Abstract
Resolvin-E1 (RvE1) has been demonstrated to promote inflammatory resolution in numerous disease models. Given the importance of epithelial cells to coordination of mucosal inflammation, we hypothesized that RvE1 elicits an epithelial resolution signature. Initial studies revealed that the RvE1-receptor (ChemR23) is expressed on intestinal epithelial cells (IECs) and that microarray profiling of cells exposed to RvE1 revealed regulation of inflammatory response gene expression. Notably, RvE1 induced intestinal alkaline phosphatase (ALPI) expression and significantly enhanced epithelial ALPI enzyme activity. One role recently attributed to ALPI is the detoxification of bacterial LPS. In our studies, RvE1-exposed epithelia detoxified LPS (assessed by attenuation of NF-κB signaling). Furthermore, in epithelial-bacterial interaction assays, we determined that ALPI retarded the growth of Escherichia coli. To define these features in vivo, we used a murine dextran sulfate sodium (DSS) model of colitis. Compared with vehicle controls, administration of RvE1 resulted in significant improvement of disease activity indices (e.g., body weight, colon length) concomitant with increased ALPI expression in the intestinal epithelium. Moreover, inhibition of ALPI activity resulted in increased severity of colitis in DSS-treated animals and partially abrogated the protective influence of RvE1. Together, these data implicate a previously unappreciated role for ALPI in RvE1-mediated inflammatory resolution.
Keywords: colitis, endotoxin, lipid mediator, mucosal, epithelia
Epithelial cells, which line mucosal organs, are uniquely positioned to serve as a direct line of communication between the immune system and the external environment (1). In their normal state, mucosal surfaces of the alimentary tract are exposed on the luminal surface to high concentrations of foreign antigens while intimately associated with the immune system via subepithelial lymphoid tissue. Consequently, the epithelium forms an important barrier, preventing the free mixing of luminal antigenic material with the lamina propria, which houses the mucosal immune system.
It is now appreciated that epithelial cells contribute significantly to coordinated inflammatory responses (2, 3). Over the past decade, the study of endogenous mediators and mechanisms of inflammation resolution have come to the forefront (4). Resolvins, protectins, and, most recently, maresins have all been demonstrated to promote resolution of ongoing inflammation (5–7). Studying these lipids as potential therapeutic modalities has advantages, including localized synthesis at sites of inflammation, high potency, and rapid degradation resulting in low endogenous toxicity (8, 9). Resolvin E1 [RvE1 (5S,12R,18R-trihydroxyeicosapentaenoic acid)], a derivative of omega-3 fatty acid, contributes to resolution of inflammation via interactions with the resolvin E1 receptor, (also called ChemR23) (10, 11). RvE1 is generated at sites of inflammation through transcellular metabolism and has been shown to potently inhibit neutrophil (PMN) transendothelial migration (12), to attenuate colonic mucosal inflammation in vivo (13, 14), and to resolve oral inflammation in a rabbit periodontitis model (15). Although most of the focus has been on the influence of RvE1 on neutrophil activity/transmigration and macrophage phagocytosis (9), relatively little is known about the influence of RvE1 on cells other than leukocytes.
Given that RvE1 biosynthesis requires the presence of PMN 5-lipoxygenase and endothelial/epithelial cyclooxygenase-2 (COX-2), we hypothesized that RvE1 exerts proresolution influences via direct actions on epithelial cells. Guided initially by microarray analysis, we identified the prominent induction of the epithelial surface enzyme intestinal alkaline phosphatase (ALPI) in RvE1-stimulated intestinal epithelia. Extensions of these studies revealed that ALPI promotes inflammatory resolution in part through the detoxification of bacterial endotoxin and that RvE1-elicited ALPI promotes protection in a murine model of mucosal disease (colitis). We demonstrate that epithelial RvE1 signaling may contribute to resolution through the induction of surface ALPI. These results suggest that epithelia may actively contribute to inflammatory resolution via RvE1 signaling.
Results
Intestinal Epithelia Transduce RvE1 Signals.
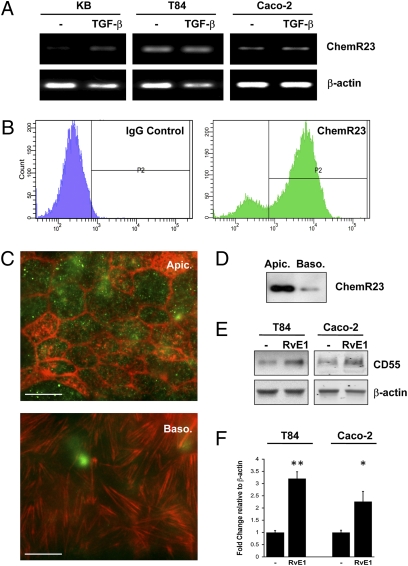
Resolvin E1 has been demonstrated to bind to and signal through ChemR23 and BLT1 receptors on neutrophils (16). Our previous studies revealed that oral epithelia (KB cells) express ChemR23 at low or even undetectable levels and that TGF-β induces such expression (17). Here, we examined other epithelial sources and whether such expression was regulated. T84 and Caco-2 intestinal epithelia (Fig. 1A) expressed ChemR23 transcript at high basal levels and such expression was not changed by TGF-β, furthermore, even though BLT1 receptor expression has been demonstrated in Caco-2 cells (18), we detected only very low levels (Fig. S1). Cell surface expression of the ChemR23 receptor in IECs was confirmed by flow cytometry (Fig. 1B) wherein ChemR23 protein was observed to be expressed at high levels on both Caco-2 and T84 cells, with a higher endogenous expression in T84 cells (83.7% of the population stained positive for ChemR23).
Fig. 1.
Intestinal epithelia express the RvE1-receptor and transduce Resolvin E1 signals.(A) RT-PCR for human ChemR23 expression in oral (KB) and intestinal epithelial cells (IECs: Caco-2 and T84). KB cells expressed ChemR23 in response to 10 ng/mL TGF-β treatment, higher basal levels of ChemR23 were detected in IECs but were not observably enhanced by TGF-β. (B) Cell surface expression of ChemR23 in IECs was confirmed by flow cytometry using anti-human ChemR23 (green) or control mouse IgG (purple). (C) Immunofluorescent microscopy of ChemR23 (green) and F-actin (red), demonstrating a predominantly apical receptor compartmentalization in T84 cells (100× magnification). (Scale bars: 10 μm.) (D) Differential biotinylation of Caco-2, apical- vs. basolateral-enriched cell surface protein isolation, blotted for ChemR23. (E) IEC transduction of RvE1-ChemR23 signals, demonstrated by an up-regulation of CD55. IECs were exposed to 100 nM RvE1 for 24 h and CD55 expression was analyzed by Western blot. (F) Densitometry for CD55 induction, graphed as mean changes in OD relative to β-actin. Data were analyzed by Student t test and are presented as mean ± SE (n = 3) (*P < 0.05, **P < 0.01).
Immunofluorescence microscopy was used to ascertain cell surface distribution of ChemR23. Confluent monolayers of T84 were grown on collagen-coated glass coverslips, fixed, permeabilized, probed with anti-human ChemR23, and counterstained with rhodamine-phalloidin. Punctate staining for ChemR23 was observed in-plane with junctional actin (Fig. 1C, Upper). Actin stress fibers were observed at the basolateral aspect (Fig. 1C Lower), but no ChemR23 staining was detectable below epithelial tight junctions. Immunofluorescence studies revealed that ChemR23 colocalizes with CD55, an apically expressed GPI-linked protein (19, 20). Differential biotinylation of apical vs. basolateral surface proteins was performed on Caco-2 cells, blotting for ChemR23 revealed enrichment on apical membranes (Fig. 1D). These findings indicate that ChemR23 is expressed at high levels in intestinal epithelia and is predominantly localized to the apical surface.
To determine whether ChemR23 transduces RvE1 signaling, we used the induction of CD55 as an endpoint. We previously established that KB cells overexpressing the ChemR23 receptor (KB-st) prominently and selectively induced CD55 expression upon RvE1 exposure (17). Here, we exposed IECs to RvE1 (100 nM for 24 h) and assessed CD55 protein levels by Western blot. RvE1 induced CD55 by 3.21 ± 0.28- (P < 0.01) and 2.26 ± 0.42-fold (P < 0.05) in T84 and Caco-2 cells, respectively (Fig. 1 E and F). Thus, surface-expressed ChemR23 readily transduces RvE1 signals in IECs.
RvE1 Stimulates Epithelial Expression of a Mucosal Protective Factor.
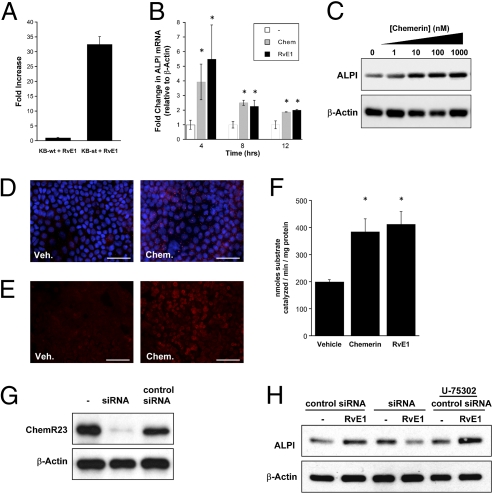
In previous work, we profiled the specific impact of RvE1 on mRNA transcripts by microarray (17). Interestingly, one of the transcripts specifically induced by RvE1 was intestinal alkaline phosphatase (ALPI)—a GPI-linked surface protein expressed primarily in mucosal epithelia (21). Indeed, microarray analysis revealed that oral epithelial ALPI was induced 32.5 ± twofold by RvE1 in KB cells over-expressing ChemR23 (Fig. 2A).
Fig. 2.
ALPI expression is up-regulated by RvE1 and chemerin. (A) Microarray analysis of RvE1 (100 nM) stimulated cells indicated a 32.5-fold induction of ALPI mRNA expression. (B) Quantitative PCR (qPCR) indicated that ALPI mRNA expression was significantly induced in a time-dependent manner in Caco-2 in response to RvE1 (100 nM) and chemerin (1 μM) treatment. Data expressed as fold-change ± SE (n = 3) (*P < 0.05). (C) Representative Western blot demonstrated a dose-dependent increase in ALPI protein levels, following exposure of Caco-2 monolayers to chemerin (0, 1, 10, 100, and 1000 nM). Corresponding β-actin is presented as a loading control. (D) Immunofluorescent microscopy revealed an increase in punctate staining for ALPI (red) in (1 μM) chemerin-treated Caco-2, nuclei were counterstained with DAPI (blue) (40× magnification). (Scale bar: 50 μm.) (E) Apical ALP activity in response to chemerin (1 μM) was demonstrated by FASTred staining, (20× magnification). (Scale bar: 100 μm.) (F) ALP activity following exposure to RvE1 (100 nM) or chemerin (1 μM) was quantified by colorimetric p-NPP assay with TLB lysates (*P < 0.05). (G) Validation of siRNA knockdown of ChemR23 in Caco-2. (H) Western blot of ALPI in control/siRNA transfected Caco-2. U-75302 (10 uM) was used to show specificity for ChemR23.
We extended these observations from oral to intestinal epithelia. A time course of RvE1 and chemerin (peptide ligand for ChemR23) exposure in Caco-2 cells (Fig. 2B) revealed a significant induction of ALPI mRNA at 4, 8, and 12 h of exposure. Such observations were confirmed by Western blot and revealed a dose-dependent increase of ALPI expression in response to chemerin (Fig. 2C). Immunofluorescent microscopy demonstrated enhanced staining for ALPI following chemerin stimulation in Caco-2 cells (Fig. 2D). To assess ALPI enzyme activity, histological alkaline phosphatase (ALP) (FASTred) (Fig. 2E) and biochemical pNitrophenylphosphate (pNPP) hydrolysis (Fig. 2F) ALP assays were used, respectively. Using both assay systems, significantly increased ALPI activity was observed in both chemerin and RvE1-exposed cells. To verify that ALPI-induction was ChemR23-mediated, we used RNAi to knockdown ChemR23 in Caco-2 cells (Fig. 2G). Although ALPI induction by RvE1 was readily detectable in control siRNA transfected cells, this induction was lost in ChemR23 knockdowns. Furthermore, treatment with U-75302 (a BLT1 antagonist) failed to attenuate the RvE1-mediated ALPI induction (Fig. 2H).
Epithelial ALPI Detoxifies Bacterial LPS.
Previous studies have implicated ALPI in the dephosphorylation of bacterial LPS (22, 23). Such dephosphorylation results in the conversion of bisphosphorylated LPS to a nontoxic, monophosphoryl form. We thus determined whether RvE1-stimulated IECs could detoxify LPS.
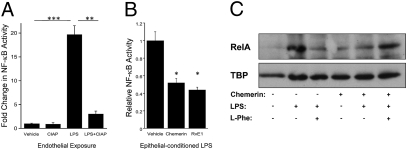
Initially, we developed an assay to examine the detoxifying bioactivity of ALPI. Exogenously administered LPS in the presence and absence of calf intestinal alkaline phosphatase (CIAP) was applied to human microvascular endothelial (HMEC) cells transfected with an NF-κB reporter assay. Significant stimulation of the NF-κB reporter was observed with LPS treatment (19.69 ± 1.84-fold increase over vehicle, P < 0.005) (Fig. 3A), which was nearly completely abolished by preincubation with CIAP (84.61 ± 3.13% decrease relative to LPS, P < 0.01). To determine if RvE1-induced epithelial ALPI could significantly detoxify LPS, a conditioned medium transfer assay was developed. Caco-2 IECs were first exposed to vehicle, chemerin, or RvE1 overnight to induce ALPI expression. Epithelia were consequently exposed for 4 h to fluorescent LPS in phenol-red-free media. To ensure equal carryover of LPS, an AlexaFluor-594 conjugated LPS was used. LPS of epithelial-conditioned media was quantified by fluorometry, concentrations normalized and applied to LPS-sensitive endothelial cells transfected with an NF-κB reporter gene assay. Such “conditioned” LPS derived from either chemerin or RvE1-stimulated epithelia resulted in significantly attenuated NF-κB activity (48 ± 5% and 56 ± 3%, respectively, P < 0.05 for both) (Fig. 3B), strongly implicating LPS detoxification by ChemR23 stimulation. Confirmation of NF-κB activity was achieved by probing endothelial nuclear preparations for RelA. Following exposure to conditioned LPS, RelA nuclear accumulation was attenuated in chemerin-treated epithelia. This effect was abrogated by coincubation with L-phenylalanine (L-Phe [ALPI inhibitor]) (24, 25) (Fig. 3C).
Fig. 3.
RvE-induced epithelial ALPI detoxifies LPS.(A) NF-κB activity was assessed in HMEC (endothelial) cells by luciferase-reporter assay, following exposure to 100 ng/mL LPS ± purified calf intestinal alkaline phosphatase (CIAP; 1 DEA unit/mL). CIAP significantly attenuated LPS-mediated NF-κB activity (**P < 0.01, ***P < 0.005). (B) LPS-transfer assay involved treatment of IECs to vehicle, chemerin (1 μM) or RvE1 (100 nM) overnight, followed by exposure to fluorescently labeled LPS for 4 h. LPS was quantified by fluorometry and applied to endothelial cells transfected with a NF-κB reporter. Conditioned media from (1 μM) chemerin- and (100 nM) RvE1-treated IECs significantly detoxified LPS (*P < 0.05). (C) Western blot of HMEC-1 nuclear RelA, following 10 min incubation in conditioned medium from Caco-2 ± 1 μM chemerin overnight and ± LPS (100 ng/mL) ± L-Phe (100 nM; ALPI inhibitor) for 4 h. TBP (TATA binding protein) was used as a loading control.
Epithelial ALPI Retards E. coli Growth.
ALP has been demonstrated to detoxify Gram-negative bacteria via dephosphorylation of the lipid A moiety of LPS (26). Thus, we sought to ascertain whether RvE1-induced epithelial ALPI could influence bacterial cell growth. Initially, we determined whether CIAP influenced growth of invasive and noninvasive Gram-positive and -negative bacteria (including Enterococcus faecalis, Salmonella typhimurium, E. coli, and Clostridium difficile). Of those bacteria tested, only E. coli growth was affected by CIAP (Fig. S2A). This growth retardation by CIAP was concentration-dependent and at higher concentrations (2 and 4 DEA units/mL) resulted in a significant inhibition (67.79 ± 3.4% and 31.15 ± 1.5%, respectively, P < 0.05 for both) of E. coli growth after 12 h (Fig. S2A). In extensions of these findings, treatment of Caco-2 IECs with RvE1 or chemerin overnight followed by inoculation with (approximately 5 × 107) E. coli resulted in a 43 ± 0.07% (P < 0.05) and 55 ± 0.05% (P < 0.005) decrease in cfu formation with RvE1 and chemerin, respectively (Fig. S2B). Direct treatment of bacteria with RvE1 or chemerin did not influence bacterial survival (P > 0.05). These findings may indicate a role for ALPI in maintenance of intestinal homeostasis by regulating E. coli growth To determine the contribution of ALPI to this influence on bacterial growth, Caco-2 cells were treated with RvE1 overnight, followed by inoculation with E. coli in the presence and absence of L-Phe. Inhibition of ALPI resulted in an incomplete abrogation of RvE1-mediated decrease in cfu formation (Fig. S2C), thus other mechanisms of RvE1-mediated bacterial killing are likely concomitantly involved.
RvE1 Ameliorates Murine DSS Colitis: The Role of ALPI.
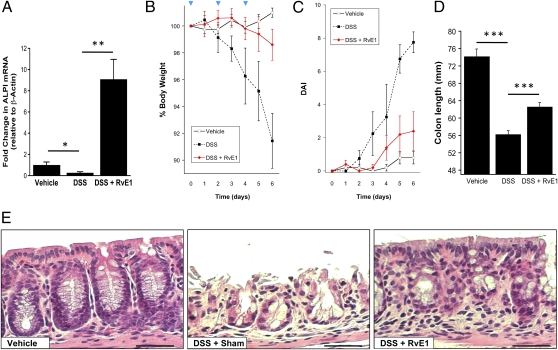
We next sought to examine the physiological relevance of RvE1-induced ALPI induction. To this end, we used the murine dextran sulfate sodium (DSS) model of colitis. Initial studies revealed that DSS significantly decreased ALPI mRNA expression by 73 ± 0.02% of vehicle control (P < 0.05) (Fig. 4A). The combination of DSS and RvE1 significantly induced colonic ALPI mRNA expression 9.08 ± 1.87-fold (P < 0.005) relative to vehicle control and 34.71 ± 8.5-fold (P < 0.005) relative to DSS (P < 0.001). These findings paralleled endpoints of colitis, namely that administration of RvE1 provided significant protection against DSS-induced weight loss (Fig. 4B, P < 0.05), disease activity indices (Fig. 4C, P < 0.01), and colonic shortening (Fig. 4D, P < 0.005). Histologically, a striking loss of epithelial cells and a distortion of crypt architecture were evident with DSS alone. RvE1 administration provided marked protection for the epithelium and somewhat protected the crypt architecture (Fig. 4F). Such observations provide a strong correlation between RvE1-induced ALPI and inflammatory resolution in DSS colitis.
Fig. 4.
RvE1 induces ALPI in vivo and is protective against DSS-induced murine colitis.(A) Colonic epithelial cells were isolated from vehicle, DSS and DSS with RvE1 injected animals. ALPI mRNA expression was quantified by qPCR (*P < 0.05, **P < 0.01). (B) Animal weights were recorded and expressed as % of starting weight. DSS caused significant loss of body weight, analyzed by two-way ANOVA (P < 0.01). DSS?treated animals did not differ significantly from vehicle mice. n = 5 mice/group. (C) DAI scoring and (D) colon lengths indicated that RvE1 was protective against colitis (***P < 0.005). (E) H&E staining of colonic sections highlight epithelial integrity of vehicle-treated animals, a striking loss of crypt architecture and epithelium with DSS. RvE1-treatment protects epithelial integrity (10× magnification). (Scale bar: 100 μm.)
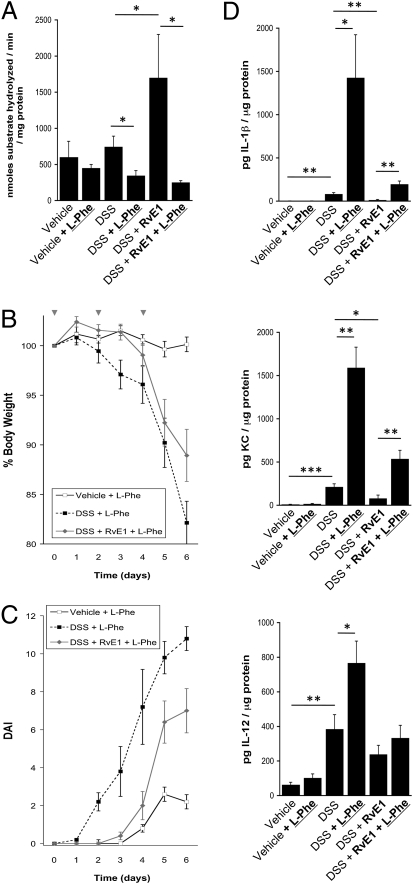
To define the role of ALPI in protection afforded by RvE1 in DSS colitis, we used orally administered L-Phe. As depicted in Fig. 5A, tissue harvest studies indicated no significant difference between vehicle and DSS-treated mice; however, a significant increase in ALP activity was observed in RvE1-treated animals (P < 0.05). Administration of L-Phe, revealed a significant loss of ALP activity elicited by L-Phe administration to either DSS or the combination of DSS and RvE1 (P < 0.05). Moreover, as shown in Fig. 5B, L-Phe reversed the protection from DSS-induced weight loss attributable to RvE1 (P < 0.05 compared with L-Phe alone). Furthermore, disease severity [measured by disease activity index (DAI)] was significantly different in DSS mice administered RvE1 and L-Phe, than vehicle mice (P < 0.01) (Fig. 5C). Similarly, as shown in Fig. 5D, tissue cytokine and chemokine profiling identified a prominent induction of IL-1β (38 ± 6-fold increase over vehicle, P < 0.01) and murine KC (human homolog of IL-8, 116 ± 17-fold increase over vehicle, P < 0.005) by DSS, both of which were nearly completely reversed by RvE1 (P < 0.01 and P < 0.05, respectively). Administration of the ALPI inhibitor L-Phe to colitic mice in combination with RvE1 resulted in a complete reversal of RvE1 protection (P < 0.01 for both). A similar trend was observed with IL-12 (Fig. 5D), with a significant induction with DSS exposure (P < 0.01) and higher induction with DSS and L-Phe together (P < 0.05); however, the protection afforded by RvE1 was not statistically significant. Taken together, these results indicate that ALPI significantly contributes to the proresolving qualities of RvE1 in this murine model of colitis.
Fig. 5.
ALPI-inhibitor ameliorates protective effects of RvE1 in DSS-induced murine colitis.(A) Oral administration of L-Phe, significantly inhibited tissue alkaline phosphatase activity. (B) Weight loss curves indicate that DSSPhe and DSS??Phe both lost significantly more weight during the time course than vehiclePhe-treated animals. (C) DAI scores indicate that L-Phe-treated DSS? mice disease severity was significantly different from vehicle mice (P < 0.05). (D) Mesoscale analysis of tissue cytokine levels revealed an increased IL-1β, KC, and IL-12 in DSS, which was reduced by RvE1 in all cases (not significant for IL-12). L-Phe increased the cytokine concentration in DSS and DSS? treated animals (*P < 0.05, **P < 0.01, ***P < 0.005).
Discussion
Mucosal epithelia provide highly specialized barrier between the host and luminal microenvironment. It is now appreciated that the maintenance of tolerance to commensal microorganisms present at the epithelial surface and the mounting of an appropriate inflammatory response is a delicate balancing act coordinated between the epithelium and immune cells of the lamina propria (27). The epithelium functions to coordinate not only inflammatory initiation but, as recently appreciated, also promotes the active resolution of inflammation (28). Of particular interest in this regard are lipid mediators, including those from arachidonic acid and omega-3 fatty acids, generated during cell–cell interactions in mucosal inflammation (29). Guided initially by microarray analysis, we identified the prominent induction of ALPI by RvE1 in intestinal epithelia. Extensions of these studies indicate both expression and function of the RvE1 receptor (ChemR23) on intestinal epithelia.
Previous studies have assigned a proresolving role for RvE1 predominantly on macrophage and neutrophil function (29). RvE1 generation at sites of inflammation requires transcellular biosynthesis involving acetylated endothelial/epithelial COX-2 (cyclooxygenase-2) and subsequent metabolism by PMN 5-lipoxygenase. Acetylation of COX-2 (e.g., with aspirin) inhibits its oxygenase activity but maintains its peroxidation function, thus favoring the generation of 18R-hydroxyeicosapentaenoic acid from eicosapentaenoic acid (EPA). Likewise, in the case of intestinal inflammation, the combination of low baseline pO2 and high O2 consumption by infiltrating PMN such conditions could favor COX-2 peroxidation of EPA in the absence of COX-2 acetylation (30). As RvE1 is generated within the microenvironment of inflammatory sites, we investigated whether cells other than leukocytes might respond to RvE1 through surface membrane signaling. Our previous work using heterologous expression systems had defined at least a minimal machinery for RvE1 signaling in oral epithelial cell lines, but native expression was low to undetectable (17). A screen of various epithelial sources revealed prominent expression of ChemR23 on human intestinal epithelial cell lines (T84 and Caco-2). Notable was the pattern of expression on polarized epithelia. These investigations revealed that ChemR23 localizes predominantly to the apical membrane surface, which was somewhat unexpected given that many other G protein-coupled receptors exhibit basolateral expression in polarized epithelia (31). Such membrane distribution of ChemR23 suggests that the localized generation of RvE1 during PMN-epithelial interactions could occur at the apical (luminal) aspect of the tissue. This is an intriguing possibility given that the other known function for RvE1 on mucosal epithelia is to promote the termination and clearance of PMN following transmigration (17), through well-characterized CD55-dependent mechanisms (19, 32). As such, the PMN-epithelial interactions that occur within the lumen of the intestine may initiate a proresolving signature to the epithelium during PMN transit through the mucosa.
Our studies using microarray analysis revealed the prominent induction of ALPI by RvE1 signaling in various epithelial sources. ALPI has long been used as a marker of gastrointestinal differentiation (21); its biological function, however, remained unclear. The intestinal isoform of ALPI that was identified by microarray was originally proposed to limit fat absorption, based on the phenotypic observation that ALPI−/− animals gain significantly more weight on a high-fat diet than wild-type mice (20, 33). However, ALPI, particularly that from calf intestine, has been linked to prevention of sepsis (23, 34, 35), via dephosphorylation of the lipid A moiety of LPS (22, 26). The resultant product of this reaction, monophosphoryl lipid A (MPLA) not only lacks toxicity associated with LPS but potentially may function as a potent immune adjuvant (36). Following the finding that RvE1-induced ALPI expression in IECs, we defined whether this signature represented a biologically significant role in detoxifying LPS. To do this required development of a bioassay. To circumvent the possibility that LPS could be internalized by epithelia, a functional and easily quantifiable source (fluorescently-labeled LPS) was used to define the role of RvE-1-induced ALPI on LPS detoxification. In conjunction with TLR4-expressing endothelia transfected with an NFκB reporter, this bioassay proved useful in defining a prominent role for surface expressed ALPI in the neutralization of the proinflammatory nature of LPS. In parallel, it was revealed that RvE1-induced, surface expressed ALPI killed E. coli. Although we do not know exact mechanisms of bacterial killing, Caccavo et al. (37) demonstrated that the glycoprotein lactoferrin is capable of exerting both bacteriostatic and bacteriocidal activities through binding to LPS lipid A. From these findings we propose that apically-expressed ALPI is capable not only of detoxifying LPS via lipid A dephosphorylation, but for limiting E. coli growth should it adhere to the epithelial surface.
As a proof of principle, we extended these findings to a mucosal inflammation model. In particular, we selected a murine model of colitis given the current understanding of inflammatory bowel disease as a multifaceted disorder, involving immune system dysregulation, genetic susceptibility, and environmental factors (i.e., luminal microbiota) (38). Several studies have investigated the association of ALPI expression with the microbiota. In zebrafish, for example, ALPI was found to be absent in germ-free animals but expression was induced following exposure to bacteria (39). Moreover, ALPI expression has been demonstrated to be maintained by enteral feeding (40) and has proven beneficial through unknown mechanisms in a rat colitis model (41). Furthermore, ALPI gene expression has been demonstrated to be suppressed or absent in ulcerative colitis and Crohn’s disease patients (41, 42). Mechanistically this may be due to a suppression of ALPI mRNA expression by the proinflammatory cytokines such as TNFα and IL-1β (43). Given that these cytokines have documented roles in human inflammatory bowel disease (IBD) and murine colitis models (44, 45), we hypothesized that ALPI expression would be lost in DSS-induced murine colitis, ultimately leading to enhanced response to bacterial LPS. Our findings support this hypothesis and demonstrate that parenteral administration of RvE1 significantly elevates intestinal ALPI and that such increases in ALPI are associated with attenuated colitis. A role for ALPI was demonstrated through the use of orally administered L-Phe, which to date is the most selective inhibitor of the intestinal isoform of ALP (46). At multiple levels, L-Phe was shown to be an inhibitor of ALPI and was demonstrated to almost completely reverse the protection afforded by RvE1 in DSS colitis. Such results strongly implicate RvE1-induced ALPI as an endogenous, surface expressed factor important in inflammatory resolution in the mucosa.
Taken together, these studies strongly suggest ALPI may be involved in the resolution of mucosal inflammation. Intestinal epithelial expression of apical ChemR23 could present a convenient target for drug delivery. Additionally, these findings could contribute to therapeutic intervention in mucosal diseases such as IBD.
Materials and Methods
ALP Activity.
ALP activity was detected histologically by using FASTred (SIGMAFAST Fast Red TR/Naphthol AS-MX cell stain; Sigma) (47) or by performing a pNPP hydrolysis assay (43) using Tris-based lysis buffer (TLB).
LPS-Transfer Assay.
Before LPS-transfer assay, Caco-2 cells were treated with RvE1 or chemerin and HMEC cells were transfected with pNFκB-Luc reporter plasmid (Stratagene). Fluorescently labeled LPS (LPS-AlexaFluor594) was diluted to 100 ng/mL in DMEM without phenol-red and applied to epithelia that had been treated for 24 h with RvE1 or chemerin. Following incubation for 1 h at 37 °C, the LPS concentration of the epithelia-exposed phenol-red free DMEM was determined fluorometrically (Cytofluor 2300; Millipore Corp.), to account for potential loss of LPS. Quantification of fluorescent-LPS concentration allowed for adjustment and normalization of LPS for transfer to HMEC cells transfected with NFkB-luc reporter. Ten nanograms/milliLiter of LPS was applied to transfected cells, and NFkB activity was measured.
Animals.
Age and weight matched female C57/B6 mice were purchased from (The Jackson Laboratory). All procedures were performed with approval of the Animal Care and Use Committee of the University of Colorado Denver. Colitis was induced by dissolving 3% DSS (MP Biomedicals) in the drinking water of mice for 6 d. Body weight, occult blood in feces, and stool consistency were recorded daily for each mouse to determine the DAI. The DAI scores were assigned as described previously (48). RvE1 (1 μg) or saline were injected i.p. on d 0, 2, and 4. All mice received a twice-daily gavage (200 μL) of either saline or L-Phe (100 mM L-phenylalanine in saline), using a 25-mm 24-gauge feeding needle (FST).
Tissue Collection and Processing.
Colons were removed and measured. Approximately 1 cm of whole colon was removed and fixed in neutral buffered formalin (Sigma) for histological analysis and 1cm was collected in TLB for mesoscale, pNPP, and Western blotting analysis. The remainder of the colon was placed in a HBSS- buffer containing EDTA, as described (49) and agitated to remove the epithelium. Enriched epithelia were stored in RNAlater (Ambion) and subsequently RNA was isolated using RNeasy kit (Qiagen). Total RNA was quantified using a (Nanodrop). Only RNA with a 260/280 ratio between 1.8 and 2.0 was used for subsequent experiments.
Supplementary Material
Acknowledgments
RvE1 was synthesized in Core C of P50-DE016191 by Dr. Nicos Petasis (University of Southern California) and validated in Core D of P50-DE016191 by Dr. Rong Yang (Brigham and Women's Hospital). We also thank Dr. Charles N. Serhan for critical analysis of this manuscript. This work was supported by National Institutes of Health Grants DE13499 and DK50189 and a grant from the Crohn's and Colitis Foundation of America.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914730107/-/DCSupplemental.
References
- 1.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 2.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 3.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 5.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, et al. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arita M, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 9.Hong S, et al. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 10.Arita M, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittamer V, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, et al. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arita M, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudert CA, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasturk H, et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 16.Arita M, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 17.Campbell EL, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: A new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 18.Ihara A, et al. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 19.Louis NA, Hamilton KE, Kong T, Colgan SP. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 2005;19:950–959. doi: 10.1096/fj.04-3251com. [DOI] [PubMed] [Google Scholar]
- 20.Nakano T, et al. Disruption of the murine intestinal alkaline phosphatase gene Akp3 impairs lipid transcytosis and induces visceral fat accumulation and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1439–G1449. doi: 10.1152/ajpgi.00331.2006. [DOI] [PubMed] [Google Scholar]
- 21.Vaishnava S, Hooper LV. Alkaline phosphatase: Keeping the peace at the gut epithelial surface. Cell Host Microbe. 2007;2:365–367. doi: 10.1016/j.chom.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Bentala H, et al. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002;18:561–566. doi: 10.1097/00024382-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Poelstra K, et al. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol. 1997;151:1163–1169. [PMC free article] [PubMed] [Google Scholar]
- 24.Fishman WH, Sie HG. Organ-specific inhibition of human alkaline phosphatase isoenzymes of liver, bone, intestine and placenta; L-phenylalanine, L-tryptophan and L homoarginine. Enzymologia. 1971;41:141–167. [PubMed] [Google Scholar]
- 25.Goldstein DJ, Rogers C, Harris H. Evolution of alkaline phosphatases in primates. Proc Natl Acad Sci USA. 1982;79:879–883. doi: 10.1073/pnas.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyama I, Matsunaga T, Harada T, Hokari S, Komoda T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem. 2002;35:455–461. doi: 10.1016/s0009-9120(02)00330-2. [DOI] [PubMed] [Google Scholar]
- 27.Lai Y, Gallo RL. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis NA, Hamilton KE, Colgan SP. Lipid mediator networks and leukocyte transmigration. Prostaglandins Leukot Essent Fatty Acids. 2005;73:197–202. doi: 10.1016/j.plefa.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wozniak M, Keefer JR, Saunders C, Limbird LE. Differential targeting and retention of G protein-coupled receptors in polarized epithelial cells. J Recept Signal Transduct Res. 1997;17:373–383. doi: 10.3109/10799899709036615. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence DW, et al. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narisawa S, et al. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol. 2003;23:7525–7530. doi: 10.1128/MCB.23.21.7525-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beumer C, et al. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther. 2003;307:737–744. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- 35.Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DK. A physiologic function for alkaline phosphatase: Endotoxin detoxification. Laboratory Investigation; a Journal of Technical Methods and Pathology. 1997;76:319–327. [PubMed] [Google Scholar]
- 36.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28:281–299. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caccavo D, et al. Lactoferrin-lipid A-lipopolysaccharide interaction: Inhibition by anti-human lactoferrin monoclonal antibody AGM 10.14. Infect Immun. 1999;67:4668–4672. doi: 10.1128/iai.67.9.4668-4672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 39.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg RF, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuin A, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58:379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 42.Torres MI, Lorite P, López-Casado MA, Ríos A. A new approach using tissue alkaline phosphatase histochemistry to identify Crohn's disease. Pathol Res Pract. 2007;203:485–487. doi: 10.1016/j.prp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Malo MS, et al. The pro-inflammatory cytokines, IL-1beta and TNF-alpha, inhibit intestinal alkaline phosphatase gene expression. DNA Cell Biol. 2006;25:684–695. doi: 10.1089/dna.2006.25.684. [DOI] [PubMed] [Google Scholar]
- 44.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 46.Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1223–G1233. doi: 10.1152/ajpgi.00313.2007. [DOI] [PubMed] [Google Scholar]
- 47.Cox WG, Singer VL. A high-resolution, fluorescence-based method for localization of endogenous alkaline phosphatase activity. J Histochem Cytochem. 1999;47:1443–1456. doi: 10.1177/002215549904701110. [DOI] [PubMed] [Google Scholar]
- 48.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Mahida YR, Wu KC, Jewell DP. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989;30:1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.