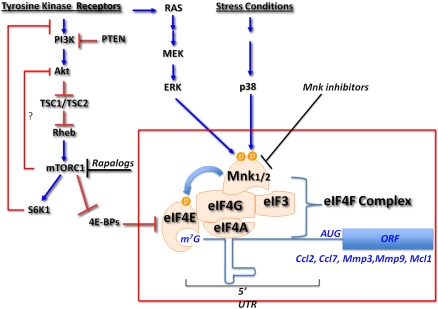
The PI3K/Akt/mTORC1 axis is perhaps the most frequently activated pathway in human cancers. Therefore, concerted efforts are being made to inhibit this pathway pharmacologically for cancer therapy. Although some success has been achieved, the multiple feedback loops that “litter” this pathway (ref. 1; Fig. 1), combined with the potential toxicity resulting from its inhibition, make the pharmacological targeting efforts very challenging. The ideal goal is to inhibit the functionality of the pathway in cancer cells without affecting normal cells and without eliciting feedback loops that could diminish the therapeutic efficacy. Back-to-back papers published in PNAS may provide this kind of therapeutic avenue (2, 3).
Fig. 1.
Schematic illustration depicting the cellular pathways that lead to eIF4E activation and phosphorylation by Mnk1/2. The PI3K/Akt/mTORC1 pathway, which is frequently activated in human cancers, releases 4E-BPs from eIF4E, and enables eIF4E to bind eIF4G, which, in turn, assembles the eIF4F complex comprising eIF4E, eIF4G, eIF4A, and eIF3. Mnk1 and Mnk2, which are activated by Erk and by the stress inducible kinase p38, use eIF4G as a docking site to phosphorylate efficiently eIF4E. The phosphorylation of eIF4E is critical for its oncogenic activity, probably through the differential translation of proteins that are required for oncogenesis. The phosphorylation of eIF4E by Mnk1/2 provides a new avenue for cancer therapy. The inhibition of eIF4E phosphorylation could have similar consequences as the inhibition mTORC1 by rapalaogs, but with the advantage that it does not elicit the activation of Akt as a result of the inhibition of the negative feedback loops mediated by mTORC1.
Previous studies suggest that the most critical target of the serine/threonine kinase Akt, required for tumorigenesis, is mTORC1 (4). The two major downstream targets of mTORC1 are S6K1 and eukaryotic initiation factor-4E binding proteins (4E-BPs), which regulate ribosomal biogenesis and mRNA translation. S6K1 is phosphorylated and activated by mTORC1, whereas 4E-BPs are phosphorylated and inactivated by mTORC1. The 4E-BPs bind to, and sequester, the eukaryotic initiation factor-4E (eIF4E), but their phosphorylation by mTORC1 induces their dissociation from eIF4E. The binding of eIF4E to the 4E-BPs prevents its interaction with the scaffold protein eIF4G, precluding the initiation of mRNA translation (ref. 5; Fig. 1). Thus, the phosphorylation of 4E-BPs by mTORC1 may constitute a rate-limiting step in mRNA translation. eIF4E is required for 5′-cap-dependent mRNA translation in general and for the translation of mRNAs with long and structured 5′ untranslated region (UTR) in particular (Fig. 1).
Efforts to determine which of the two major downstream effectors of mTORC1, S6K1 or 4E-BPs, has the predominant role in cell proliferation and tumorigenesis show that it is likely the 4E-BPs (6), implying that the activity of eIF4E is the major determinant of mTORC1 tumorigenic activity. Supporting a major prooncogenic role of eIF4E are observations that it can contribute to the oncogenic transformation both in vitro and in vivo and that it is highly expressed in diverse types of cancer (reviewed in ref. 5). Collectively, the results imply that targeting eIF4E should have a major impact on the ability of the PI3K/Akt/mTORC1 pathway to maintain cancer cells. But how would it be possible to inhibit eIF4E without introducing any toxicity? Fortunately, eIF4E is posttranslationally modified by phosphorylation, and this phosphorylation is important for its tumorigenic activity. The kinases that phosphorylate eIF4E on a conserved serine 209 are MAP kinase-interacting kinases, Mnk1 and Mnk2, which are activated by Erk and p38 (reviewed in ref. 7). Because Mnk is using eIF4G as a docking site to facilitate eIF4E phosphorylation (8), the phosphorylation of eIF4E by Mnk is apparently occurring after eIF4E-eIF4G binding and in concert with the assembly of the eIF4F complex (Fig. 1).
The requirement of Mnk for Drosophila development underscores the importance of this phosphorylation for the function of eIF4E (9). Surprisingly, however, mice that lack both Mnk1 and Mnk2 do not have any apparent phenotype (11), which may cast doubt on whether phosphorylation by Mnk has any impact on the functionality of the mammalian eIF4E (7). Nevertheless, the phosphorylation of eIF4E by Mnk on Ser-209 is critical for the oncogenic activity of eIF4E (10). The most straightforward interpretation is that in mammalian cells, Ser-209 phosphorylation is not essential for the activity of eIF4E in normal cells but is required in cancer cells. Thus, targeting Mnks could be the ideal therapeutic approach that selectively affects cancer cells, and not normal cells, without toxicity. As a proof of concept of this possibility, Ueda et al. crossed mice lacking Mnk1 and Mnk2 with mice deficient in Pten, specifically in T cells (3). Pten loss in T cells induces early onset of T-cell lymphoma and with no tumor-free survival after 125 days. The deletion of Mnk1 and Mnk2 significantly and markedly decreased tumor incidence and increased tumor free survival. All of the lymphomas had high eIF4E expression and phosphorylation in comparison with normal thymocytes, but the phosphorylation of eIF4E was completely abolished in the absence of Mnk1 and Mnk2. Because human glioblastoma is one tumor type in which the PI3K/Akt/mTORC1 axis is frequently activated through the loss of Pten, Ueda et al. knocked down Mnk1 in glioblastoma cells and subjected them to xenografts assay. The knockdown of Mnk1 was sufficient to diminish the high level of eIF4E phosphorylation in these cells and significantly attenuated the growth of the tumors.
As a prelude to these studies, Ueda et al. (3) characterized Mnk1/Mnk2 double knockout cells. Mnks-deficient cells have the same proliferation rate as Mnks-proficient cells under standard culture conditions and are comparable in their response to stress conditions such as hypoxia and reduced glucose or glutamine levels. However, the ability of Mnks-deficient cells expressing oncogenic Ras to grow in an anchorage-independent manner is significantly impaired when compared with Mnks-proficient cells expressing oncogenic Ras. These results reinforced the notion that, although Mnk is not required for normal cell maintenance, it is important for oncogenic transformation. As noted by Ueda et al., their results cannot completely exclude the possibility that Mnk affects tumorigenesis through other targets, such as Sprouty2, cPLA2, and hnRNPA1, in addition to eIF4E. The complementary approach used by Furic et al., however, strongly suggests that the effect of Mnk on tumorigenesis is largely, if not exclusively, through the phosphorylation of eIF4E.
Furic et al. engineered knock-in mice with the normal allele of eIF4E replaced by a mutant eIF4E, in which Ser-209 was converted to alanine, rendering it resistant to phosphorylation by Mnk (2). Consistent with the phenotype of Mnk1/2 DKO mice, the knock-in mice do not have any obvious phenotype. However, cells derived from these mice are relatively resistant to oncogenic transformation by activated Ras. Like the Mnk1/2 DKO cells, the knock-in cells are not impaired in cell proliferation under standard conditions. To verify the effect of eIF4E phosphorylation on tumorigenesis in vivo, the knock-in mice were crossed with mice in which Pten is selectively deleted in the prostate. The loss of Pten in the mouse prostate elicits early onset of high-grade prostate intraepithelial neoplasia (PIN) with some incidence of invasive neoplasia. In mice expressing the phosphorylation-resistant eIF4E, the development of high grade PINs was substantially attenuated, with no incidence of invasive neoplasia. These results are relevant to human prostate cancer, because Pten is frequently mutated in human prostate cancer. Consistently, Furic et al. showed that eIF4E phosphorylation is greatly elevated in human prostate carcinomas, particularly in hormone-refractive carcinomas, and is positively correlated with high Gleason scores.
In summary, the two complementary studies provide convincing evidence that eIF4E phosphorylation by Mnk is critical for the maintenance of cancer cells displaying a hyperactive PI3K/Akt/mTORC1 axis. Importantly, the studies provide strong support for the use of pharmacological inhibition of Mnk to treat cancers displaying mTORC1 activation and eIF4E phosphorylation as a valuable diagnostic and prognostic marker in these cancers. Nevertheless, several important questions remain to be resolved. First, it is still unclear why the phosphorylation of eIF4E enhances its activity. One possibility is that the phosphorylation weakens its interaction with its repressors the 4E-BPs. Additionally, it was shown that eIF4E facilitates the export of certain mRNAs from the nucleus to the cytoplasm and that this activity of eIF4E is enhanced by its phosphorylation (12). Second, it is not certain why eIF4E phosphorylation is preferentially required for tumorigenesis, although it is not required for normal cell proliferation and survival. Furic et al. analyzed mRNAs preferentially affected by eIF4E phosphorylation. Among these mRNAs are the chemokines Ccl2 and Ccl7 that contribute to tumor progression, and the matrix metalloproteases, MMP3 and MMP9, which contribute to tumor invasiveness. The effect on the metalloproteases mRNA translation could explain the lack of invasiveness of the prostate neoplasia in eIF4E phosphorylation-resistant knock-in mice and the relatively high expression of MMP3 in high grade PINs in the mouse and in human prostate carcinomas. Furthermore, Furic et al. found a significant correlation between eIF4E phosphorylation and high expression of MMP3 in human prostate carcinoma samples. However, more studies are needed to establish why phosphorylation of eIF4E is preferentially required for tumorigenesis.
A final question remains because the studies do not address whether the effect of the eIF4E phosphorylation inhibition on the tumors is cytostatic, cytolytic, or both. This question is important because the rapalogs (rapamycin analogs), which are used as mTORC1 inhibitors, have mostly a cytostatic effect on cancer cells. Another disadvantage of the rapalogs is that they inhibit the negative feedback loop induced by mTORC1 (Fig. 1), resulting in activation of Akt and increased cell survival. Thus, one advantage of Mnk inhibitors over the rapalogs is that they do not lead to Akt activation, but do they also kill the cells? Furic et al. show that prostate tumors derived from the eIF4E knock-in mice are less proliferative as judged by Ki-67 staining, suggesting a cytostatic effect, but cell death was not measured. Interestingly, previous studies showed that overexpression of eIF4E, as well as eIF4E phosphorylation, promote cell survival, at least in part, through the elevation of the anti-apoptotic protein Mcl-1 (10). Mcl-1 is a Bcl2 family member with a very short half-life, and Mcl-1 mRNA translation highly depends on eIF4E. Thus, it is possible that the inhibition of eIF4E phosphorylation by Mnk induces the killing of the tumor cells, as shown for Myc-induced lymphoma (10). Notably, tuberous sclerosis complex (TSC)-deficient cells are resistant to cell death in the absence of growth factors, in part because of the high levels of Mcl-1 due to mTORC1 and eIF4E activation in these cells. Silencing eIF4E in these cells induces high levels of cell death (13). Thus, pharmacological inhibition of Mnks could be an attractive therapeutic approach for tuberous sclerosis complex.
Finally, the task ahead is to find a specific and selective pharmacological inhibitor of Mnks. Although some inhibitors are used for research purposes, it is not certain whether these inhibitors are highly specific and whether they do not have off targets.
Footnotes
References
- 1.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Furic L, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA. 2010;107:14134–14139. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda T, et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA. 2010;107:13984–13990. doi: 10.1073/pnas.1008136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skeen JE, et al. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Sonenberg N. eIF4E, the mRNA cap-binding protein: From basic discovery to translational research. Biochem Cell Biol. 2008;86:178–183. doi: 10.1139/O08-034. [DOI] [PubMed] [Google Scholar]
- 6.Dowling RJ, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyronnet S, et al. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachance PE, Miron M, Raught B, Sonenberg N, Lasko P. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol Cell Biol. 2002;22:1656–1663. doi: 10.1128/MCB.22.6.1656-1663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendel HG, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: The role of the eukaryotic translation initiation factor eIF4E. Cell Cycle. 2007;6:65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 13.Bhaskar PT, et al. mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3beta inhibition. Mol Cell Biol. 2009;29:5136–5147. doi: 10.1128/MCB.01946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]