Abstract
Background
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) demonstrated that implantable cardioverterdefibrillator (ICD) therapy reduces all-cause mortality in patients with New York Heart Association class II/III heart failure and a left ventricular ejection fraction ≤35% on optimal medical therapy. Whether ICD therapy reduced sudden death caused by ventricular tachyarrhythmias without affecting heart failure deaths in this population is unknown.
Methods and Results
SCD-HeFT randomized 2521 subjects to placebo, amiodarone, or shock-only, single-lead ICD therapy. Over a median follow-up of 45.5 months, a total of 666 deaths occurred, which were reviewed by an Events Committee and initially categorized as cardiac or noncardiac. Cardiac deaths were further adjudicated as resulting from sudden death presumed to be ventricular tachyarrhythmic, bradyarrhythmia, heart failure, or other cardiac causes. ICD therapy significantly reduced cardiac mortality compared with placebo (adjusted hazard ratio, 0.76; 95% confidence interval, 0.60 to 0.95) and tachyarrhythmia mortality (adjusted hazard ratio, 0.40; 95% confidence interval, 0.27 to 0.59) and had no impact on mortality resulting from heart failure or noncardiac causes. The cardiac and tachyarrhythmia mortality reductions were evident in subjects with New York Heart Association class II but not in subjects with class III heart failure. The reduction in tachyarrhythmia mortality with ICD therapy was similar in subjects with ischemic and nonischemic disease. Compared with placebo, amiodarone had no significant effect on any mode of death.
Conclusions
ICD therapy reduced cardiac mortality and sudden death presumed to be ventricular tachyarrhythmic in SCD-HeFT and had no effect on heart failure mortality. Amiodarone had no effect on all-cause mortality or its cause-specific components, except an increase in non-cardiac mortality in class III patients.
Keywords: cardiomyopathy; death, sudden; heart failure; mortality; tachyarrhythmias
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) demonstrated superiority of shock-only, single-lead implantable cardioverter-defibrillator (ICD) therapy over placebo for reducing all-cause mortality in a primary prevention population with New York Heart Association (NYHA) class II or III ischemic or nonischemic heart failure (HF) and a left ventricular ejection fraction ≤35%.1,2 Amiodarone had no effect on total mortality compared with placebo.
A beneficial effect of ICD therapy on arrhythmic death has been demonstrated in a number of clinical trials.3–7 However, this benefit may be offset by an increase in cardiac nonarrhythmic outcomes, as seen in the Defibrillator in Acute Myocardial Infarction Trial (DINAMIT)8 and the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial.9 This study is an analysis of cause-specific mortality outcomes in SCD-HeFT.
Methods
The study design, subject demographics, and main study outcomes of SCD-HeFT have been reported previously.1,2 Briefly, a total of 2521 subjects were randomized in equal proportions to receive a single-lead ICD programmed in a shock-only mode, amiodarone, or placebo. The amiodarone and placebo arms in the trial were double blinded. The randomization scheme was stratified by NYHA class and HF type. Subjects were >18 years of age, had chronic stable NYHA class II or III HF (assigned by the site investigator) resulting from ischemic or nonischemic causes, had a left ventricular ejection fraction ≤0.35, and were on optimal HF medical therapy. Enrollment was from September 16, 1997, to July 18, 2001, and subjects were followed up for a median of 45.5 months. Vital status was available for 100% of subjects at the end of the study.
Event Adjudication
All deaths were classified as witnessed or unwitnessed, as sudden or nonsudden, as cardiac or noncardiac, and when the event was cardiac, as resulting from ventricular tachyarrhythmia, bradyarrhythmia, HF, or other cardiac causes according to predetermined SCD-HeFT events definitions. This adjudication method was modified from the approach used in the Multicenter Unsustained Tachycardia Trial (MUSTT)10 and was based on the Hinkle-Thaler11 system. The modifications, described below, were designed to refine discernment of HF events.
Clinical records pertaining to outcome events were reviewed by an independent Events Committee composed of 6 electrophysiologists and 6 HF cardiologists. These records included available histories, physical examinations, hospitalization records, laboratory data, paramedic and emergency room records, ECG rhythm strips at the onset of the event (or first available monitored rhythm after the onset of the event), autopsy reports, and a detailed narrative event summary provided by the physician investigator at the enrolling site. A dedicated events nurse reviewed these documents and removed all information identifying randomized therapy assignment before Events Committee adjudication to maintain blinding. Moreover, ICD interrogation data results were not provided to the Events Committee to further avoid bias in the determination of the cause of death.
During the first year of the trial, all outcome events were reviewed by the entire Events Committee. Subsequently, a complete outcome event documentation package was reviewed by 2 members of the committee (1 electrophysiologist and 1 HF cardiologist). No further review was required for those cases with complete adjudication agreement between reviewers. Cases with adjudication disagreement were resolved by the entire Events Committee.
Death or Cardiac Arrest Event Classification
A cardiac arrest was defined as the sudden loss of consciousness requiring transthoracic defibrillation and/or cardiopulmonary resuscitation to stabilize blood pressure and rhythm. A death occurring within 48 hours of a cardiac arrest was considered a single event. A death occurring >48 hours after a cardiac arrest was classified separately. A witnessed event was defined as an event occurring in the presence of or heard by another observer (ie, family member or medical staff). An event was considered unwitnessed if the subject had not been seen, heard from, or monitored for >5 minutes before the event. Events were considered sudden if they occurred within 1 hour of the onset of major accelerating symptoms.
Cardiac or Noncardiac Event Classification
Events were classified as cardiac or noncardiac to establish the most proximate cause of death or arrest. An outcome event in a subject already hospitalized because of a serious, independent, noncardiac problem such as cancer, renal or liver failure, sepsis, or a vascular event accompanied by substantial disease-specific morbidity was considered to be noncardiac in origin. When a hospitalized subject developed HF or an unexpected arrhythmia as the mechanism of immediate demise, the outcome event was considered to be cardiac in origin. In the setting of equal cardiac and noncardiac considerations, the event was ascribed to a cardiac cause.
Event Subclassification
Cardiac events were subclassified as sudden death presumed to be ventricular tachyarrhythmic, bradyarrhythmic, HF related, or a result of other cardiac causes. An instantaneous or nearly instantaneous death was classified as being due to a ventricular tachyarrhythmia in the absence of a clear indication of an alternative mode of death. Death during sleep was considered to be due to a ventricular tachyarrhythmia if the event was unexpected and occurred in the absence of acceleration of HF symptoms. Deaths resulting from the sequelae of a cardiac arrest or occurring within 30 days of and related to a device implantation were considered to be due to a ventricular tachyarrhythmia. An outcome event was considered to be due to a bradyarrhythmia only when the subject's rhythm demonstrated a bradyarrhythmia at the onset of the event.
Death occurring in a subject with progressively worsening HF over the preceding 3 to 4 months, in whom long-term survival was not expected, was considered to be due to HF even when death was sudden or associated with a terminal ventricular tachyarrhythmia event. This adjudication required the absence of evidence that the cause of progressive HF was not a sustained supraventricular or ventricular tachyarrhythmia. Events deemed related to HF therapy such as those triggered by digoxin toxicity or inotrope-related ventricular tachyarrhythmia were also characterized as being due to HF.
Nonarrhythmic non-HF cardiac deaths included those with strong evidence for acute myocardial infarction, accelerating angina without evidence of a myocardial infarction, an arrhythmic death in a hospitalized subject within 48 hours of a cardiac surgical procedure or percutaneous intervention, or a death within 30 days and as a direct result of any other cardiovascular procedure.
Noncardiac Events
Deaths classified as noncardiac included vascular events such as a stroke, peripheral arterial embolism, pulmonary embolism, aneurysm rupture, and acute hemorrhage and nonvascular events such as those underlying serious lung, liver, kidney or other organ failure, cancer, and sepsis. The death was considered noncardiac even if a ventricular tachyarrhythmia occurred but was considered secondary to the underlying noncardiac cause of death.
Statistical Analysis
All treatment comparisons were performed according to the intention-to-treat principle with 2-tailed statistical testing. Cumulative mortality rates were calculated with the Kaplan–Meier method.12 Follow-up time for each patient, including the time until death, was measured from the point of randomization. Treatment differences in all-cause and cause-specific mortality were assessed with Cox proportional-hazards models13 adjusted for NYHA class and HF type. Treatment effects (relative risks) were characterized through the use of hazard ratios (HRs) and associated 95% confidence intervals (CIs) derived from the Cox models. These models were also used to test interactions between HF class and treatment and between HF type and treatment. Wald χ2 tests were used for tests of treatment effects, and likelihood-ratio χ2 tests were used for interactions. The proportional-hazards assumption was tested for each randomized treatment in each end point model by the inclusion of separate treatment-by-time interactions; all models met the proportional-hazards assumption.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Total and Cardiac Mortality
During follow-up, deaths occurred in 182 of 829 subjects (22.0%) randomized to receive an ICD, in 240 of 845 subjects (28.4%) randomized to receive amiodarone, and in 244 of 847 subjects (28.8%) randomized to receive placebo (the Table). As previously reported, there was a 23% reduction in all-cause mortality with ICD therapy versus placebo (P=0.007), whereas there was no significant difference in all-cause mortality between subjects randomized to amiodarone and subjects randomized to placebo.1
Table.
Mode of Death by Treatment Group
| ICD Therapy (n=829), n (%) | Amiodarone (n=845), n (%) | Placebo (n=847), n (%) | All Subjects, n (%) | |
|---|---|---|---|---|
| All deaths | 182 (22.0) | 240 (28.4) | 244 (28.8) | 666 (100) |
| Cardiac | 122 (67) | 162 (68) | 167 (68) | 451 (68) |
| Tachyarrhythmic | 37 (20) | 75 (31) | 95 (39) | 207 (31) |
| Bradyarrhythmic | 1 (<1) | 5 (2) | 3 (1) | 9 (1) |
| HF | 72 (40) | 67 (28) | 66 (27) | 205 (31) |
| Nonarrhythmic, non-HF | 9 (5) | 10 (4) | 2 (1) | 21 (3) |
| Cardiac but unable to classify further | 3 (2) | 5 (2) | 1 (<1) | 9 (1) |
| Noncardiac | 48 (26) | 54 (23) | 53 (22) | 155 (23) |
| Vascular | 11 (6) | 10 (4) | 12 (5) | 33 (5) |
| Nonvascular | 37 (20) | 44 (18) | 41 (17) | 122 (18) |
| Unknown | 12 (7) | 24 (10) | 24 (10) | 60 (9) |
Values in parentheses are percent of deaths within the treatment group.
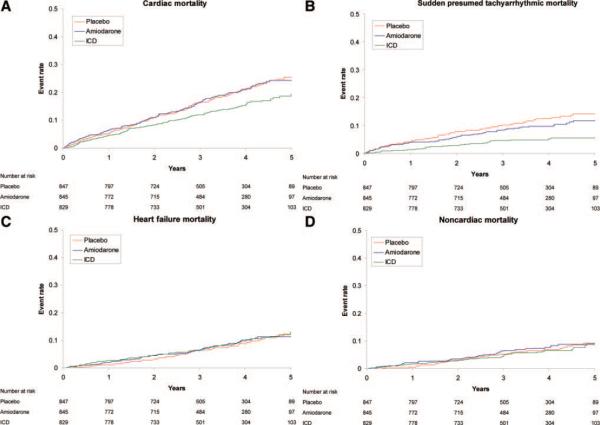
Cardiac mortality occurred in 122 subjects (14.7%) randomized to ICD, 162 subjects (19.2%) randomized to amiodarone, and 167 subjects (19.7%) randomized to placebo (the Table). The adjusted HR describing the benefit of ICD therapy versus placebo for cardiac mortality was 0.76 (95% CI, 0.60 to 0.95; P=0.018). There was no difference in cardiac mortality between subjects randomized to amiodarone and those randomized to placebo (Figures 1A and 2).
Figure 1.
Kaplan–Meier estimates in each treatment arm. A, Cardiac mortality. B, Cardiac mortality resulting from sudden death presumed to be ventricular tachyarrhythmic. C, Cardiac mortality resulting from HF. D, Noncardiac mortality. See text for HRs.
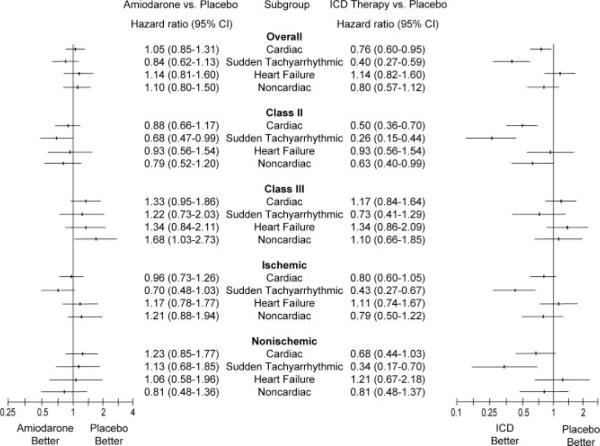
Figure 2.
HRs for the comparison of amiodarone and ICD therapy for cardiac, sudden death presumed to be ventricular tachyarrhythmic, HF, and noncardiac mortalities in the entire cohort and for specified subgroups adjusted for NYHA class and HF type.
Cardiac Death Subclassification
Cardiac mortality resulting from sudden death presumed to be ventricular tachyarrhythmic occurred in 37 subjects (4.5%) randomized to ICD, in 75 subjects (8.9%) randomized to amiodarone, and in 95 subjects (11.2%) randomized to placebo (Figure 1B). The adjusted HR describing the benefit of ICD therapy versus placebo for cardiac mortality caused by tachyarrhythmia was 0.40 (95% CI, 0.27 to 0.59; P<0.001). The modest difference between the rates of sudden death presumed to be ventricular tachyarrhythmic in subjects randomized to amiodarone and those randomized to placebo was not statistically significant (adjusted HR, 0.84; 95% CI, 0.62 to 1.13; P=0.25). Subjects randomized to receive an ICD showed a reduction in sudden death presumed to be the result of ventricular tachyarrhythmia almost immediately after device implantation (Figure 1B).
Cardiac mortality caused by HF occurred in 72 of 829 subjects (8.7%) randomized to an ICD, in 67 of 845 subjects (7.9%) randomized to amiodarone, and in 66 of 847 subjects (7.8%) randomized to placebo (Figure 1C). There were no statistically significant differences in cardiac mortality resulting from HF among treatment groups. Likewise, there were no apparent differences in cardiac mortality caused by bradyarrhythmias or cardiac mortality resulting from other causes among treatment groups, although event rates were too low to permit formal testing (the Table).
Noncardiac and Unknown Modes of Death
A total of 23% of all deaths were from noncardiac causes, most of which were nonvascular causes (the Table). There were no statistical differences in noncardiac mortality between treatment groups (Figure 1D).
The cause of death could not be fully determined for 69 of the 666 deaths (10.4%). Some of these cases could be classified as cardiac or noncardiac but could not be further subclassified (the Table). There were no statistical differences in mortality from unknown causes between treatment groups.
Impact of HF Class on Mode of Death
There were a total of 362 deaths in those with NYHA class II HF and 304 deaths in those with NYHA class III HF. The impact of HF functional class on cardiac, sudden death presumed to be ventricular tachyarrhythmic, HF, and noncardiac mortalities is shown in Figure 2. The interaction between ICD therapy and NYHA class was significant for cardiac mortality (P=0.0004) and sudden death presumed to be ventricular tachyarrhythmic (P=0.0091) but not for HF (P=0.29) or noncardiac (P=0.11) mortalities. In subjects with NYHA class II HF, ICD therapy reduced cardiac mortality (adjusted HR, 0.50; 95% CI, 0.36 to 0.70) and sudden death presumed to be ventricular tachyarrhythmic (adjusted HR, 0.26; 95% CI, 0.15 to 0.44) compared with placebo, whereas there was no effect on HF mortality (adjusted HR, 0.93; 95% CI, 0.56 to 1.54). ICD therapy had no effect on any mode of death in those with NYHA class III HF.
Amiodarone therapy had trends toward an interaction between HF classes with respect to cardiac mortality (P=0.064) and sudden death presumed to be ventricular tachyarrhythmic (P=0.073). Those with NYHA class II HF had a trend toward a lower mortality. There was no interaction between amiodarone therapy and HF class for HF mortality (P=0.30). There was a significant interaction of amiodarone therapy on noncardiac mortality between NYHA classes (P=0.020), with an increase in noncardiac mortality seen in those with NYHA Class III HF (adjusted HR, 1.68; 95% CI, 1.03 to 2.73).
Impact of Type of HF on Mode of Death
There were 432 deaths in those with an ischemic type of HF and 234 deaths in those with a nonischemic type. Of those, there were 221 deaths in those with NYHA class II HF of ischemic type, 141 deaths in those with NYHA class II HF and nonischemic type, 211 deaths in those with NYHA class III HF and ischemic type, and 93 deaths in those with NYHA class III of nonischemic type.
The impact of ischemic versus nonischemic causes of HF on cardiac, sudden death presumed to be ventricular tachyarrhythmic, HF, and noncardiac mortalities is shown in Figure 2. There was no significant interaction of ICD therapy with the type of HF in cardiac (P=0.53), sudden death presumed to be ventricular tachyarrhythmic (P=0.58), HF (P=0.82), or noncardiac (P=0.92) mortalities. ICD therapy demonstrated a trend toward a reduction in cardiac mortality in ischemic (adjusted HR, 0.80; 95% CI, 0.60 to 1.05) and nonischemic (adjusted HR, 0.68; 95% CI, 0.44 to 1.03) types of HF, whereas there was a significant reduction in sudden death presumed to be ventricular tachyarrhythmic in ischemic (adjusted HR, 0.43; 95% CI, 0.27 to 0.67) and nonischemic (adjusted HR, 0.34; 95% CI, 0.17 to 0.70) types of HF.
No interaction was seen with amiodarone therapy and type of HF in cardiac (P=0.29), sudden death presumed to be ventricular tachyarrhythmic (P=0.14), HF (P=0.79), and noncardiac (P=0.15) mortalities.
Discussion
Principal Findings
This mode-of-death analysis of SCD-HeFT demonstrates that the reduction in all-cause mortality associated with ICD therapy was due exclusively to a reduction in cardiac mortality from sudden death presumed to be ventricular tachyarrhythmic.
The goal of ICD therapy, to reduce death from otherwise fatal tachyarrhythmias, has been demonstrated in both primary and secondary studies. Similar to the findings in our trial, the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II)14 showed a significant reduction in all-cause, total cardiac, and sudden cardiac mortality in patients with coronary artery disease and reduced left ventricular function. Neither nonsudden nor noncardiac mortality was affected by ICD therapy compared with conventional medical therapy. A meta-analysis of secondary prevention trials of ICDs,15 including the Antiarrhythmics Versus Implantable Defibrillators (AVID) study,3 Cardiac Arrest Study Hamburg (CASH),16 and Canadian Implantable Defibrillator (CIDS) study,4 demonstrated that compared specifically with amiodarone, ICD therapy reduced all-cause and arrhythmic mortality with no change in nonarrhythmic mortality.
Two clinical trials of ICD therapy, however, have not demonstrated a reduction in all-cause mortality. In DINAMIT, a decrease in arrhythmic death was offset by an increase in cardiac nonarrhythmic death in patients at high risk for ventricular tachyarrhythmias enrolled 6 to 40 days after a myocardial infarction.8 Similar findings were seen in the Coronary Artery Bypass Graft Patch (CABG-Patch) trial, in which subjects with a low left ventricular ejection fraction and an abnormal signal-averaged ECG undergoing CABG surgery were randomized to receive or not receive an ICD after CABG.17 Although ICD therapy was associated with a decrease in arrhythmic mortality, all-cause mortality was not affected because of a low arrhythmic event rate and no effect of the ICD on nonarrhythmic mortality.
ICDs Do Not Prevent All Sudden Deaths
This analysis also indicates that despite the presence of an ICD, sudden death may still occur. In SCD-HeFT, 20% of total deaths in the ICD group were classified as sudden deaths presumed to be ventricular tachyarrhythmic, which, although significantly less than in the placebo group (39% of all deaths), is still notable. It is understood that not all terminal arrhythmic events can be prevented with ICD therapy and that some arrhythmic events may even be caused by an ICD.18 Postshock pulseless electrical activity, incessant ventricular tachyarrhythmias, and shock failure have been among the causes of failure of ICD therapy.19,20 Myocardial infarction has also been shown in an autopsy series to be a significant cause of sudden death in HF patients.21 Finally, catastrophic noncardiac events such as pulmonary embolus, dissecting aortic aneurysm, and intracerebral hemorrhage may be the inciting sudden death event.
Impact of HF Class
Perhaps surprising was the observation, consistent with the main trial results,1 that there was no benefit of ICD therapy in reducing ventricular tachyarrhythmic death in subjects with NYHA class III HF, the only subgroup that did not show a benefit. This may be partially explained by a lower percentage of deaths resulting from ventricular tachyarrhythmia compared with HF in this population. More important, however, an analysis using the Seattle Heart Failure Model demonstrated that the lack of benefit from ICD therapy on sudden death was only in the decile with the highest predicted mortality, indicating that the lack of benefit in the NYHA class III HF population was likely skewed by this group.22 The other 90% of the study population had a reduction in sudden deaths.
Impact of Underlying Cause of HF
The benefit of ICD therapy was similar regardless of whether the origin of HF was ischemic or nonischemic. Again, the benefit in both those with ischemic and those with nonischemic HF was due solely to a reduction in sudden deaths presumed to be ventricular tachyarrhythmic with no effect on HF deaths. Amiodarone had no effect on all-cause mortality or on any of its components in those with either type of HF.
The only other trial to examine the role of ICD therapy as a primary prevention strategy for reducing sudden cardiac death in patients with a nonischemic cause of HF was the relatively small Defibrillator in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) study.5 Subjects randomized to receive an ICD did not demonstrate a reduction in all-cause mortality, although on posthoc analysis, there was a statistically significant reduction in sudden death with no difference in HF mortality.
Amiodarone Therapy
SCD-HeFT was unique among primary prevention trials of ICD therapy for including a placebo-controlled antiarrhythmic drug therapy arm. At the time of trial design, 2 studies had suggested conflicting results relative to the potential benefit of amiodarone as a primary prevention strategy to reduce sudden cardiac death in HF patients, the Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure (CHF-STAT)23 and Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA).24 SCD-HeFT clearly resolved this question when amiodarone was shown to have no benefit on reducing all-cause mortality. Given the antiarrhythmic properties of amiodarone, a reduction in ventricular tachyarrhythmic mortality might have been expected but was not observed.
A statistically significant increase in noncardiac mortality was observed in NYHA class III HF subjects randomized to receive amiodarone versus placebo therapy. The specific causes for this increased risk are not clear.
Limitations
Although SCD-HeFT was unique with regard to the mechanism of event adjudication by both electrophysiologists and HF cardiologists, the main limitation of this study is the inherent difficulty in defining causality and the associated classification. This limitation is particularly relevant to the category of cardiac mortality resulting from ventricular tachyarrhythmias. Although such deaths were, by definition, sudden, some events classified as ventricular tachyarrhythmias may have been due to other causes, including bradyarrhythmias, pulseless electrical activity, myocardial infarction,21 and noncardiac causes. Information from ICD interrogations was purposely not made available for event adjudication because it may have affected the Events Committee's decision using data not available in other treatment groups. Only a limited number of subjects, however, had their device interrogated at the time of death; therefore, this information was not used in event adjudication. In addition, sparse information precluded causality adjudication in 9% of deaths, which may have affected the study results.
Conclusions
In SCD-HeFT, ICD therapy was associated with a reduction in all-cause mortality in subjects with NYHA class II or III HF and LEFT VENTRICULAR EJECTION FRACTION ≤35%. This study demonstrated that the benefit was due solely to a reduction in sudden deaths presumed to be ventricular tachyarrhythmic and was not offset by an adverse effect on HF or noncardiac deaths. Furthermore, the dominant beneficiaries of these advantages are patients with NYHA class II HF. NYHA class III patients in SCD-HeFT had a high rate of death resulting from progressive HF, which may have offset any benefit of the ICD in reducing death from ventricular tachyarrhythmias. Amiodarone increased noncardiac mortality in subjects with NYHA class III HF but otherwise had a neutral effect on cause-specific mode of death.
CLINICAL PERSPECTIVE.
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) demonstrated that implantable cardioverter-defibrillator therapy reduces all-cause mortality in patients with New York Heart Association class II/III heart failure and a left ventricular ejection fraction ≤35% on optimal medical therapy. This report examined the mode of death in SCD-HeFT. A total of 2521 subjects were randomized to placebo, amiodarone, or shock-only, single-lead implantable cardioverter-defibrillator therapy. Over a median follow-up of 45.5 months, 666 deaths were reviewed by an Events Committee and categorized as sudden death presumed to be ventricular tachyarrhythmic, heart failure related, bradyarrhythmic, nonarrhythmic non–heart failure related, or noncardiac. Implantable cardioverter-defibrillator therapy reduced cardiac and ventricular tachyarrhythmic mortality and had no impact on mortality resulting from heart failure or noncardiac causes compared with placebo. The cardiac and ventricular tachyarrhythmic mortality reductions were evident in subjects with New York Heart Association class II but not in subjects with class III heart failure. The reduction in ventricular tachyarrhythmic mortality with implantable cardioverter-defibrillator therapy was similar in subjects with ischemic and nonischemic disease. Amiodarone compared with placebo had no significant effect on any mode of death, although there was an increase in noncardiac mortality in those with New York Heart Association class III heart failure who were receiving amiodarone. Implantable cardioverter-defibrillator therapy has a beneficial effect on reducing sudden death presumed to be due to ventricular tachyarrhythmias without an effect on heart failure mortality.
Acknowledgments
Source of Funding This work was supported by grants (UO1 HL55766, UO1 HL55297, and UO1 HL55496) from the National Heart, Lung, and Blood Institute/National Institutes of Heath, Medtronic, and Wyeth Pharmaceuticals.
Disclosures Dr Packer reports receiving consulting fees from Medtronic and Boston Scientific/Guidant, royalties from St Jude Medical, and grant support from Medtronic, St Jude Medical, and Boston Scientific. Dr Mitchell reports receiving research support and resident support from and serving on the speakers' bureau of Medtronic and St Jude Medical. Dr Rogers reports being on the speakers' bureau of Medtronic and Boston Scientific. Dr Walsh reports being on the speakers' bureau of Medtronic and receiving consulting fees from Boston Scientific, Medtronic, United Health Care, Biocontrol, and St Jude Medical. Dr Poole reports receiving lecture fees from Medtronic and Boston Scientific/Guidant, grant support from Biotronik, and consulting fees from Philips. Dr Carlson is the Cardiac Rhythm Management Division Chief Medical Officer with St. Jude Medical. Dr Mark reports receiving consulting fees, lecture fees, and research grants from Medtronic. Dr Lee reports receiving grant support and consulting fees from Medtronic. Dr Bardy reports receiving grant support from and having intellectual property rights with Medtronic, receiving consulting fees and grant support from Philips, receiving grant support from Laerdal, and being a founder and board member of and having equity and intellectual property rights with Cameron Health.
Footnotes
Clinical Trial Registration Information—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00000609.
The other authors report no conflicts.
References
- 1.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, Fishbein DP. The Sudden Cardiac Death–Heart Failure Trial (SCD-HeFT) In: Woosley RL, Singh SN, editors. Arrhythmia Treatment and Therapy: Evaluation of Clinical Trial Evidence. Marcel Dekker; New York: 2000. pp. 323–342. [Google Scholar]
- 3.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias: the Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O'Brien B. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 5.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia: Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 9.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–3123. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 10.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease: Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 11.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Cox D. Regression models and life-tables. J R Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 14.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) J Am Coll Cardiol. 2004;43:1459–1465. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, Gent M, Roberts RS. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials: AVID, CASH and CIDS studies: Antiarrhythmics vs Implantable Defibrillator Study: Cardiac Arrest Study Hamburg: Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 16.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 17.Bigger JT, Jr, Whang W, Rottman JN, Kleiger RE, Gottlieb CD, Namerow PB, Steinman RC, Estes NA., 3rd Mechanisms of death in the CABG Patch trial: a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation. 1999;99:1416–1421. doi: 10.1161/01.cir.99.11.1416. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney MO, Ruetz LL, Belk P, Mullen TJ, Johnson JW, Sheldon T. Bradycardia pacing-induced short-long-short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J Am Coll Cardiol. 2007;50:614–622. doi: 10.1016/j.jacc.2007.02.077. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell LB, Pineda EA, Titus JL, Bartosch PM, Benditt DG. Sudden death in patients with implantable cardioverter defibrillators: the importance of post-shock electromechanical dissociation. J Am Coll Cardiol. 2002;39:1323–1328. doi: 10.1016/s0735-1097(02)01784-9. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi H, Weil M, Tang W, Kamohara T, Jin X, Bisera J. Myocardial dysfunction after electrical defibrillation. Resuscitation. 2002;54:289–296. doi: 10.1016/s0300-9572(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 21.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the Assessment of Treatment With Lisinopril and Survival (ATLAS) trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 22.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozfarrian D, Linker DT, Maggioni A, Anand I, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Use of Seattle Heart Failure Model to identify benefit vs harm from a primary prevention ICD. Heart Rhythm. 2008;5(suppl):S78. [Google Scholar]
- 23.Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia: Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 24.Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure: Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) Lancet. 1994;344:493–498. doi: 10.1016/s0140-6736(94)91895-3. [DOI] [PubMed] [Google Scholar]