Abstract
Background
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) demonstrated that implantable cardioverter-defibrillator (ICD) therapy significantly improved survival compared with medical therapy alone in stable moderately symptomatic heart failure patients with an ejection fraction (EF) ≤35%. The purpose of this report is to describe the outcomes in African-Americans (AA) and other minorities.
Methods
Of 2521 patients enrolled, 23% were minorities and 17% were AA. Baseline demographic, clinical variables, socioeconomic status and long-term outcomes were compared according to race. Two major prespecified subgroups were examined: heart failure cause (ischemic vs. non-ischemic) and NYHA class (II vs. III).
Results
At baseline, compared with Caucasians, AA were younger and had more non-ischemic heart failure, lower EFs, worse NYHA functional class, and higher prevalence of a history of non-sustained ventricular tachycardia. Comparable percentages of Caucasians and AA held paid jobs but Caucasians had a significantly higher educational level and household income (p=0.001). Compliance with ICD implantation and medical therapy was comparable in both subgroups. No significant difference was observed in the rate of ICD discharge among Caucasians and AAs. Adjusted mortality risk was significantly higher in AA compared with Caucasians (hazard ratio = 1.27, p=0.038). Mortality was equally reduced in both race groups receiving ICD therapy compared to placebo (hazard ratio 0.65 in AA and 0.73 in Caucasians).
Conclusions
Survival benefits from ICD therapy in SCD-HeFT were not dependent on race. In addition, in this clinical trial setting, there was no evidence that AA were less willing to accept ICD therapy than Caucasians.
Keywords: Sudden cardiac death, African Americans, minorities, ICD, amiodarone, heart failure
INTRODUCTION
Demographic projections for the US population by race and ethnicity suggest that by the year 2050 there will be no majority populations (1). Currently about 30% of the US population is minority (2). This minority percentage will increase especially in the Hispanic and African American (AA) segments of the US population (1).
A higher prevalence of HF in AA vs. the general population with heart failure exists; 3% of the AA population have HF vs. 2% of the general US population (3). However, minorities have been severely underrepresented in most major, large randomized heart failure clinical trials, averaging only 15% (2). The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), a randomized, placebo-controlled trial with 23% minority enrollment and long-term follow-up provides important new data on the treatment of heart failure in minorities (4). The purpose of this report is to describe the minority patients in SCD-HeFT along with their outcomes according to randomized treatment. Since the majority of the minority patients were AA, their results will be reported in detail. Other minority groups were too small to use for treatment-based comparisons and their data are presented primarily for descriptive purposes.
METHODS
Study Design and Primary Clinical Results
As previously described, SCD-HeFT was a National Heart, Lung, and Blood Institute sponsored, multi-center, randomized trial that tested whether amiodarone (dose based on weight) or a shock only, single lead ICD reduced all-cause mortality compared to placebo (4). All patients received state-of-the-art medical therapy. Key inclusion criteria included age 18 years or greater with either ischemic or non-ischemic stable NYHA functional Class II or III heart failure and an ejection fraction ≤ 35%. Examination of the trial results by race subgroups was prespecified. The investigational review board of each participating institution approved the protocol. After informed written consent from all patients and before randomization, all patients underwent resting 12-lead electrocardiography, a 6-minute walk test, 24-hour ambulatory electrocardiography, liver and thyroid-function studies, and chest radiography.
The primary endpoint was all-cause mortality. Average length of follow-up was 45.5 months and vital status was known on all 2521 patients at last follow-up. Overall, ICD therapy reduced all-cause mortality by 23% relative to medical therapy alone (p=0.007). Amiodarone had no effect on survival (hazard ratio = 1.06, p=0.529).
Patients
From 148 centers in the United States, Canada and New Zealand, 2521 patients were randomized into the trial. Of these, 425 (17%) were self-identified AA and an additional 164 (6%) belonged to another minority group, primarily Hispanic.
The purpose of this analysis was to assess whether there were any differences in the benefit of randomized therapy according to race, both overall and in major prespecified subgroups.
Statistical Analysis
Categorical baseline and follow-up variables were summarized using percentages and were compared between Caucasians and AA using the likelihood ratio chi-square test. Continuous baseline variables were summarized as median (25th and 75th percentiles) and were compared using the Wilcoxon rank sum test. Differences in medication use between the race groups were further examined to determine if they were due to different proportions of patients with ischemic and non-ischemic heart failure etiology.
Mortality-rate estimates as a function of follow-up time were generated using the method of Kaplan and Meier (5) A Cox proportional hazards model (6) was used to examine the association of race (Caucasians vs. AA) with mortality risk. This model was adjusted for randomized treatment assignment and other predictors of mortality in SCDHeFT, including heart failure etiology, NYHA class, age, gender, ejection fraction, diabetes mellitus, systolic blood pressure, murmur of mitral regurgitation, past substance abuse, time since diagnosis of heart failure, 6-minute-walk distance, Duke Activity Status Index (7), ACE-inhibitor use, digoxin use, renal insufficiency, PR interval, QTc interval, and presence of IVCD on the baseline ECG. In addition, the model was adjusted for other baseline imbalances between the race groups: hyperlipidemia, history of hypertension, atrial fibrillation or flutter, non-sustained ventricular tachycardia, syncope, QRS duration, diastolic blood pressure, heart rate, and socioeconomic status. Risk relationships were expressed as hazard ratio (95% confidence interval) (HR [CI]).
To determine whether the effect of either randomized treatment was different for Caucasians vs. AAs overall and within subgroups, interaction terms were tested in the model. Interactions between race and all other variables in the model were also tested. An unadjusted Cox model was used to assess the relationship of race to risk of ICD discharge among those patients who received an ICD.
RESULTS
Demographics and Baseline Clinical Data
Among the 2521 patients randomized in the trial, 589 (23%) were minorities. Subdivision of this group included 425 (72%) African Americans, 111 (19%) Latin Americans, and 53 (9%) other minorities (30 Asian, 17 Native American, 6 Pacific Islander). Significant differences in the demographic and clinical data were evident across racial groups (Table 1). AA patients with heart failure were younger (56 vs. 61 years, p=0.001) with a higher percentage of women (35% vs. 21%, p=0.001). AA patients had some markers of higher risk, including a higher percentage of NYHA functional class III (34% vs. 29%, p=0.032), lower ejection fractions (median 20% vs. 25%, p=0.001), and lower 6-minute walk distance (6MW), AA 970 feet (ft)/Caucasians 1155 ft, (p=0.001). AA compared with Caucasians were significantly (p=0.001) more likely to have a non-ischemic cause for their heart failure (70% vs. 43%).
TABLE 1.
Baseline Characteristics
| Caucasian (N= 1932) |
African- American (N=425) |
Latin- American (N=111) |
Other minority† (N=53) |
|
|---|---|---|---|---|
| Age (years) | 61 (53, 69) | 56 (47, 65)* | 57 (49, 67) | 55 (46, 66) |
| Female | 21% | 35%* | 24% | 17% |
| NYHA Class III | 29% | 34% | 32% | 32% |
| 6-minute walk distance (ft) |
1155 (898, 1400) |
970 (735, 1232)* |
1033 (720, 1320) |
1175 (930, 1400) |
| DASI^ | 24 (15, 35) | 20 (13, 31)* | 19 (17, 29) | 21 (13, 32) |
| Ejection fraction (%) | 25 (20, 30) | 20 (15, 27)* | 21 (18, 28) | 25 (19, 32) |
| Non-ischemic HF etiology |
43% | 70% | 51% | 51% |
| High cholesterol | 56% | 38%* | 47% | 53% |
| Diabetes | 29% | 34%* | 45% | 36% |
| Pulmonary disease | 20% | 19% | 14% | 11% |
| Hypertension | 51% | 78%* | 61% | 43% |
| Weight (lbs) | 190 (165, 218) | 195 (163, 229) | 175 (150, 208) | 169 (145, 208) |
| Systolic BP | 118 (105, 130) | 121 (110, 140)* | 116 (104, 130) | 110 (104, 120) |
| Diastolic BP | 70 (61, 78) | 74 (65, 82)* | 70 (62, 80) | 70 (60, 80) |
| Heart rate (bpm) | 72 (64, 83) | 78 (69, 88)* | 72 (64, 83) | 76 (64, 87) |
| Atrial fibrillation /flutter |
17% | 12%* | 12% | 17% |
| Syncope | 7% | 4%* | 3% | 8% |
| QRS≥120 ms | 44% | 27%* | 38% | 40% |
| Non-Sustained VT | 22% | 31%* | 20% | 21% |
| EP study | 15% | 21%* | 16% | 11% |
| Randomized treatment | ||||
| Amiodarone | 34% | 30% | 45% | 32% |
| Placebo | 33% | 34% | 31% | 47% |
| ICD | 33% | 36% | 24% | 21% |
| Working at a paid job | 27% | 26% | 17% | 30% |
| Household income | * | |||
| ≤$10,000 | 9% | 29% | 36% | 15% |
| $10,001–$20,000 | 18% | 21% | 21% | 26% |
| $20,001–$30,000 | 17% | 13% | 20% | 9% |
| $30,001–$45,000 | 19% | 13% | 11% | 13% |
| $45,001–$60,000 | 14% | 8% | 14% | 9% |
| ≥$60,001 | 19% | 9% | 3% | 15% |
| Years of education | 12 (12, 14) | 12 (11, 14)* | 12 (8, 14) | 12.5 (12, 15) |
30 Asian, 17 Native American, 6 Pacific Islander
African-Americans different from Caucasians at p<0.05.
Continuous variables shown as median (25th, 75th percentiles).
DASI—Duke Activity Status Index (Higher scores reflect better functioning)
AA patients were significantly less likely to have a history of high cholesterol (38% vs. 56%, p=0.001) but more likely to have a history of diabetes (34% vs. 29%, p= 0.019) and hypertension (78% vs. 51%, p= 0.001). Higher systolic and diastolic blood pressure were present in AA patients on enrollment (median 121 vs. 118 systolic, p=0.001 and 74 vs. 70 diastolic, p=0.001). Median resting heart rate was also notably higher in AA (78 vs. 72 bpm, p=0.001).
There was a lower percentage of AA patients with a history of atrial fibrillation or flutter (12% vs. 17%, p=0.008). The AA patients also had a lower percentage with a QRS duration greater than 120 msec (27% vs. 44%, p=0.001) or to have a history of documented syncope (4% vs. 7%, p=0.003) at the time of enrollment. Conversely, the presence of non-sustained ventricular tachycardia (31% vs. 22%, p=0.001) and the performance of an electrophysiology study (21% vs. 15%, p=0.003) were more common in AA prior to enrollment.
While the rate of employment for pay was the same in AA and Caucasians, the distribution of income was significantly lower in AA and highest grade reached in school was also lower (Table 1).
Comparisons of Heart Failure Medications
Comparison of Caucasians and AA with respect to use of medications showed similar use of ACEI +/or ARB at baseline and end of study and comparable use of beta-blocker at the last follow-up visit (Table 2). ASA and statin therapies were used significantly more frequently at both time points of the trial in Caucasians compared with AA patients (p<0.05 for both). When ASA use was adjusted for HF etiology, the difference in use became non-significant (p=0.07), indicating that the difference in ASA use was partly due to the higher proportion of ischemic heart failure patients among Caucasians. However, with statin use, a significant difference remained after controlling for etiology. Digoxin use was more common in AA. Median baseline digoxin serum level was significantly lower in AA compared with Caucasians (0.80 ng/ml vs. 0.90 ng/ml, p=0.001).
TABLE 2.
Medication Use
| Caucasian | African- American |
Latin American |
Other minority† |
||
|---|---|---|---|---|---|
| N | (Start) | 1932 | 425 | 111 | 53 |
| (End) | 1919 | 418 | 110 | 53 | |
| Beta blocker | (Start) | 70% | 64%* | 71% | 70% |
| (End) | 78% | 75% | 75% | 81% | |
| ACEI/ARB | (Start) | 96% | 96% | 98% | 100% |
| (End) | 86% | 89% | 84% | 94% | |
| ASA | (Start) | 59% | 46%* | 53% | 42% |
| (End) | 57% | 46%* | 47% | 51% | |
| Statin | (Start) | 42% | 24%* | 39% | 32% |
| (End) | 51% | 30%* | 43% | 36% | |
| Digoxin# | (Start) | 68% | 74%* | 78% | 68% |
| (End) | 60% | 67%* | 58% | 66% |
30 Asian, 17 Native American, 6 Pacific Islander
African-Americans different from Caucasians at p<0.05.
Median baseline digoxin level (25,75%): 0.90 ng/ml (0.60, 1.25) for Caucasians, 0.80 (0.50, 1.20) for AA, p=0.001.
Compliance
No difference was seen in the non-compliance rate for study drug therapy, defined as the discontinuation of either placebo or amiodarone at the time of the last follow-up (31% for AA vs. 32% for Caucasian, p=0.61). Rates of ICD refusal among patients randomized to ICD were not different (4% for AA vs. 2% for Caucasian, p=0.11).
Prognosis According to Race
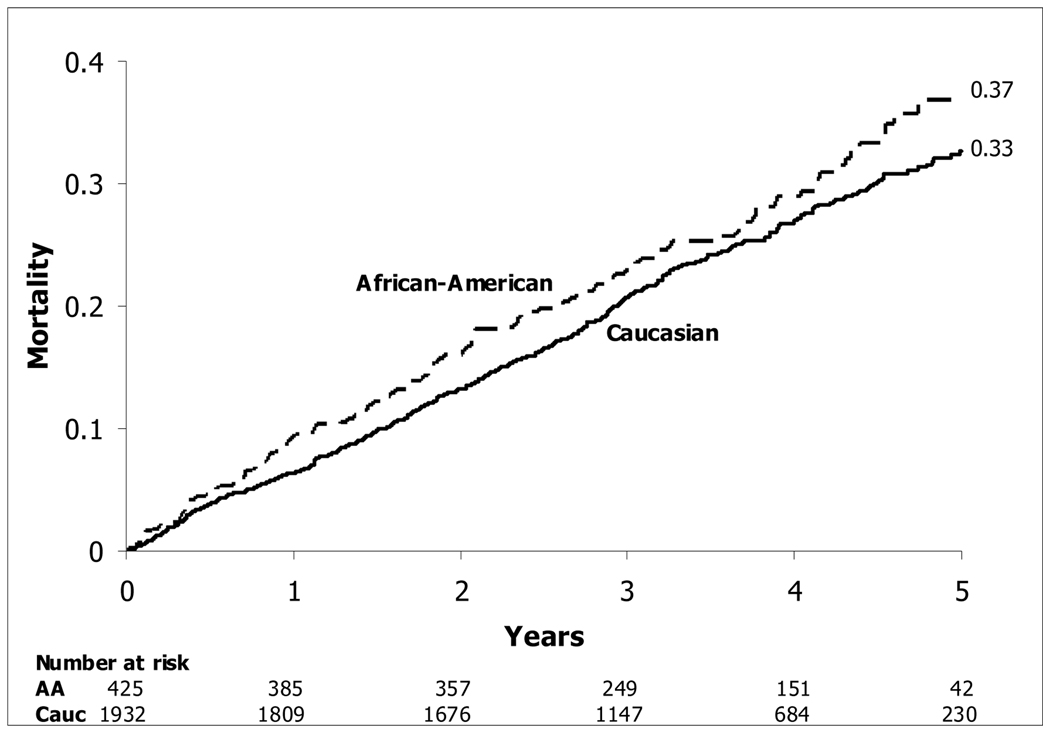
A higher risk of mortality was seen in AA compared with Caucasians (HR[CI] = 1.27 [1.01, 1.58], p=0.038) (Figure 1). This difference persisted after adjustment for randomized treatment assignment, other predictors of mortality, baseline imbalances between the races, and indicators of socioeconomic status.
Figure 1.
Kaplan-Meier Estimates of Death from Any Cause—-Caucasians and AA
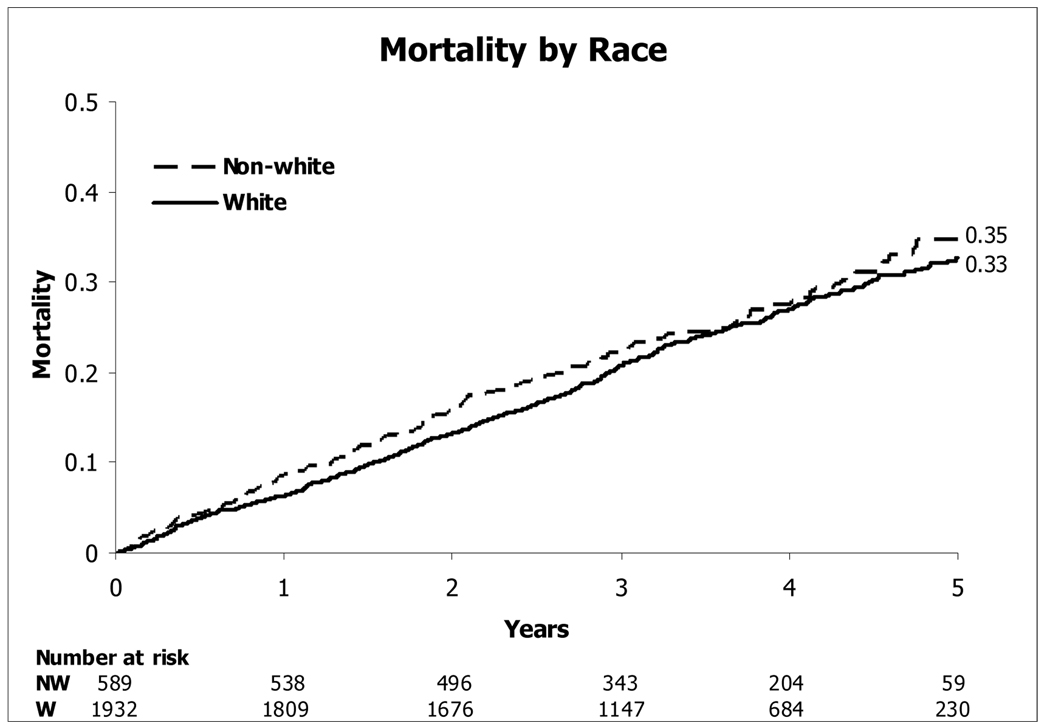
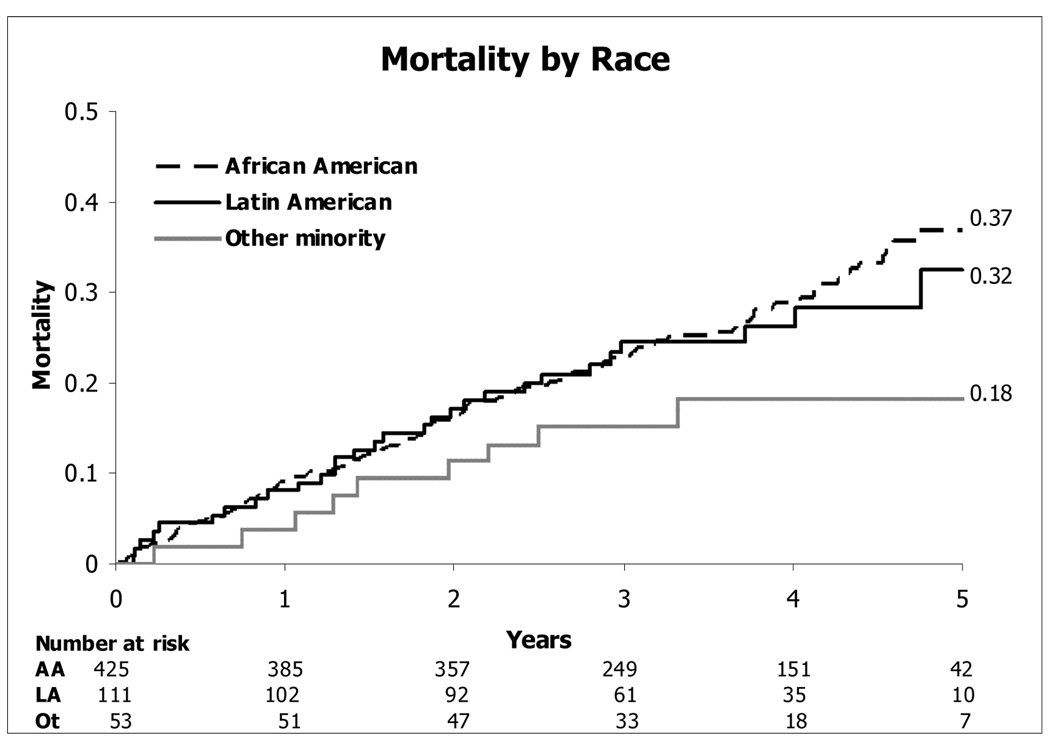
Overall, a higher risk of mortality was not seen in the full minority cohort compared with Caucasians (HR [CI] = 1.14 [0.94 to 1.39]; p=0.18) (figure 2). Within this minority cohort, the highest mortality was seen in the African American subset, the second highest mortality in the Latin American group, and the lowest mortality in the “other minority” subset (Figure 3).
Figure 2.
Kaplan-Meier Estimates of Death from Any Cause—-Caucasians and Minorities
Figure 3.
Kaplan-Meier Estimates of Death from Any Cause for the Minority Subgroups.
Intention-to-treat Comparisons According to Race
AA and Caucasians patients were evenly distributed across the placebo, amiodarone, and ICD arms (Table 1). There was no significant interaction between either randomized treatment and race (test for ICD-vs.-placebo different across race groups, p=0.53; for amiodarone-vs.-placebo different across race groups, p=0.71), indicating that treatment effects were consistent across the two racial groups. ICD therapy reduced the risk of death to a similar extent in both racial groups. The HR for ICD vs. placebo was 0.65 (0.43, 0.99) for AA and 0.73 (0.58, 0.90) for Caucasians. Similarly, the HR for amiodarone vs. placebo was 1.11 (0.90, 1.37) for Caucasians and 1.08 (0.71, 1.64) for AA. No significant difference was observed in ICD discharges between AA and Caucasians (HR[CI] = 1.10 [0.80, 1.51], p=0.56).
Interactions Between Race and Other Mortality Predictors
For the prespecified subgroups, there were no significant interactions between race and either NYHA class (p=0.95) or heart failure etiology (p=0.80), indicating that the relative risk of mortality for AA vs. Caucasians was similar across the subgroups. Although minority patients were more likely, compared with white patients, to be in class III, the majority of patients from both the white and nonwhite groups were in class II.
Among the other variables in the model, two showed a significant interaction with race: history of documented syncope (p=0.032) and history of atrial fibrillation or flutter (p=0.042). Among AA, patients with prior syncope had a higher mortality risk (HR[CI] = 2.18 [1.06, 4.28]) while among whites there was no association of prior syncope with mortality (0.92 [0.73, 1.14] for syncope vs. no syncope). Similarly, AA with a history of atrial fibrillation or flutter had a higher mortality risk (HR[CI] = 1.58 [0.97, 2.57]) while among whites there was no association of prior atrial fibrillation with mortality (0.91 [0.65, 1.28]).
There were no other significant interactions between race and other variables, indicating that the associations of these predictors with mortality risk were consistent across racial groups.
DISCUSSION
Our study is the first to examine in detail the experience of a large cohort of minority patients in a clinical trial of primary prevention with ICD therapy. Two findings are particularly important. First, we found no evidence that ICD therapy was less effective in the prevention of all-cause mortality among African-Americans compared with Caucasians, as evidenced by equivalent ICD:placebo hazard ratio estimates. Further, among ICD patients, there was no difference by race in the proportion with ICD shocks. Second, in SCD-HeFT, AA had higher mortality than Caucasians even after adjustment for recognized potential confounders. AA entering SCD-HeFT had factors suggesting both lower risk (younger age and less ischemic heart failure) and higher risk (more NYHA class III patients, lower mean EF, shorter 6MW and lower socioeconomic status), but the differences in survival persisted after adjustment for these and other known baseline imbalances.
Differences in the use of evidence-based medicines, with the exception of statin, were not significant after controlling for HF etiology. Evidence of benefit with statin use in ischemic heart disease is firmly established but the verdict is still pending for its use in nonischemic systolic heart failure (8,9). Digoxin use was more common in AA and the drug level was lower in AA compared to Caucasians. Post hoc analysis of the Digoxin Trial (10) found that higher serum concentration of digoxin was associated with higher mortality and conversely, lower levels were associated with lower mortality in most subgroups, with the exception of non-whites (adjusted P-value for interaction was equal to 0.006). However, controlling for medication in our analysis, digoxin use revealed no substantive change in hazard ratios of AA vs. Caucasian.
There was no evidence that minorities were less willing to accept ICD therapy, once randomized to this treatment, than Caucasians, nor to discontinue medical therapy.
Prior Randomized Trial Data on Minorities and ICD Therapy
MADIT II recently published a comparative analysis by race (11). This trial, enrolled patients ≥1 month after acute myocardial infarctions with left ventricular ejection fractions ≤0.30. ICD therapy in MADIT-II was associated with a reduction in total mortality, cardiac death, and sudden cardiac death in whites but not in blacks. However, in this non-prespecified post hoc analysis only 102 AA patients were enrolled and therefore the analysis had very low statistical power to examine race-based differences in outcome. The DEFINITE Trial of ICD therapy in non-ischemic cardiomyopathy patients enrolled 34% minorities, including 26% African-American. However, the small sample size (119 AA patients) precludes more detailed analysis by race (12).
Prior Observational Studies on Minorities and ICD Therapy
In the comparison of whites versus blacks in The Multicenter UnSustained Tachycardia Trial (MUSTT), blacks had lower ICD implantation rate and worse survival than whites (13). However the lower implantation rate for ICDs was based on blacks appearing to respond more favorably to antiarrhythmic drugs at EPS testing and blacks were also more likely than whites to refuse ICD implantation when it was recommended in that trial.
A recent analysis of 2002 CMS data showed that among elderly patients with presumed ischemic cardiomyopathy, more whites than blacks received an ICD (14). This analysis is limited, however, by the need to use claims data to identify clinical diagnoses and by the fact that it uses data that predate the publication of the major evidence for primary prevention ICD therapy. Examination of this same issue in even earlier CMS data showed that there was a large black-white disparity in the use of ICD therapy during the 1990s but that there was also evidence that this disparity was decreasing towards the end of that decade (15).
A large black-white disparity was also found in the use of ICD for survivors of a cardiac arrest in 2000 and 2001, although less than half of all survivors were discharged with an ICD, for reasons that are not clear from the data (16).
And earlier, an analysis of hospitalized records in California between 1992 and 1994 found that after controlling for potential confounding factors, black patients were significantly less likely than white patients to undergo EPS or ICD implantation (17). Blacks also had the highest initial hospital and one-year mortality rates. Patients’ insurance status and hospital capabilities were some of the factors found to impact use of EPS procedures or ICD implantation.
SCD-HeFT was conducted in hospitals with the capability of performing EPS procedures and ICD implantation and insurance status had no influence on the randomization. Unlike some of the prior mentioned studies, no significant difference was seen in SCD-HeFT in the proportion of nonwhites and whites randomized to the various treatment groups and no difference was observed among racial groups in the rate of refusal for ICD implantation after randomization.
This equal acceptance of ICD implantation may reflect the more established nature of the devise therapy (for secondary prevention) at the time of SCDHeFT. Because of historical abuse in clinical trials involving minorities there has been a level of mistrust and reluctance among certain groups to participate in clinical trials. However, in SCDHeFT’s setting of patient education and full disclosure, committed investigators achieved unprecedented minority recruitment and participation.
Limitations
Race was a prespecified subgroup analysis for SCD-HeFT. Prespecification, however, does not remedy low statistical power. A study, such as SCD-HeFT, that is powered for the overall primary comparison by intention-to-treat will usually not have significant power to detect modest treatment-related differences in subgroups. In this analysis, we cannot therefore rule out small quantitative differences in treatment benefit. Nonetheless, the consistency of ICD benefit between AA and Caucasians provides some reassurance that substantial and clinically important differences in ICD benefit by race are unlikely. For racial/ethnic groups other than AA, the number enrolled was too small to permit reliable treatment comparisons within subgroups.
It is now well recognized that racial phenotype is a poor predictor of important biologic variability. In addition, race has complex correlations with socioeconomic and cultural factors that can affect the delivery and response to medical care. With the data available to us, however, we were able to examine only limited surrogates of socioeconomic status.
Conclusion
In the SCD-HeFT trial, African-Americans with moderately severe stable heart failure had a worse prognosis that was not explained by differences in measured baseline disease severity or intensity of evidence-based medical therapy. Importantly, the acceptance of ICD implantation by AA was at a similar rate to Caucasians and they benefited from the therapy to the same extent. With worsened clinical outcome of AA with HF and our study showing similar acceptance of ICD therapy and equal benefit, policies need to be implemented to correct the lower devise implantation rate in this population compared to Caucasians.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. http://www.census.gov/population/www/projections/popproj.html.
- 2.Heiat A, Gross CP, Krumholz HM. Representation of the Elderly, Women, and Minorities in Heart Failure Clinical Trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW. Heart failure in African Americans: a cardiovascular enigma. J Card Fail. 2000;6:183–186. doi: 10.1054/jcaf.2000.17610. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, et al. Amiodarone or an Implantable Cardioverter-Defibrillator for Congestive Heart Failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 6.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 7.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional cpacity (the Duke Activity Status Index) AJC. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 8.Strey CH, Young JM, Lainchbury JH, Frampton CM, Nicholls MG, Richards AM, et al. Short-term statin therapy improves endothelial function and neurohormonal imbalance in normocholesteraemic patients with non-ischaemic heart failure. BMJ. 2006 doi: 10.1136/hrt.2005.082560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ip JH, Dickinson MG, Olshansky B, Hellkamp A, Anderson J, et al. Does Statin Therapy Decrease Mortality in Patients with Cardiomyopathy? Unanticipated Observations from SCDHeFT. Circulation. 2005 Oct 25;Vol 112(No 17) [Google Scholar]
- 10.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vorobiof G, Goldenberg I, Moss AJ, Zareba W, Mcnitt S. Effectiveness of The Implantable Cardioverter Defibrillator in Blacks Versus Whites (from MADIT-II) AJC. 2006 Nov;Vol 98(Issue 10):1383–1386. doi: 10.1016/j.amjcard.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Kadish A, Dyer A, Daubert JP, et al. for the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 13.Russo AM, Hafley GE, Lee KL, Stamato NJ, Lehmann MH, et al. Racial Differences in Outcome in the Multicenter UnSustained Tachycardia Trial (MUSTT)—A Comparison of Whites vs. Blacks. Circulation. 2003;108:67–72. doi: 10.1161/01.CIR.0000078640.59296.6F. [DOI] [PubMed] [Google Scholar]
- 14.Gauri AJ, Davis A, Hong T, Burke MC, Knight BP. Disparities in the Use of Primary Prevention and Defibrillator Therapy Among Blacks and Women. AJM92006) 2006 Feb;:119. doi: 10.1016/j.amjmed.2005.08.021. 167 AJM. [DOI] [PubMed] [Google Scholar]
- 15.Groeneveld PW, Heidenreich PA, Garber AM. Trends in implantable cardioverter-defibrillator racial disparity: The importance of geography. JACC. 2005;45:72–8. 15. doi: 10.1016/j.jacc.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 16.Voigt A, Ezzeddine R, Barrington W, Obiaha-Ngwu O, Ganz LI, London B, Daba S. Utilization of implantable cardioverter-defibrillators in survivors of cardiac Arrest in the United States from 1996 to 2001. JACC. 2004;44:855–858. doi: 10.1016/j.jacc.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 17.Alexander M, Baker L, Clark C, McDonald DM, Rowell R, et al. Management of Ventricular Arrhythmias in Diverse Populations in California. Am Heart J. 2002;144:431–439. doi: 10.1067/mhj.2002.125500. [DOI] [PubMed] [Google Scholar]