCOFF markers srt-45 and srt-47 and the AWCON markers srt-26 and srt-28 were examined in mutants that affect srsx-3 and str-2 expression. In nsy-7 mutants, all three AWCOFF markers were expressed in both AWCs, and all three AWCON markers were reduced or absent (Fig. 6B). As predicted by earlier studies, this result suggests that, in nsy-7 mutants, AWCON is transformed into AWCOFF.
hmbx-1(ky777) regulated AWCOFF chemoreceptor expression in a cell-wide manner, reducing expression of all three AWCOFF-specific markers, but sparing the three AWCON markers (Fig. 6B). The symmetric AWC signaling genes odr-3 and odr-1 were expressed normally in AWCOFF neurons in hmbx-1(ky777) mutants, and AWCOFF morphology appeared normal, indicating that the AWCOFF neurons lost their asymmetric identity, but did not die or degenerate (Supplemental Fig. S5; data not shown).
Both daf-1 TGFβ mutants and odr-1 cGMP olfactory transduction mutants showed a distinct, gene-specific pattern of regulation of AWCOFF and AWCON chemoreceptors. In daf-1 mutants, expression of the AWCOFF markers srsx-3 and srt-47 was reduced, but the AWCOFF marker srt-45 and all AWCON markers were expressed at wild-type levels (Fig. 6B). odr-1 mutants were defective for expression of all three AWCOFF markers and the AWCON marker str-2, but expression of the AWCON markers srt-26 and srt-28 was largely intact (Fig. 6B). Thus, the TGFβ and cGMP-dependent pathways regulate subsets of chemoreceptor genes in both AWCOFF and AWCON neurons.
The relationships between the different transcriptional pathways were probed by examining double mutants, using the multicopy str-2 and srsx-3 reporters and the srsx-3 single-copy reporter. In general, double mutants recapitulated single mutant phenotypes in simple patterns, suggesting that hmbx-1, nsy-7, and daf-3 act independently of each other (Supplemental Fig. S6). odr-1 defects were partly suppressed by hmbx-1(tm1274), suggesting that these two pathways are additive and independent; hmbx-1(ky777) and nsy-7 effects were also additive.
The seven asymmetric AWC promoters had many potential binding sites for AWC-regulating proteins (Fig. 6C). All seven promoter fragments had predicted binding sites for CEH-36, the AWC terminal selector gene, and potential binding sites for DAF-3; six of seven promoters had matches to the consensus binding site for HMBX-1. NSY-7 sites were only present in the three AWCOFF promoters. In previous studies, analysis of the srsx-3 promoter suggested that NSY-7 binding was necessary for asymmetric expression (Lesch et al. 2009). The identification of additional transcriptional repressors raised the question of whether NSY-7 binding was also sufficient for asymmetric expression. To address this question, a single copy of the CCTTAAC sequence was inserted into an odr-1∷GFP reporter, which is ordinarily expressed in both AWC neurons (and also in AWB neurons). Single-copy odr-1∷GFP reporters with and without CCTTAAC sequences were inserted at a defined site on chromosome II using the MosSCI method. The wild-type single-copy odr-1∷GFP fusion was expressed in both AWC neurons, but an odr-1 plasmid with a CCTTAAC site 200 bp upstream of the ATG was expressed asymmetrically in a single AWC neuron (Fig. 6D). Coexpression of this transgene with srsx-3 but not str-2 reporters indicated that the CCTTAAC site repressed expression in AWCON. When crossed into a nsy-7 mutant, the modified transgene was again expressed in both AWC neurons (Fig. 6D). A more distal insertion of the NSY-7 site was expressed bilaterally. These results support the hypothesis that NSY-7 is a transcriptional repressor, and demonstrate that a single promoter-proximal NSY-7-binding site is sufficient to repress gene expression in AWCON.
Discussion
Multiple environmental and cell-intrinsic influences converge at the transcriptional level to regulate chemoreceptors in AWC neurons. Together with the symmetric AWC terminal selector gene ceh-36 (Kim et al. 2010), at least four different systems for transcriptional regulation contribute to adult AWCOFF-specific expression of the chemoreceptor gene srsx-3. First, the previously identified transcriptional repressor NSY-7, which is expressed preferentially in AWCON, can repress srsx-3 expression by direct binding to a consensus site. Second, cGMP signaling promotes srsx-3 expression via a cGMP-dependent protein kinase, a cell-autonomous cGMP-gated channel, and unknown transcriptional regulators (Lesch et al. 2009). Third, density-dependent dauer pheromones repress expression of the secreted TGFβ homolog DAF-7, which otherwise acts continuously to maintain srsx-3 expression. The TGFβ pathway regulates multiple chemoreceptor genes; in agreement with this observation, binding sites for SMAD transcription factors such as DAF-3 are among the most common sequences found upstream of chemoreceptor start sites (McCarroll et al. 2005). Finally, the conserved transcription factor hmbx-1 represses srsx-3 expression preferentially in adult AWCON neurons, although it is expressed in both AWC neurons and can act in AWCOFF when bearing the ky777 point mutation or when overexpressed.
Genetic analysis of hmbx-1 suggests that it is involved in long-term maintenance of a specific cell identity, not the developmental establishment of that identity or the regulation of specific genes. The original hmbx-1(ky777) mutation is a recessive gain-of-function allele with effects that resemble those of hmbx-1 overexpression. Because of its nature and its dosage sensitivity, it was identified only by whole-genome sequencing. Although recessive gain-of-function alleles are relatively rare, they have been described in genes encoding potassium channels and their regulators, tyrosine kinase receptors, and the ETS domain transcription factor LIN-1 (Klingler et al. 1988; Jacobs et al. 1998; Perez de la Cruz et al. 2003). LIN-1-recessive gain-of-function mutations disrupt a negative regulatory domain; it is possible that ky777 does the same to HMBX-1.
The analysis of a single-copy insertion of the srsx-3∷GFP reporter uncovered a phenotype for the hmbx-1-null mutant that was not evident with a multicopy reporter, demonstrating that hmbx-1 normally represses AWCOFF chemoreceptor genes in AWCON neurons. Single-copy reporter genes also identified a predicted HMBX-1-binding site required for repression of srsx-3 by hmbx-1. Multicopy integrated and extrachromosomal arrays are commonly and successfully used to analyze gene expression in C. elegans. However, high-copy transgenes are subject to repeat-induced silencing in the germline and, to a lesser extent, somatic tissues (Hsieh and Fire 2000), and this leads to altered genetic requirements for their expression. For example, specific genes including tam-1, a gene isolated in our screen, are required for efficient expression of high-copy transgenes, but not of the corresponding endogenous genes (Hsieh et al. 1999). Moreover, high-copy transgenes have a high propensity to form heterochromatin, which is not observed with low-copy transgenes of the same sequence (Meister et al. 2010). We suggest that the heterochromatic state of the high-copy transgene may bypass the normal requirement for hmbx-1 and perhaps other repressors that maintain gene silencing. The ability to introduce single-copy transgenes into defined genomic locations by MosSCI represents a significant advance for controlling copy number and genomic context effects on gene expression (Frokjaer-Jensen et al. 2008).
hmbx-1 has effects that are temporally distinct from those of nsy-7, a transcription factor that acts in AWCON beginning in the L1 larval stage. The relative importance of wild-type hmbx-1 is greater in older compared with younger adults, and, likewise, the elevated repressive activity of the hmbx-1(ky777) mutant increases in adults over time. It may be that NSY-7 loses activity in older animals, or that other changes in gene expression alter the relative importance of the two transcription factors as the animal ages.
The results described here point to a significant role for single transcription factor-binding sites in chemoreceptor expression. The presence of a single binding site for NSY-7 is sufficient to repress expression of an odr-1 promoter ordinarily expressed in both AWC neurons. Likewise, deletion or mutation of the HMBX-1-binding site in the srsx-3 promoter permitted misexpression of srsx-3 in AWCON. The relative ease with which sensory receptor expression can be altered suggests genetic malleability and potentially evolutionary flexibility in the specificity of GPCR expression in chemosensory neurons (Jovelin 2009). Particularly in C. elegans, where ectopic expression of a gene in a single additional neuron can dramatically change behaviors, altered expression due to new mutations might have significant consequences for the animal's behavior and fitness (Troemel et al. 1997).
Despite the potent effects of single binding sites, many additional candidate binding sites were identified in each asymmetric AWC gene. Functional studies of asymmetrically expressed promoters in ASE neurons have uncovered multiple repressor and activator elements in each promoter (Etchberger et al. 2009). In ASE, che-1-binding sites with different affinities contribute to left–right asymmetry of gene expression as well as general ASE identity (Etchberger et al. 2009); it may be informative that the AWCON-specific promoters contain more predicted CEH-36-binding sites than the AWCOFF-specific promoters.
hmbx-1 is expressed in C. elegans sensory neurons, and its human and mouse homologs also display high levels of nervous system expression (Chen et al. 2006). The human homolog exhibits repressor activity in vitro, and associates with telomeric sequences in several human cell lines (Chen et al. 2006; Dejardin and Kingston 2009). Telomeres are zones of strong transcriptional repression, suggesting a possible repressor function for HMBOX-1 at these locations (Gottschling et al. 1990). The sequence conservation of HMBOX-1 genes between worm and mammalian homologs, their conserved repressor activity, the apparently conserved binding site specificity, and their neuronal expression pattern all hint at possible conserved neuronal functions. With this in mind, it will be interesting to determine the identities of hmbx-1 target genes, particularly those that are important in older adult animals, and to explore the association of human HMBOX1 with telomeric repeats during normal growth and aging.
Materials and methods
Genetics and strains
C. elegans strains were cultured using standard methods (Brenner 1974). All strains were grown at 20°C unless otherwise specified. Mutants were isolated by direct inspection of GFP and dsRed fluorescence following mutagenesis of the strain CX7894 kyIs408 [srsx-3∷GFP;str-2∷dsRed2;elt-2∷GFP] II (Lesch et al. 2009). Mutagenesis was performed with ethyl methane sulfonate (EMS) using standard protocols (Brenner 1974).
Among the new mutations, tax-4 and tax-2 alleles differed in phenotype from tax-4 and tax-2 mutants characterized previously using the kyIs140 [str-2∷GFP] I transgene (Troemel et al. 1999; Lans and Jansen 2006). Whereas expression of str-2∷GFP from kyIs140 was lost in most tax-2 and tax-4 mutants, the effect of tax-2 and tax-4 on str-2∷DsRed from kyIs408 was milder. Analysis of the canonical tax-4(p678)-null mutant indicated that the difference was due to the transgene and not to the nature of the mutant alleles (Fig. 1B). kyIs140 fluorescence is much dimmer than that of kyIs408, so it may be more sensitive to small reductions in expression level.
A strain list appears as Supplemental Table S1.
Molecular biology
Standard molecular biology techniques were used. The gfp-pest reporter was made by overlap extension PCR between the GFP template from the pSM-GFP expression vector and the PEST domain template from a commercially available destabilized GFP vector (d1EGFP-N1, Clontech). The product was then cloned into MCS2 of pSM with the srsx-3 promoter in MCS1 to create the srsx-3∷gfp-pest vector.
A PCR fragment containing the hmbx-1 genomic coding region and 7 kb of upstream sequence was amplified from N2 lysate using the primers 5′-CATATCACATCCTCCTGTTTTCAAG-3′ and 5′-CCAACAAAATTTCAGGGCAAGC-3′. HMBX-1 cDNAs were obtained by PCR from a C. elegans poly(A) cDNA library using primers at the beginning and end of the predicted ORF (5′-ATGCTATTCACAATTGAGCAACTG-3′ and 5′-TCACTCAGACTTCAAAATAGAAG-3′), and were cloned into MCS2 of a modified pSM expression vector (containing a NotI site in MCS2 instead of MCS1) using the restriction sites SalI and NotI.
The hmbx-1(7kb)∷GFP, srt-45∷GFP, and srt-47∷GFP reporters were created by PCR fusion of the promoter, amplified from N2 lysate, with GFP template amplified from pPD95.75. Reactions were carried out as described in Hobert (2002) with the following primers: hmbx-1(7kb): A, 5′-TGCCGATTGCCAAAAACTTCC-3′, and A*, 5′-CATATCACATCCTCCTGTTTTCAAG-3′, and B, 5′-AGTCGACCTGCAGGCATGCAAGCTGTGTAAAATAAAATTTACTACGAGGTT-3′; srt-45: A, 5′-CTTTTAAGTATTGTCACTCCGGC-3′, and A*, 5′-TTGGACATGTAATCATGCATG-3′, and B, 5′-AGTCGACCTGCAGGCATGCAAGCTTGCGGAATCTGAAGTTTTTCG-3′; srt-47: A, 5′-GCGTAAAACACAGCAGAAAA-3′, and A*, 5′-ATCTTTAAAGGTGCATTTTATTTGG-3′, and B, 5′-AGTCGACCTGCAGGCATGCAAGCTTTGGTGATAAAGAGAGGTTATAG-3′.
To create the srt-26∷GFP, srt-28∷GFP, and srt-29∷GFP reporters, the srt-26, srt-28, and srt-29 promoter sequences were amplified by PCR and cloned into MCS1 of the pSM-GFP expression vector using the FseI and AscI restriction sites.
For MosSCI experiments, NSY-7-binding sites were added to the odr-1∷GFP expression vector, and the srsx-3∷GFP plasmid was modified to generate the srsx-3(ΔH)∷GFP reporter by site-directed mutagenesis using the the QuikChange II Site-Directed Mutagenesis Kit (Stratagene). odr-1∷GFP or srsx-3∷GFP sequences were cut out of pSM using the FseI and SpeI restriction sites, and were cloned into a pCFJ151 MosSCI insertion vector (Frokjaer-Jensen et al. 2008) that had been modified to include a FseI site in the MCS.
Whole-genome sequencing
Whole-genome sequencing of the ky777 mutant was performed by the Rockefeller Genomics Resource Center using Solexa-Illumina Genome Analyzer technology. SNP mapping was used to determine a genetic interval of ∼440 kb for the mutation. Purified genomic DNA was prepared from 1000–2000 worms, and was used for library preparation. fastq sequences were aligned to the ce6 genome assembly at the University of California at Santa Cruz using the Mosaik alignment program (http://bioinformatics.bc.edu/marthlab/Mosaik#Current_Documentation), and single-base changes, insertions, or deletions were predicted using GigaBayes polymorphism detection software (http://bioinformatics.bc.edu/marthlab/GigaBayes). High-probability single-base changes that were predicted to fall within an exon, a 3′UTR, or a 5′UTR were checked by PCR and conventional (Sanger) sequencing.
Sequence alignment
The hmbx-1 homeodomain sequence was aligned with HMBOX1 homeodomain sequences using ClustalW at Pôle BioInformatique Lyonnais (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_clustalw.html). UniProt ID numbers are as follows: for C. elegans, Q9TYT0; for Brugia malayi, A8QCW9; for Danio rerio, Q4V904; for Mus musculus, Q8BJA3; for Homo sapiens, A8K3R8.
RNAi
RNAi was performed by injection of dsRNA. All assays were performed in an eri-1(mg366);lin-15B(n744)-sensitized background (Sieburth et al. 2005). For injection, a dsDNA template corresponding to an exon of the target ORF was amplified from N2 lysate with a T7 sequence (TAATACGACTCACTATAGGGAGA) added at the 5′ ends. The following gene-specific sequences were used for these primers: nsy-7 (exon 2), 5′-GTTGCGAAAGGATATTCAGATG-3′ and 5′-CTTAGCAAACAAGTTGGTGAGT-3′; hmbx-1 (exon 3), 5′-ACAAGCTCCCGTAACACAAC-3′ and 5′-TCACTCAGACTTCAAAATAGAAGCC-3′.
Transcription was performed using the T7 RiboMAX Express RNAi System (Promega) according to instructions, and the unpurified reaction mix was injected into the body cavity, gut, or gonad of adult hermaphrodites. F1 progeny from eggs laid at least 24 h after the injection were scored after 3–4 d for str-2 and srsx-3 expression. Control experiments established the normal RNAi response of hmbx-1(ky777) animals: RNAi against nsy-7 in ky777 mutants resulted in a 100% loss of str-2 expression (44 out of 44 RNAi+ vs. zero out of 90 RNAi− animals; P < 0.0001 [Fisher's exact test]), consistent with the phenotype of hmbx-1(ky777);nsy-7(tm3080) double mutants.
MosSCI integrations
Mos single-copy integrants were generated using the direct insertion protocol described in Frokjaer-Jensen et al. (2008). Thirty to 50 EG4322 ttTi5605; unc-119(ed3) worms were injected with rab-3∷mCherry, myo-2∷mCherry, myo-3∷mCherry, pJL43.1 (a vector containing the Mos1 transposase under the control of the germline promoter glh-2), and a vector containing the specific promoter∷GFP sequence to be inserted flanked by sequences homologous to the insertion site. Animals that were rescued for the unc-119 phenotype (array-positive) were allowed to starve out twice, and then unc-119 rescued animals that lacked the three mCherry coinjection markers (integrant-positive, array-negative) were cloned out from separate plates to found independent integrated lines. These lines were outcrossed twice to wild-type animals, and the presence of the intact insertion was verified by PCR and sequencing.
Microscopy
For all microscopy, live animals were immobilized on an agarose pad containing 5 mM NaN3.
Fluorescence microscopy was carried out on a Zeiss Axioplan2 imaging system with a Hamamatsu Photonics C2400 CCD camera, or a Zeiss Axio Imager.Z1 with ApoTome with a Zeiss AxioCam MRm CCD camera. Most animals were scored under a 20× or 40× Plan-Neofluar objective, where “bright” and “faint” fluorescence were scored qualitatively, and photographs were taken under a 40× Plan-Neofluar or 63× Plan-Apochromat objective. Confocal microscopy (Fig. 5A) was done under a 40×/1.2 W C-Apochromat water immersion objective on a Zeiss LSM 510 confocal imaging system using the Zeiss LSM 510 version 3.2 confocal software.
Developmental timing
To evaluate marker expression in the L1 larval stage, larvae were staged by hatch-off. Late embryos were picked to an NGM plate seeded with the Escherichia coli strain OP50. After 30 min, just-hatched L1s were transferred to a fresh plate and grown for 14 h at 20°C (for L1s) or ∼70 h at 20°C (for adults). To compare young and old adults, 25 L4 animals were picked per plate and grown for 12 h (1 d) at 20°C, 36 h (2 d) at 20°C, 84 h (4 d) at 20°C, 156 h (7 d) at 20°C, or 228 h (10 d) at 20°C before scoring. Animals were transferred to new plates every 24 h to prevent crowding and starvation.
Pheromone assays
Ascarosides C3, C6, and C9 (generously provided by R. Butcher and J. Clardy, Harvard Medical School) were added to liquid agar at the concentrations indicated. For negative controls, the same volume of solvent (ethanol) was added to the agar. Ten milliliters of agar was poured into 6-cm culture dishes and allowed to cool. Plates were then seeded with 100 μL of OP50 bacteria and dried in a hood for 1.5–2 h. These plates were either used immediately or stored overnight at 4°C. Twenty srsx-3∷gfp-pest array-positive young adults (older than L4, with no eggs yet visible in the gonad) were picked to each plate and incubated for 4 h at 25°C. Animals were scored for presence or absence of GFP in AWC neurons under a 40× objective.
Acknowledgments
We thank Shohei Mitani for the hmbx-1(tm1274) strain, Rebecca Butcher and Jon Clardy for ascaroside pheromones, and Mike Chiorazzi and Diana McKeage for contributing to the mutant characterization. This work was supported by NIDCD grant DC004089. B.J.L. was supported by MSTP grant GM07739, and by an individual NRSA (F30MH084482). C.I.B is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1932610.
Supplemental material is available at http://www.genesdev.org.
References
- Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, Pena-Castillo L, Alleyne TM, Mnaimneh S, Botvinnik OB, Chan ET, et al. 2008. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133: 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R 1991. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65: 175–187 [DOI] [PubMed] [Google Scholar]
- Busturia A, Morata G 1988. Ectopic expression of homeotic genes caused by the elimination of the Polycomb gene in Drosophila imaginal epidermis. Development 104: 713–720 [DOI] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J 2007. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol 3: 420–422 [DOI] [PubMed] [Google Scholar]
- Butcher RA, Ragains JR, Kim E, Clardy J 2008. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci 105: 14288–14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI 2007. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450: 63–70 [DOI] [PubMed] [Google Scholar]
- Chang S, Johnston RJ Jr, Hobert O 2003. A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev 17: 2123–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Johnston RJ Jr, Frokjaer-Jensen C, Lockery S, Hobert O 2004. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature 430: 785–789 [DOI] [PubMed] [Google Scholar]
- Chen S, Saiyin H, Zeng X, Xi J, Liu X, Li X, Yu L 2006. Isolation and functional analysis of human HMBOX1, a homeobox containing protein with transcriptional repressor activity. Cytogenet Genome Res 114: 131–136 [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR 2000. Candidate taste receptors in Drosophila. Science 287: 1830–1834 [DOI] [PubMed] [Google Scholar]
- Coburn CM, Mori I, Ohshima Y, Bargmann CI 1998. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult C. elegans: A distinct pathway for maintenance of sensory axon structure. Development 125: 249–258 [DOI] [PubMed] [Google Scholar]
- Colosimo ME, Tran S, Sengupta P 2003. The divergent orphan nuclear receptor ODR-7 regulates olfactory neuron gene expression via multiple mechanisms in Caenorhabditis elegans. Genetics 165: 1779–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Graca LS, Zimmerman KK, Mitchell MC, Kozhan-Gorodetska M, Sekiewicz K, Morales Y, Patterson GI 2004. DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF β pathway to regulate C. elegans dauer development. Development 131: 435–446 [DOI] [PubMed] [Google Scholar]
- Dejardin J, Kingston RE 2009. Purification of proteins associated with specific genomic loci. Cell 136: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al. 2007. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol 25: 663–668 [DOI] [PubMed] [Google Scholar]
- Etchberger JF, Lorch A, Sleumer MC, Zapf R, Jones SJ, Marra MA, Holt RA, Moerman DG, Hobert O 2007. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev 21: 1653–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchberger JF, Flowers EB, Poole RJ, Bashllari E, Hobert O 2009. Cis-regulatory mechanisms of left/right asymmetric neuron-subtype specification in C. elegans. Development 136: 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Russel S, Ruvkun G 2005. Functional genomic analysis of C. elegans molting. PLoS Biol 3: e312 doi: 10.1371/journal.pbio.0030312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40: 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet J, Mango SE 2002. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295: 821–825 [DOI] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL 1990. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell 61: 635–645 [DOI] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA 1990. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell 63: 751–762 [DOI] [PubMed] [Google Scholar]
- Hobert O 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730 [DOI] [PubMed] [Google Scholar]
- Hobert O 2008. Regulatory logic of neuronal diversity: Terminal selector genes and selector motifs. Proc Natl Acad Sci 105: 20067–20071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Fire A 2000. Recognition and silencing of repeated DNA. Annu Rev Genet 34: 187–204 [DOI] [PubMed] [Google Scholar]
- Hsieh J, Liu J, Kostas SA, Chang C, Sternberg PW, Fire A 1999. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes Dev 13: 2958–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Beitel GJ, Clark SG, Horvitz HR, Kornfeld K 1998. Gain-of-function mutations in the Caenorhabditis elegans lin-1 ETS gene identify a C-terminal regulatory domain phosphorylated by ERK MAP kinase. Genetics 149: 1809–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK 2005. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433: 541–545 [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Hobert O 2003. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426: 845–849 [DOI] [PubMed] [Google Scholar]
- Johnston RJ Jr, Chang S, Etchberger JF, Ortiz CO, Hobert O 2005. MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc Natl Acad Sci 102: 12449–12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovelin R 2009. Rapid sequence evolution of transcription factors controlling neuron differentiation in Caenorhabditis. Mol Biol Evol 26: 2373–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Colosimo ME, Yeung H, Sengupta P 2005. The UNC-3 Olf/EBF protein represses alternate neuronal programs to specify chemosensory neuron identity. Dev Biol 286: 136–148 [DOI] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P 2009. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 326: 994–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim R, Sengupta P 2010. The HMX/NKX homeodomain protein MLS-2 specifies the identity of the AWC sensory neuron type via regulation of the ceh-36 Otx gene in C. elegans. Development 137: 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler M, Erdelyi M, Szabad J, Nusslein-Volhard C 1988. Function of torso in determining the terminal anlagen of the Drosophila embryo. Nature 335: 275–277 [DOI] [PubMed] [Google Scholar]
- Lanjuin A, Sengupta P 2002. Regulation of chemosensory receptor expression and sensory signaling by the KIN-29 Ser/Thr kinase. Neuron 33: 369–381 [DOI] [PubMed] [Google Scholar]
- Lanjuin A, VanHoven MK, Bargmann CI, Thompson JK, Sengupta P 2003. Otx/otd homeobox genes specify distinct sensory neuron identities in C. elegans. Dev Cell 5: 621–633 [DOI] [PubMed] [Google Scholar]
- Lans H, Jansen G 2006. Noncell- and cell-autonomous G-protein-signaling converges with Ca2+/mitogen-activated protein kinase signaling to regulate str-2 receptor gene expression in Caenorhabditis elegans. Genetics 173: 1287–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch BJ, Gehrke AR, Bulyk ML, Bargmann CI 2009. Transcriptional regulation and stabilization of left-right neuronal identity in C. elegans. Genes Dev 23: 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Li H, Bargmann CI 2005. Identification of transcriptional regulatory elements in chemosensory receptor genes by probabilistic segmentation. Curr Biol 15: 347–352 [DOI] [PubMed] [Google Scholar]
- Meister P, Towbin BD, Pike BL, Ponti A, Gasser SM 2010. The spatial dynamics of tissue-specific promoters during C. elegans development. Genes Dev 24: 766–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola I, Heavey B, Horcher M, Busslinger M 2002. Reversion of B cell commitment upon loss of Pax5 expression. Science 297: 110–113 [DOI] [PubMed] [Google Scholar]
- Muller J, Kassis JA 2006. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr Opin Genet Dev 16: 476–484 [DOI] [PubMed] [Google Scholar]
- Nokes EB, Van Der Linden AM, Winslow C, Mukhopadhyay S, Ma K, Sengupta P 2009. Cis-regulatory mechanisms of gene expression in an olfactory neuron type in Caenorhabditis elegans. Dev Dyn 238: 3080–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KM, Sarafi-Reinach TR, Horne JG, Saffer AM, Sengupta P 2002. The DAF-7 TGF-β signaling pathway regulates chemosensory receptor gene expression in C. elegans. Genes Dev 16: 3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Heavey B, Rolink AG, Busslinger M 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401: 556–562 [DOI] [PubMed] [Google Scholar]
- Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G 1997. The DAF-3 Smad protein antagonizes TGF-β-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev 11: 2679–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckol EL, Troemel ER, Bargmann CI 2001. Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans. Proc Natl Acad Sci 98: 11032–11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de la Cruz I, Levin JZ, Cummins C, Anderson P, Horvitz HR 2003. sup-9, sup-10, and unc-93 may encode components of a two-pore K+ channel that coordinates muscle contraction in Caenorhabditis elegans. J Neurosci 23: 9133–9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BC, Ye B, Zackhary R, Schrader K, Seydoux G, Reed RR 1998. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development 125: 1561–1568 [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL 1996. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science 274: 1389–1391 [DOI] [PubMed] [Google Scholar]
- Rens-Domiano S, Hamm HE 1995. Structural and functional relationships of heterotrimeric G-proteins. FASEB J 9: 1059–1066 [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R 2004. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet 38: 413–443 [DOI] [PubMed] [Google Scholar]
- Sagasti A, Hobert O, Troemel ER, Ruvkun G, Bargmann CI 1999. Alternative olfactory neuron fates are specified by the LIM homeobox gene lim-4. Genes Dev 13: 1794–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafi-Reinach TR, Sengupta P 2000. The forkhead domain gene unc-130 generates chemosensory neuron diversity in C. elegans. Genes Dev 14: 2472–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafi-Reinach TR, Melkman T, Hobert O, Sengupta P 2001. The lin-11 LIM homeobox gene specifies olfactory and chemosensory neuron fates in C. elegans. Development 128: 3269–3281 [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH 1996. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17: 719–728 [DOI] [PubMed] [Google Scholar]
- Sengupta P, Colbert HA, Bargmann CI 1994. The C. elegans gene odr-7 encodes an olfactory-specific member of the nuclear receptor superfamily. Cell 79: 971–980 [DOI] [PubMed] [Google Scholar]
- Shibata Y, Takeshita H, Sasakawa N, Sawa H 2010. Double bromodomain protein BET-1 and MYST HATs establish and maintain stable cell fates in C. elegans. Development 137: 1045–1053 [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL 2002. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci 25: 251–281 [DOI] [PubMed] [Google Scholar]
- Sieburth D, Ch'ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, Dupuy D, Rual JF, Hill DE, Vidal M, et al. 2005. Systematic analysis of genes required for synapse structure and function. Nature 436: 510–517 [DOI] [PubMed] [Google Scholar]
- Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS 1995. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J Mol Biol 247: 377–389 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI 1997. Reprogramming chemotaxis responses: Sensory neurons define olfactory preferences in C. elegans. Cell 91: 161–169 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Sagasti A, Bargmann CI 1999. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell 99: 387–398 [DOI] [PubMed] [Google Scholar]
- Tucker M, Sieber M, Morphew M, Han M 2005. The Caenorhabditis elegans aristaless orthologue, alr-1, is required for maintaining the functional and structural integrity of the amphid sensory organs. Mol Biol Cell 16: 4695–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden AM, Nolan KM, Sengupta P 2007. KIN-29 SIK regulates chemoreceptor gene expression via an MEF2 transcription factor and a class II HDAC. EMBO J 26: 358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden A, Wiener S, You YJ, Kim K, Avery L, Sengupta P 2008. The EGL-4 PKG Acts with the KIN-29 SIK and PKA to regulate chemoreceptor gene expression and sensory behaviors in Caenorhabditis elegans. Genetics 180: 1475–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R 1999. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96: 725–736 [DOI] [PubMed] [Google Scholar]
- Wes PD, Bargmann CI 2001. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 410: 698–701 [DOI] [PubMed] [Google Scholar]