Abstract
Thymine glycol (Tg) is the most common DNA lesion of thymine induced by interaction with reactive oxygen species. Because of the addition of hydroxyl groups at C5 and C6 in a Tg lesion, the damaged base loses its aromatic character and becomes nonplanar; consequently, the C5 methyl group protrudes in an axial direction and that prevents the stacking of the 5′ base above the Tg lesion. Because Tg presents a severe block to continued synthesis by replicative DNA polymerases, we determine here how human cells manage to replicate through this lesion. Using a duplex plasmid system where bidirectional replication ensues from an origin of replication, we show that translesion synthesis (TLS) makes a prominent contribution to Tg bypass and that it occurs in a predominantly error-free fashion. Also, we provide evidence that Polκ and Polζ function together in promoting error-free replication through the lesion, and based on structural and biochemical information, we propose a role for Polκ at the insertion step and of Polζ at the extension step of Tg bypass. We discuss the implications of these observations and suggest that human cells have adapted the TLS machinery to function in a much more error-free fashion than could have been predicted from the intrinsic catalytic efficiencies and fidelities of TLS polymerases.
Keywords: DNA polymerases kappa and zeta, error-free bypass of thymine glycol, replicative lesion bypass, thymine glycol bypass in humans
Thymine glycol (Tg) is the most common oxidation product of thymine (1–3). Hydroxyl radicals, the principal DNA damaging species formed from aerobic respiration or from exposure to chemical oxidizing agents or ionizing radiation, interact directly with thymine primarily at the 5,6 double bond, generating a thymine glycol. Although Tg lesions in humans can be removed by base excision repair (BER) involving the action of the DNA glycosylase/AP lyase NTH1 or by nucleotide excision repair (NER) (4, 5), if the damaged base is not removed by these repair processes and goes through the replication fork, that presents a severe block to continued synthesis by eukaryotic replicative DNA polymerases (Pols) (6, 7).
Because of the addition of hydroxyl groups at C5 and C6 in a Tg, the damaged base loses its aromatic character and becomes nonplanar (8–10). As a consequence, the C5 methyl group protrudes in an axial direction that prevents the base 5′ to the Tg lesion from stacking above it. Hence a Tg lesion presents a block not only for nucleotide (nt) insertion opposite it but also for the subsequent extension step. Steady-state kinetic analyses of Tg bypass by human translesion (TLS) Pols have indicated that Polη can insert an A opposite Tg with the same catalytic efficiency as for insertion of an A opposite undamaged T; moreover, Polη can also extend from the Tg:A base pair with only an ∼15-fold reduction in efficiency (7). However, Polη incorporates the wrong nts opposite Tg rather frequently as the misincorporation of G or a C residue occurs with only an ∼8-, and 20-fold reduction in efficiency compared with the efficiency for A incorporation. Polη can also extend from these base pairs with the same magnitude of reduction in efficiency as that seen for their insertion (7). Hence although Polη can promote efficient TLS through a Tg lesion by promoting both the insertion and extension steps, it does so in quite an error-prone manner. Polκ can promote error-free TLS opposite Tg by inserting an A and extending from there, but its efficiency at the insertion step is reduced ∼50-fold, and at the extension step it is inhibited ∼250-fold (11). Interestingly, even though Polκ is less efficient at both steps of TLS than Polη, it performs TLS in a more accurate manner—as it incorporates nts other than an A with greatly reduced catalytic efficiency (11). The other two human Y family Pols, Rev1 and Polι, are unlikely to function in TLS opposite Tg because of their highly specialized roles in DNA synthesis as Rev1 specifically incorporates a C opposite template G and Polι is highly inefficient and error-prone opposite pyrimidine template bases, particularly opposite a template T (12).
Polζ, a B-family Pol, comprised of Rev3 catalytic subunit and Rev7 accessory subunit (13), is highly specialized for extending from nts inserted opposite a lesion site by another DNA Pol (12). Because it has not been possible to purify human Polζ, all the inferences for the TLS role of Polζ in human cells have been drawn from biochemical studies done with yeast Polζ. These studies have indicated that Polζ can extend efficiently opposite from a diverse array of DNA lesions, as for example, a cis-syn TT dimer (14), a (6-4) photoproduct (14, 15), an abasic site (16), 1,N6-etheno deoxyadenosine (ϵdA) (17), and others (18); however, Polζ is inhibited at inserting nts opposite these lesions. Steady-state kinetic analyses have indicated that Polζ can also extend from the Tg:A base pair as efficiently as from the T:A base pair (6).
These biochemical observations with the TLS Pols would suggest a role of Polη, Polκ, and Polζ in promoting replication through a Tg lesion, in which Polη could carry out efficient but highly mutagenic TLS, whereas Polκ could act primarily at the insertion step with the subsequent extension step being performed by Polζ. To elucidate the roles of various Pols in promoting replication through a Tg lesion in human cells, here we examine TLS using a duplex plasmid in which bidirectional replication ensues from a replication origin (19, 20) and proceeds through a site-specific Tg lesion carried on the leading or the lagging DNA strand template. From these analyses, we make the following observations: (i) On both the DNA strands, TLS makes a prominent contribution to the replicative bypass of Tg lesion; (ii) in normal human cells, TLS through the lesion occurs in a predominantly error-free fashion; and (iii) Pols κ and ζ function together in promoting error-free TLS through the Tg lesion. We discuss the implications of these observations for the role of TLS in promoting error-free lesion bypass during replication and we consider the contributions of TLS Pols to Tg bypass in relation to their intrinsic efficiency and fidelity opposite this lesion.
Results
The Plasmid System Containing a Site-Specific Tg Lesion and TLS Analyses.
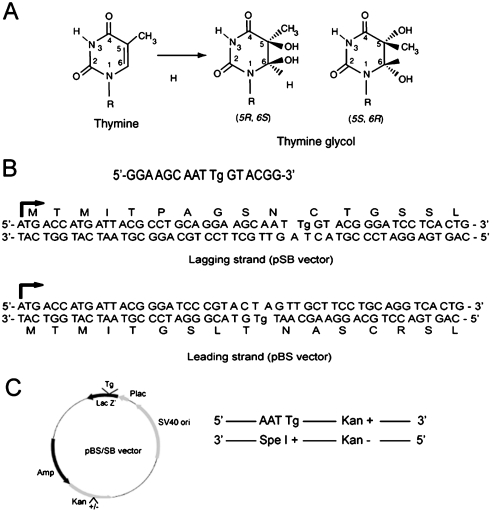
Tg is one of the major stable products of thymine modification produced by cellular oxidative damage or by exposure to ionizing radiation. Tg exists in two cis isomer forms: 5R 6S and 5S 6R (21) (Fig. 1A), and both of these isomers block synthesis by eukaryotic replicative Pols. We have incorporated each of these isomers in the duplex plasmid in the leading strand or the lagging strand DNA template (Fig. 1B). Because the DNA strand that has the Tg lesion also carries the kan+ gene and because the lacZ sequence on this strand is in-frame (Fig. 1 B and C), TLS through the Tg will produce a blue colony on LB/kan plates with IPTG and X-gal. The other DNA strand without the lesion is marked with the kan- gene, and it carries an Spe1 sequence opposite from the lesion on the other strand, which puts the lacZ gene out of frame (Fig. 1 B and C). Hence replication through the Tg lesion via template switching will produce white colonies. Removal of the lesion by BER or NER before replication also would generate a white colony because that would result from repair synthesis using the nondamaged Spe1 DNA strand as the template. The TLS frequencies are determined from the number of blue colonies among the total kan+ colonies.
Fig. 1.
Strategy for examining TLS opposite a Tg lesion carried on the leading or lagging strand DNA template of a duplex plasmid. (A) Structures of 5R,6S and 5S,6R Tg. (B) The target 16-mer sequence containing a Tg, and the sequences of the N-terminal portion of the lacz′ gene in the lagging strand (pSB) and the leading strand (pBS) vector with a Tg lesion are shown. (C) Strategy for TLS assays. (Left) The locations of the Tg, the lacZ′ gene, SV40 origin, and the kan gene are shown on the vector. (Right) In the duplex plasmid, the DNA strand containing the Tg also carries the wild type kanamycin gene (Kan+) so that TLS through the Tg will produce a blue colony on LB/Kan plates with IPTG and X-gal. The other DNA strand harbors an SpeI sequence opposite the Tg and carries the kan- gene. Because the SpeI sequence puts the lacZ′ sequence out of frame, replication of the Tg-containing strand by template switching would generate white colonies.
For determining the roles of various Pols in TLS through the Tg lesion, we carried out parallel experiments with undamaged and damaged plasmid where the levels of particular Pol(s) were depleted by siRNA knockdowns, and as expected, whereas with the undamaged plasmid the frequency of blue colonies among the kan+ colonies remained the same regardless of which Pol had been depleted, with the Tg containing plasmid, a reduction in the frequency of blue colonies occurred upon the siRNA knockdown of some but not of other Pols, indicative of the role of particular Pol(s) in mediating TLS through the Tg lesion.
Requirement of Pols κ and ζ for TLS Opposite Tg.
As shown in Table 1, in normal human cells treated with control (NC) siRNA, TLS through the Tg lesion accounts for ∼23% of lesion bypass on the leading strand and for ∼19% on the lagging strand. Hence the contribution of TLS to the bypass of the 5R,6S lesion on both the DNA strands is quite similar. The TLS frequency for the lesion carried on either the leading DNA strand or the lagging strand template remained about the same in Polη siRNA treated cells as in cells treated with control siRNA. We also verified that TLS on both the DNA strands in xeroderma pigmentosum variant (XPV) cells occurred as frequently as in normal human cells. The siRNA knockdown of Polι also conferred no reduction in TLS frequencies. To exclude the possibility that Pols η and ι provide alternate means of TLS opposite the 5R,6S Tg lesion, we examined whether the simultaneous knockdown of both these Pols has an effect on TLS frequencies. However, because TLS occurs as frequently upon depletion of both these Pols as in normal cells (Table 1), Pols η and ι do not provide alternative competing pathways for TLS opposite this DNA lesion. On the other hand, the siRNA depletion of Polκ led to an ∼50% reduction in TLS frequencies opposite the 5R,6S lesion carried on either DNA strand, and because the simultaneous depletion of either Polκ and Polη or of Polκ and Polι caused no further reduction in TLS frequencies than that observed upon the knockdown of Polκ alone (Table 1), we conclude that Polη and ι play no significant role in TLS opposite the 5R,6S Tg lesion whereas Polκ makes a prominent contribution to promoting replication through this lesion.
Table 1.
Effects of siRNA knockdowns of TLS Pols on the replicative bypass of 5R,6S Tg lesion carried on the leading or lagging strand DNA template in human cells
| Leading strand | Lagging strand | |||||
| siRNA | # Kan+ colonies | # blue colonies among Kan+ | TLS, % | # Kan+ colonies | # blue colonies among Kan+ | TLS, % |
| NC | 648 | 152 | 23.4 | 626 | 121 | 19.4 |
| Polη | 546 | 110 | 20.1 | 645 | 118 | 18.3 |
| Polι | 536 | 134 | 25.0 | 542 | 123 | 22.7 |
| Polκ | 602 | 66 | 10.9 | 624 | 58 | 9.4 |
| Rev3 | 620 | 77 | 12.4 | 584 | 60 | 10.3 |
| Rev7 | 586 | 74 | 12.6 | 623 | 66 | 10.6 |
| Polη + Polι | 526 | 128 | 24.4 | 621 | 132 | 21.2 |
| Polη + Polκ | 572 | 59 | 10.4 | 584 | 57 | 9.8 |
| Polι + Polκ | 610 | 66 | 10.8 | 526 | 54 | 10.2 |
| Polκ + Rev3 | 642 | 75 | 11.7 | 589 | 62 | 10.6 |
| Polκ + Rev7 | 516 | 59 | 11.4 | 612 | 56 | 9.2 |
To determine whether Polκ acts in TLS opposite 5R,6S Tg alone or together with another DNA Pol, next we examined whether depletion of Polζ had an effect on TLS frequencies. As shown in Table 1, depletion of either the Rev3 catalytic subunit or the Rev7 accessory subunit conferred a reduction in TLS frequencies similar to that seen upon Polκ depletion, which suggested that Pols κ and ζ collaborate in promoting TLS opposite the Tg lesion. To verify this possibility, we examined the effects of simultaneous depletion of Polκ with Rev3 and of Polκ with Rev7. Because the reduction in TLS frequencies observed upon the simultaneous depletion of Pols κ and ζ is very similar to those observed upon depletion of either Pol, this epistatic interaction signifies that the two Pols act together in promoting replication through the 5R,6S Tg lesion.
Biochemical studies have suggested that human TLS Pols can react differently to the 5R,6S vs. 5S,6R isoforms of Tg; for example, the 5R,6S stereoisomer is less inhibitory to TLS by Polη than the 5S,6R form (7), and Polκ misincorporates a G with a higher frequency opposite the 5R,6S form (11). Hence, we verified whether our conclusion for the combined role of Polκ and Polζ in TLS opposite the 5R,6S form applies also to the 5S,6R form. As shown in Table 2, TLS opposite the 5S,6R form carried on the leading strand template occurs as frequently as opposite the 5R,6S isoform and the various TLS Pols operate very similarly opposite both forms of Tg lesion. Thus, whereas depletion of Polη or Polι has no effect, depletion of either Polκ or Polζ confers an over 50% reduction in TLS frequencies opposite the 5S,6R form as well.
Table 2.
Effects of siRNA knockdowns of TLS Pols on the replicative bypass of 5S,6R Tg lesion carried on the leading strand template in human cells
| siRNA | # Kan+ colonies | # blue colonies among Kan+ | TLS, % |
| NC | 475 | 115 | 24.2 |
| Polη | 326 | 77 | 23.6 |
| Polι | 312 | 79 | 25.3 |
| Polκ | 512 | 57 | 11.1 |
| Rev3 | 612 | 65 | 10.6 |
| Rev7 | 586 | 60 | 10.2 |
Pols κ and ζ Mediate Error-Free Bypass of a Tg Lesion.
Next, we determined the mutagenicity of TLS opposite Tg in normal human cells. We find that opposite the 5R,6S isoform TLS occurs in a predominantly error-free manner regardless of whether the lesion is on the leading or on the lagging strand DNA template, as only ∼2% of TLS events had incurred mutational changes that resulted from an insertion of a G, C, or T opposite the Tg site rather than the correct A (Table 3). As expected from the lack of involvement of Polη or Polι in TLS opposite Tg, the incidence of mutational TLS events was not affected upon knockdown of either of these Pols. Interestingly, the knockdowns of either Polκ or of Rev3 or of Rev7 conferred an over 2-fold increase in mutagenic TLS events as the mutation frequencies rose to ∼5% (Table 3). In particular, a T is incorporated somewhat more frequently in cells depleted of Polκ or Polζ than in normal cells (Table 3). Because the frequency and pattern of TLS events (mutagenic or nonmutagenic) are very similar for the lesion carried on either the leading or the lagging template strands, pooling the data for the two strands from control (NC) siRNA treated cells shows 8 TLS events resulting from T insertion among a total of 852 TLS, whereas in Polκ-depleted cells 22 T insertions were observed among a total of 888 TLS events, and in cells depleted of either of the subunits of Polζ, 22 T insertions occurred among the 861 TLS events. A χ2 comparison of these data for control cells vs. Polκ-depleted cells or for control cells vs. Polζ-depleted cells indicates that the increase in the frequency of T insertion in Polκ- or Polζ-depleted cells is significantly different (p < 0.025) from that in normal cells.
Table 3.
Effects of siRNA knockdowns of TLS Pols on mutation frequencies and nucleotides inserted opposite a 5R,6S Tg carried on the leading or the lagging strand DNA template in human cells
| Nucleotide inserted | ||||||||
| DNA strand | siRNA | # of Kan+ blue colonies sequenced* | A | G | C | T | Other† | Mutation frequency (%) |
| Leading | NC | 356 (8) | 348 | 2 | 2 | 3 | 1 | 2.2 |
| Polη | 164 (4) | 160 | 1 | 1 | 2 | 0 | 2.4 | |
| Polι | 192 (5) | 187 | 1 | 1 | 3 | 0 | 2.6 | |
| Polκ | 392 (17) | 375 | 4 | 2 | 9 | 2 | 4.3 | |
| Rev3 | 213 (10) | 203 | 2 | 3 | 4 | 1 | 4.7 | |
| Rev7 | 268 (14) | 254 | 3 | 2 | 8 | 1 | 5.2 | |
| Lagging | NC | 496 (10) | 486 | 2 | 2 | 5 | 1 | 2.0 |
| Polη | 224 (5) | 219 | 1 | 1 | 3 | 0 | 2.2 | |
| Polι | 144 (3) | 141 | 0 | 0 | 2 | 1 | 2.1 | |
| Polκ | 496 (23) | 473 | 4 | 3 | 13 | 3 | 4.6 | |
| Rev3 | 208 (9) | 199 | 1 | 2 | 5 | 1 | 4.3 | |
| Rev7 | 172 (8) | 164 | 1 | 2 | 5 | 0 | 4.7 | |
*Numbers of mutational TLS events are shown in parenthesis.
†Mutation at the flanking 5′T to C.
We also tested the roles of TLS Pols in mutagenic vs. error-free lesion bypass opposite the 5S,6R isoform carried on the leading strand template. As shown in Table 4, opposite this stereoisomer also only ∼2% of TLS events were mutagenic, and the frequency of mutagenic TLS rose to ∼5% upon siRNA knockdown of either Polκ or Polζ. Hence we conclude that TLS Pols κ and ζ mediate predominantly error-free TLS opposite both the 5R,6S and 5S,6R isoforms of Tg.
Table 4.
Effects of siRNA knockdowns of TLS Pols on mutation frequencies and nucleotides inserted opposite a 5S,6R Tg carried on the leading strand DNA template in human cells
| Nucleotide inserted | |||||||
| siRNA | # of Kan+ blue colonies sequenced* | A | G | C | T | Other† | Mutation frequency (%) |
| NC | 380 (9) | 371 | 2 | 2 | 4 | 1 | 2.4 |
| Polη | 188 (4) | 184 | 1 | 0 | 3 | 0 | 2.1 |
| Polι | 190 (4) | 186 | 1 | 1 | 2 | 0 | 2.1 |
| Polκ | 238 (12) | 226 | 2 | 1 | 7 | 2 | 5.0 |
| Rev3 | 192 (9) | 183 | 2 | 2 | 4 | 1 | 4.7 |
| Rev7 | 240 (11) | 229 | 1 | 2 | 6 | 2 | 4.6 |
*Numbers of mutational TLS events are shown in parenthesis.
†Mutation at the flanking 5′T to C.
Discussion
Role of TLS in Promoting Replication Through a Tg Lesion.
Here we show that TLS in normal human cells can account for ∼20–25% of replicative bypass of a Tg lesion. The overall contribution of TLS to Tg bypass, however, may be significantly higher because in repair proficient cells, the lesion could have been removed from the duplex plasmid by BER or NER before its transit through the replication fork. Previously, from our studies with cis-syn TT dimer and a (6-4) TT photoproduct, we showed that whereas in NER defective human cells TLS opposite both these lesions contributes to ∼30–40% of lesion bypass, in NER proficient human cells TLS accounts for ∼20% of lesion bypass (19, 20, 22).
Opposite the Tg lesion, TLS on the leading DNA strand occurs only somewhat more frequently then on the lagging strand; however, the differences in TLS frequencies on the two strands are rather small and not statistically significant. Although TLS opposite a cis-syn TT dimer and (6-4) TT photoproduct was observed to occur somewhat more frequently on the leading strand than on the lagging strand, opposite both these lesions also TLS accounted for a very significant fraction of lesion bypass on both the DNA strands (19, 20). Furthermore, from the observations that the genetic control of TLS on the two DNA strands is almost identical for the Tg lesion, and a similar phenomenon is observed for TLS opposite a cis-syn TT dimer and a (6-4) TT photoproduct (19, 20), we conclude that TLS makes a prominent contribution to lesion bypass on both the DNA strands and that similar genetic mechanisms modulate TLS on the two strands.
From studies of replication products of SV40 DNA in UV irradiated human cells, and from the analysis of replication products from human cell-free extracts replicating SV40-derived plasmids, it has been inferred that a UV lesion located on the leading strand template presents a severe block to replication fork movement, whereas a UV lesion on the lagging strand does not significantly inhibit replication fork progression but inhibits the completion of Okazaki fragments (23, 24). Because the gap remaining in the Okazaki fragment opposite the DNA lesion could be filled in by template switching mechanisms using the newly synthesized leading strand as the template for synthesis, one might have anticipated TLS to be more active on the leading strand than on the lagging strand, because that could have provided for a more rapid completion of replication through the lesion, whereas a gap on the lagging strand could have been subsequently filled in by template switching. However, our observations that TLS occurs nearly equally frequently and has the same genetic control on the two DNA strands raises the possibility that when the replication fork stalls at a lesion site on either DNA strand, the action of the TLS Pols is somehow coordinated with the stalled replisome, irrespective of whether the lesion is located on the leading or the lagging DNA strand.
TLS Promotes Predominantly Error-Free Bypass Through a Tg Lesion.
Here we show that in human cells replicatve bypass of a Tg lesion occurs in a predominantly error-free fashion as only ∼2% of TLS events incur mutations that result from the incorporation of a C, a G, or a T instead of an A. Although because of the low fidelity of TLS Pols it has been assumed that lesion bypass by them would be a highly mutagenic process, and this idea may be generally valid for Escherichia coli and to a lesser extent for yeast (25), our studies in human cells have pointed to a more error-free role of TLS in promoting lesion bypass during replication. Similar to the observations of predominantly error-free TLS through a Tg lesion, we showed previously that human TLS Pols promote predominantly error-free replication through a cis-syn TT dimer as well as a (6-4) TT photoproduct (19, 20). Although different sets of TLS Pols promote replication through these UV induced lesions and through the Tg lesion, our observations that mutations occur in only ∼2% of TLS events opposite any of these lesions would suggest that in human and other mammalian cells, mechanisms have evolved that adapt TLS Pols to act in a more highly error-free fashion than could have been predicted from their intrinsic fidelities.
Pols κ and ζ Function Together in Promoting Error-Free Replicative Bypass of a Tg Lesion in Human Cells.
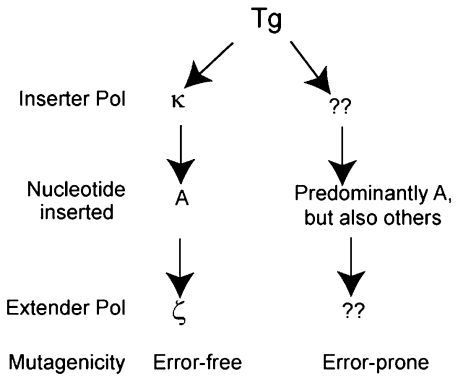
From studies with siRNA knockdowns of Pols η, ι, κ, and ζ, singly and in combinations, we conclude a role for Pols κ and ζ in promoting replication through a Tg lesion. However, because depletion of either Polκ, or of the Rev3 or Rev7 subunit of Polζ confers an ∼50%–60% reduction in TLS opposite the lesion carried on either the leading or the lagging DNA strand, we are led to infer the additional involvement of as yet unidentified Pol(s) in TLS opposite this lesion (Fig. 2). Hence, we propose that TLS through a Tg lesion occurs via a Polκ/ζ-dependent pathway and via another pathway mediated by Pols that remain to be identified.
Fig. 2.
Pathways for replicative bypass of a Tg (5R,6S or 5S,6R) lesion in human cells. The combined action of Polκ and Polζ, in which Polκ inserts an A opposite Tg and Polζ extends from the Tg:A base pair, promotes error-free replication through the lesion. In the alternative pathway controlled by TLS Pols that remain to be identified, although A is the predominant nt incorporated, other nts, in particular a T, are also inserted but with a low frequency; hence the alternative pathway is somewhat more mutagenic.
Because biochemical studies with human Polκ have indicated that it is more proficient at the insertion step than at the extension step (11), and biochemical studies with yeast Polζ have shown that it is highly proficient at extending from an A placed opposite Tg (6), we propose a role for Polκ at the insertion step and of Polζ at the extension step of Tg bypass (Fig. 2). A Tg lesion is a block to replication at both the insertion and extension steps because the C5 methyl group protrudes in the axial direction that prevents the base 5′ to the Tg lesion from stacking above it and that weakens the ability of the 5′ template base for Watson–Crick hydrogen bonding (8–10). The structure of Polκ bound to DNA and dNTP suggests that it is well suited for inserting an A opposite the Tg lesion (26). The axial orientation of the C5 methyl group is unlikely to impinge on the 5′ base in the Polκ active site because the 5′ unpaired template strand is directed out of the active site cleft and is stabilized via interactions with the residues in the N-clasp of Polκ; additionally, the Tg lesion could be stabilized via interactions between its C5 methyl group and Met135 emanating from the fingers domain in Polκ active site (26). Because Polζ promotes proficient extension from nts opposite a variety of DNA lesions that distort the DNA helix and/or impair Watson–Crick base pairing, we presume that the inability of the 5′ template base pair to properly stack above the Tg:A base pair and to form Watson–Crick hydrogen bonding is also not inhibitory for synthesis by Polζ.
Although TLS opposite a Tg lesion generates only ∼2% mutational events, our observations that this error frequency increases by over 2-fold in cells depleted of either Polκ or Polζ would suggest that Pols κ and ζ mediate a more error-free mode of TLS than that mediated by the other as yet unidentified pathway (Fig. 2). The identification of Pols involved in the alternative TLS pathway could be important to establish the degree of error-free lesion bypass by Pols κ and ζ, because then one could determine whether upon the inactivation of the alternative pathway, TLS becomes so highly error-free that almost no mutational events are recovered.
Other Considerations.
Our observations that Pols κ and ζ promote predominantly error-free replication through a Tg lesion raise a number of important issues. Because biochemical studies have indicated that Polη is highly efficient at inserting an A opposite the Tg (5R,6S) lesion and it extends from the Tg:A base pair with only an ∼15-fold reduction in efficiency (7), whereas Polκ inserts an A opposite the Tg (5R,6S) lesion with an ∼50-fold reduction in efficiency and is inhibited at extending from the Tg:A base pair by ∼250-fold (11), one would have expected Polη to function in TLS opposite the Tg lesion because then this Pol alone could have carried out both the insertion and extension steps. What then might have been the underlying basis for human cells to have evolved a strategy whereby they utilize two different polymerases—Polκ and Polζ, instead of the highly efficient Polη to perform TLS opposite the Tg lesion. These considerations might suggest that human and other mammalian cells have adapted the TLS machinery such that a more error-free Pol or a combination of Pols is utilized over a more efficient but also more error-prone Pol. Thus, although Polη is very efficient at both the insertion and extension steps of TLS opposite the Tg lesion, it also is quite error-prone as it inserts a G opposite Tg and extends from the Tg:G base pair with only an ∼8–10 fold reduction in efficiency (7). Polκ on the other hand is reduced by ∼3,000-fold in inserting a G opposite the 5R,6S isomer compared to insertion of an A opposite undamaged T (11). Hence, we presume that mechanisms exist in human cells that enable Polκ and not Polη to function in TLS opposite the Tg lesion. Also, because Polκ is inhibited at the extension step, Polζ-mediated extension from the Tg:A base pair would lead to error-free lesion bypass.
How could an inherently mutagenic process such as TLS be made to act in a predominantly error-free manner in human cells? Whereas the basic structural features could account for the specificity, and to a certain degree for the efficiency and fidelity of lesion bypass by human TLS Pols, as for example, the ability of Polη to accommodate the two templating residues of a cis-syn TT dimer in its active site (27, 28), or the ability of Polι to push the templating purine bases into a syn conformation and the resultant Hoogsteen base pairing (17, 29, 30), or the structural features of Polκ that could allow it to insert an A opposite Tg (26), the catalytic efficiencies and fidelities of these TLS Pols may be further modulated by proteins that these Pols associate with to carry out TLS in conjunction with the replication ensemble. Hence we presume that whereas such protein–protein interactions improve the catalytic efficiency and fidelity of Polκ opposite Tg, they might have a more negative impact upon Polη’s ability to act opposite Tg. Such a conjecture implies that in humans and presumably in other mammalian cells, a complex network of protein–protein interactions affects the roles of TLS Pols such that an intrinsically less efficient but more error-free Pol is selected to carry out lesion bypass over a Pol that is more efficient but also more error-prone.
In summary, in spite of their intrinsic error-proneness, the mutagenic contributions of TLS Pols to the replicative bypass of DNA lesions are minimized not just by limiting their access to the replication ensemble only to when the replisome stalls at a lesion site and proliferating cell nuclear antigen (PCNA) is ubiquitylated (12), but also by their structural features providing for a high degree of specificity in lesion bypass. Protein–protein interactions and other modifications could additionally contribute to enhance their catalytic efficiency and fidelity opposite DNA lesions.
Materials and Methods
Construction of Plasmid Vectors Containing a Thymine Glycol.
The 16-mer oligonucleotides containing the (5R, 6S) or the (5S, 6R) isomer of thymine glycol (Fig. 1A) were synthesized and purified. (for detailed information on method see SI Text). The in-frame target sequences of lacZ′ gene in the resulting vectors are shown in Fig. 1B. Because in the DNA strand containing the Tg lesion, the lacZ′ gene sequence is in-frame (Fig. 1B) and this strand carries also the kan+ gene (Fig. 1C), TLS through the Tg lesion will produce a blue colony on LB/kan plates with IPTG and X-gal. Because the other DNA strand has the SpeI sequence opposite the lesion and carries the kan- gene (Fig. 1C), and the lacZ′ sequence is out of frame in this strand (Fig. 1B), replication by template switching would generate white colonies.
Translesion Synthesis Assays and Mutational Analyses of TLS Products from Human Cells.
Human fibroblast cells were plated in 6-well plates at 70% confluence (approximately, 3 × 105 cells per well) and transfected with 100 pmole duplex siRNAs and the efficiency of siRNA inhibition of TLS Pols (Fig. S1) determined as described (19). For the simultaneous siRNA knockdown of two genes, 100 pmole siRNAs for each gene were mixed and transfected. After 48 h incubation, the heteroduplex target vector DNA (1 μg) and 50 pmole of siRNA (second transfection) were cotransfected with Lipofectamine 2000 (Invitrogen). After 30 h incubation, plasmid DNA was rescued from cells by the alkaline lysis method and digested with DpnI to remove unreplicated plasmid DNA. The plasmid DNA was then transformed into E. coli XL1-Blue super competent cells (Stratagene). Transformed bacterial cells were diluted in 1 mL super optimal broth with catabolite repression (SOC) media and plated on both LB/amp (50 μg/mL ampicillin, Sigma) and LB/kan (25 μg/mL kanamycin, Sigma) plates containing 1 μM isopropyl-1-thio-β-D-galactopyranoside (IPTG) (Roche) and 100 μg/mL of X-Gal (Roche). After 16 h incubation at 37 °C, blue and white colonies were counted from kanamycin plates. The TLS frequency was determined from the number of blue colonies out of total colonies growing on LB/kan plates. Plasmid DNA obtained from blue colonies was analyzed to determine the mutation frequency and the mutational changes incorporated during TLS. The details of methods for construction of lesion-containing duplex plasmids, for siRNA knockdowns and for checking that the depletion of various TLS Pols was highly efficient, and for TLS and mutational analyses in human cells have been described previously (19).
Supplementary Material
Acknowledgments.
We thank Dr. Richard Hodge (UTMB, Galveston, TX) for providing Tg containing DNAs, and Dr. Thomas Wood (UTMB, Galveston, TX) for sequencing the TLS products. This work was supported by National Institutes of Environmental Health Sciences (NIEHS) Grant ES012411. The Tg containing DNAs were constructed by Dr. Richard Hodge in the Synthetic Organic Chemistry Core Laboratory at UTMB, supported by NIEHS Center Grant P30-ES06676.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007795107/-/DCSupplemental.
References
- 1.Adelamn R, Saul RL, Ames BN. Oxidative damage to DNA: Relation to species metabolic rate andlife span. Proc Natl Acad Sci USA. 1988;85:2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenkel K, Goldstein MS, Teebor GW. Identification of the cis-thymine glycol moeity in chemically oxidized and gamma-irradiated deoxyribonucleotic acid by high-pressure liquid chromatography analysis. Biochemistry. 1981;20:7566–7571. doi: 10.1021/bi00529a035. [DOI] [PubMed] [Google Scholar]
- 3.Teoule R, Bert C, Bonicel A. Thymine fragment damage retained in the DNA polynucleotide chain after gamma irradiation in aerated solutions. II. Radiat Res. 1977;72:190–200. [PubMed] [Google Scholar]
- 4.Ikeda S, et al. Purification and characterization of human NTH1, a homolog of Escherichia coli endonuclease III. Direct identification of Lys-232 as the active nucleophilic residue. J Biol Chem. 1998;273:21585–21593. doi: 10.1074/jbc.273.34.21585. [DOI] [PubMed] [Google Scholar]
- 5.Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: Possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc Natl Acad Sci USA. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RE, Yu S-L, Prakash S, Prakash L. Yeast DNA polymerase zeta (ζ) is essential for error-free replication past thymine glycol. Gene Dev. 2003;17:77–87. doi: 10.1101/gad.1048303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusumoto R, Masutani C, Iwai S, Hanaoka F. Translesion synthesis by human DNA polymerase η across thymine glycol lesions. Biochemistry. 2002;41:6090–6099. doi: 10.1021/bi025549k. [DOI] [PubMed] [Google Scholar]
- 8.Kao JY, Goljer I, Phan TA, Bolton PH. Characterization of the effects of a thymine glycol residue on the structure, dynamics, and stability of duplex DNA by NMR. J Biol Chem. 1993;268:17787–17793. [PubMed] [Google Scholar]
- 9.Kung HC, Bolton PH. Structure of a duplex DNA containing a thymine glycol residue in solution. J Biol Chem. 1997;272:9227–9236. doi: 10.1074/jbc.272.14.9227. [DOI] [PubMed] [Google Scholar]
- 10.McNulty JM, Jerkovic B, Bolton PH, Basu AK. Replication inhibition and miscoding properties of DNA templates containing a site-specific cis-thymine glycol or urea rsidue. Chem Res Toxicol. 1998;11:666–673. doi: 10.1021/tx970225w. [DOI] [PubMed] [Google Scholar]
- 11.Fischhaber PL, et al. Human DNA polymerase κ bypasses and extends beyond thymine glycols during translesion synthesis in vitro, preferentially incorporating correct nucleotides. J Biol Chem. 2002;277:37604–37611. doi: 10.1074/jbc.M206027200. [DOI] [PubMed] [Google Scholar]
- 12.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 13.Nelson JR, Lawrence CW, Hinkle DC. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RE, Haracska L, Prakash S, Prakash L. Role of DNA polymerase h in the bypass of a (6-4) TT photoproduct. Mol Cell Biol. 2001;21:3558–3563. doi: 10.1128/MCB.21.10.3558-3563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haracska L, et al. Roles of yeast DNA polymerases δ and ζ and of Rev1 in the bypass of abasic sites. Gene Dev. 2001;15:945–954. doi: 10.1101/gad.882301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Hoogsteen base pair formation promotes synthesis opposite the 1,N6-ethenodeoxyadenosine lesion by human DNA polymerase ι. Nat Struct Mol Biol. 2006;13:619–625. doi: 10.1038/nsmb1118. [DOI] [PubMed] [Google Scholar]
- 18.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Protein-template directed synthesis across an acrolein-derived DNA adduct by yeast Rev1 DNA polymerase. Structure. 2008;16:239–245. doi: 10.1016/j.str.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Yoon J-H, Prakash L, Prakash S. Highly error-free role of DNA polymerase h in the replicative bypass of UV induced pyrimidine dimers in mouse and human cells. Proc Natl Acad Sci USA. 2009;106:18219–18224. doi: 10.1073/pnas.0910121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon J-H, Prakash L, Prakash S. Error-free replicative bypass of (6-4) photoproducts by DNA polymerase ζ in mouse and human cells. Gene Dev. 2010;24:123–128. doi: 10.1101/gad.1872810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig MJ, Cadet J, Boorstein RJ, Teebor GW. Synthesis of the diasteromers of thymidine glycol, determination of concentrations and rates of interconversion of theis cis-trans epimers at equilibrium and demonstration of differential alkali lability within DNA. Nucleic Acids Res. 1992;20:4839–4845. doi: 10.1093/nar/20.18.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acharya N, Yoon J-H, Hurwitz J, Prakash L, Prakash S. DNA polymerase η lacking the ubiquitin-binding domain promotes replicative lesion bypass in human cells. Proc Natl Acad Sci USA. 2010;107:10401–10405. doi: 10.1073/pnas.1005492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezzina M, Mench CFM, Courtin P, Sarasin A. Replication of simian virus 40 DNA after UV irradiation: Evidence of growing fork blockage and single-stranded gaps in daughter strands. J Virol. 1988;62:4249–4258. doi: 10.1128/jvi.62.11.4249-4258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarasin AR, Hanawalt PC. Replication of ultraviolet-irradiated simian virus 40 in monkey kidney cells. J Mol Biol. 1980;138:299–319. doi: 10.1016/0022-2836(80)90288-0. [DOI] [PubMed] [Google Scholar]
- 25.Bresson A, Fuchs RPP. Lesion bypass in yeast cells: Pol η participates in a multi-DNA polymerase process. EMBO J. 2002;21:3881–3887. doi: 10.1093/emboj/cdf363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lone S, et al. Human DNA polymerase κ encircles DNA: Implications for mismatch extension and lesion bypass. Mol Cell. 2007;25:601–614. doi: 10.1016/j.molcel.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein TD, et al. Structural basis for the suppression of skin cancers by DNA polymerase η. Nature. 2010;465:1039–1044. doi: 10.1038/nature09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trincao J, et al. Structure of the catalytic core of S. cerevisiae DNA polymerase h: implications for translesion DNA synthesis. Mol Cell. 2001;8:417–426. doi: 10.1016/s1097-2765(01)00306-9. [DOI] [PubMed] [Google Scholar]
- 29.Nair DT, Johnson RE, Prakash S, Prakash L, Aggarwal AK. Replication by human DNA polymerase ι occurs via Hoogsteen base-pairing. Nature. 2004;430:377–380. doi: 10.1038/nature02692. [DOI] [PubMed] [Google Scholar]
- 30.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Human DNA polymerase ι incorporates dCTP opposite template G via a G. C+ Hoogsteen base pair. Structure. 2005;13:1569–1577. doi: 10.1016/j.str.2005.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.