Abstract
Deinococcus radiodurans is among a small number of bacterial species that are extremely resistant to ionizing radiation, UV light, toxic chemicals, and desiccation. We measured proteome oxidation (i.e., protein carbonylation, PC) in D. radiodurans as well as in standard and evolved resistant strains of Escherichia coli exposed to ionizing radiation or UVC light and found a consistent correlation with cell killing. The unique quantitative relationship between incurred PC and cell death holds over the entire range of killing for all tested bacteria and for both lethal agents, meaning that both bacterial species are equally sensitive to PC. We show that the extraordinary robustness of D. radiodurans depends on efficient proteome protection (but not DNA protection) against constitutive and radiation-induced PC consisting of low molecular weight cytosolic compounds. Remarkably, experimental evolution of resistance to ionizing radiation in E. coli coevolves with protection against PC. The decline in biosynthetic efficacy of the cellular proteome, as measured by the loss of reproduction of undamaged bacteriophage λ in irradiated standard and evolved ionizing radiation-resistant E. coli, correlates with radiation-induced oxidative damage to host cells and their sensitivity to ionizing radiation. This correlation suggests that cell death by radiation is caused primarily by oxidative damage with consequential loss of maintenance activities including DNA repair.
Keywords: carbonylation, robustness, UV
A small number of unrelated prokaryotic and eukaryotic radiation-resistant species, such as the bacterium Deinococcus radiodurans, are unusually resistant to killing following exposure to ionizing radiation and other noxious conditions and environments such as desiccation (1). This remarkable robustness is embodied in the exceptional ability to repair hundreds of DNA double-strand breaks (DSB) generated by high doses of ionizing radiation (1). Remarkably, ionizing radiation-induced DSB are generated with equal efficiency in all prokaryotic and eukaryotic cells examined (~0.005 DSB/Mbase/Gy irradiation) (2, 3). Thus, high radiation resistance is associated with high efficacy of DNA repair.
There is no documented evidence that proteins typically involved in DSB repair (such as RecA and PolA) are present at higher concentrations in D. radiodurans than in bacteria such as Escherichia coli (2, 4). Nor is there evidence for increased specific activity of such proteins in Deinococcus. These observations suggest the intriguing possibility that the extraordinary resistance of this organism to radiation damage reflects the existence of a highly efficient biological response to cellular damage or a protection against molecular damage caused, for instance, by ionizing radiation-induced reactive oxygen species (ROS) (5, 6).
To explore the existence and nature of such putative protective mechanisms, we quantitatively examined oxidation (carbonylation) of the proteomes of sensitive and resistant strains of D. radiodurans and E. coli (including two radiation-resistant variants of E. coli evolved experimentally by exposure of surviving cells to successively increasing doses of ionizing radiation (20 cycles) (7). We show here that killing of D. radiodurans and E. coli strongly correlates with proteome carbonylation (PC) and that this correlation is independent of the extent of cellular resistance or the type of lethal agent used. We provide evidence that the oxidative damage is the cause, rather than a consequence, of radiation-induced cell death and show that selection for increased resistance to ionizing radiation in E. coli coselects for the acquisition of a protection system against PC.
Results
Oxidative damage to the proteome reflected by levels of PC in D. radiodurans and E. coli was measured immediately after exposure to radiation of cells maintained in ice. This parameter correlates with cell survival after incubation of irradiated cells on nutrient agar plates (Figs. 1 A and B). We consistently observed a correlative relationship between the dose-response of PC and cell killing induced by either ionizing radiation or UVC light that was independent of the extent of cellular resistance to these lethal agents (Fig. 1 A and B).
Fig. 1.
Cell death induced by ionizing and UV radiation correlates with ROS production and protein carbonylation in E. coli (Eco) and D. radiodurans (Dra). (A) Cell survival (cfu) and PC versus γ radiation dose. (B) Cell survival and PC versus UVC radiation dose. (C) Intracellular ROS production by γ radiation measured by intracellular DHR in two bacterial species. (D) Intracellular ROS production by UVC radiation measured by intracellular DHR in two bacterial species. Cell survival and PC are shown as the mean and SD of two duplicate experiments (see also Fig. S1).
The dose-response of radiation-induced PC in D. radiodurans lags by a factor of 21 for ionizing radiation and by a factor of 25 for UVC light (Table S1) relative to that observed in E. coli, suggesting the operation of distinct mechanisms of PC and/or differences in proteome protection against radiation-induced oxidation. At exposure to high levels of ionizing radiation and UVC, the similar PC saturation levels in E. coli and D. radiodurans indicate similar intrinsic susceptibilities of the E. coli and D. radiodurans proteomes to oxidation, supporting the proteome protection hypothesis (Fig. 1 A and B). Because cellular proteome protection systems are unlikely to be activated by radiation on ice, we conclude that the observation of ~4-fold lower levels of PC in unirradiated D. radiodurans compared with E. coli (0.4 nmol/mg versus 1.5 nmol/mg protein, respectively; Table S1 and Fig. S1, both related to Fig. 1) reflects their constitutive levels protecting the proteome up to a given radiation exposure on ice. Although the measured proportions of PC levels are robust, the quantification of PC may have significant limitations, because it is based on the standard curve for a single carbonylated protein not revealed by the commercial provider.
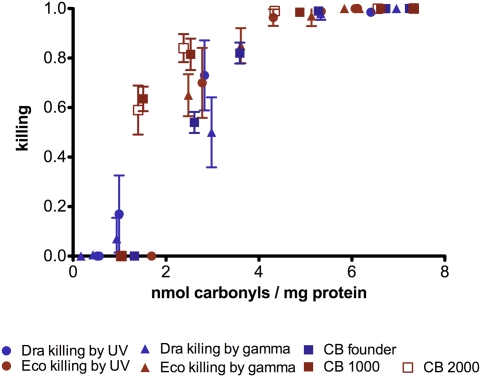
Levels of PC in both D. radiodurans and E. coli increased as a function of dose in parallel with the generation of ROS monitored by oxidation of the intracellular dye 2,4-dihydrorhodamine (DHR) to a form that fluoresces at 530 nm (8) (Fig. 1 C and D). However, the generation of ROS did not level off at exposures at which PC levels saturated (Fig. 1 C and D), indicating that all sensitive sites in the proteome were carbonylated. Based on these observations, we conclude that proteome protection against oxidative damage primarily, if not exclusively, involves suppression of the generation of ROS and/or their neutralization during irradiation on ice. Remarkably, the extent of cell killing as a function of PC shows the same hyperbolic relationship for both bacterial species and for both ionizing radiation and UVC light (Fig. 2). The question remains: Is the PC the cause or the consequence of death?
Fig. 2.
Single correlation between cell killing and PC. A similar correlation is observed for the two bacterial species, including the resistant E. coli strains CB1000 and CB2000, and for both means of irradiation. Cell survival and PC are shown as the mean and SD of two duplicate experiments.
We hypothesized that the progressive decay of cellular robustness culminating in cell death is a direct result of the progressive accumulation of oxidative damage to the proteome, a scenario formally equivalent to the instantaneous acquisition of thousands of weak, “leaky” mutations in the proteome. To assess proteome fitness more directly, we measured the capacity of irradiated E. coli host cells to propagate unirradiated bacteriophage λ, irrespective of cell survival. After injection of its genome, phage λ redirects the host cell biosynthetic machinery to its own reproduction. This process involves molecular events associated with transcription, translation, and DNA replication, all of which require multiple protein interactions and catalytic reactions that are expected to be sensitive to PC.
The capacity of E. coli to propagate phage λ after exposure to UVC light or γ radiation before phage infection is shown in Fig. 3 A and B, respectively. These experiments demonstrate a progressive decline in the ability of irradiated E. coli infected by unirradiated phage λ to generate infective centers as a function of increasing radiation exposure, reflecting a progressive decay in the functional efficacy of the cellular biosynthetic machinery and its correlation with the extent of PC. Indeed, the capacity for producing viable phage λ reveals the deleterious effects of PC with greater sensitivity than does cell killing (Fig. 3), even though the phage genome was not exposed to exogenous sources of damage. (A comparable phage–host relationship was not discovered for D. radiodurans, thus precluding similar experiments.) Our conclusion that the progressive decay in the generation of λ infective centers reflects functional degeneracy of the proteome is strengthened further by the results of single-burst size experiments (i.e., decay of the number of viable phage released by a single infected cell) (Fig. 3 and Fig. S2 A and B).
Fig. 3.
Correlations between radiation-induced protein carbonylation (▲), cellular capacity to produce phage λ (○), and cell death (●) in E. coli. (A) UVC radiation. (B) γ-radiation. Host cell survival (cfu), the cellular capacity to produce phage (infective centers, IC) and PC are presented as the mean and SD of two duplicate experiments (see also Fig. S2).
To determine further whether PC is a cause rather than a consequence of cell death in D. radiodurans, we examined the correlation between radiation-induced cell death and PC following elimination of the recA gene, which encodes a key protein required for the repair of DNA damage. This perturbation markedly increased cell killing without affecting protection against PC (Fig. 4). We therefore conclude that the increase in PC as a function of radiation dose has a causal rather than a consequential relationship to death of wild-type cells.
Fig. 4.
Cell death and protein carbonylation induced by (A) ionizing and (B) UVC radiation in ΔrecA mutants of D. radiodurans. (A) Cell survival (cfu) and total protein carbonylation versus γ irradiation dose. (B) Cell survival (cfu) and total protein carbonylation versus UVC irradiation dose. Cell survival and PC are shown as the mean and SD of two duplicate experiments.
What are the mechanisms of proteome protection in D. radiodurans? It is known that proteins in irradiated D. radiodurans are highly protected from oxidation but lose their resistance when purified from the cells (6). To discriminate between possible intrinsic resilience of D. radiodurans proteome to ROS (perhaps by robust enzymatic detoxification active at 4 °C) and/or the active involvement of some intracellular compound(s), we showed that, when extracts of E. coli and D. radiodurans are mixed in a 1:1 ratio, the elevated constitutive level of PC in E. coli is dominant (Table 1). In contrast, irradiated mixed extracts show the low levels of PC typical of D. radiodurans. This protective effect of D. radiodurans extract is eliminated by centrifugal filtration of either mixed or unmixed extracts of D. radiodurans before irradiation but not by such treatment of E. coli extracts. Hence, the putative antioxidant protector in D. radiodurans appears to be a diffusible low molecular weight moiety (that can pass through a 3-kDa cutoff filter) that protects E. coli and D. radiodurans proteins with similar efficacy.
Table 1.
Protein carbonylation in γ and UVC irradiated E. coli and D. radiodurans cell extracts
| Carbonyls/mg protein (nmol) | ||||||
| Dose of UV radiation (J/m2) | Dose of γ radiation (Gy) | |||||
| 0 | 270 | 1,900 | 0 | 800 | 7,000 | |
| E. coli | ||||||
| Nondialyzed | 1.05 ± 0.12 | 7.25 ± 0.52 | 6.68 ± 0.52 | 1.69 ± 0.22 | 5.14 ± 0.43 | 8.11 ± 0.53 |
| Dialyzed | 1.1 ± 0.21 | 7.84 ± 0.48 | 7.96 ± 0.49 | 1.62 ± 0.24 | 5.17 ± 0.45 | 9.01 ± 0.59 |
| D. radiodurans | ||||||
| Nondialyzed | 0.23 ± 0.19 | 2.08 ± 0.27 | 6.12 ± 0.55 | 0.34 ± 0.09 | 0.24 ± 0.06 | 2.10 ± 0.18 |
| Dialyzed | 0.31 ± 0.21 | 8.24 ± 0.57 | 6.46 ± 0.52 | 0.28 ± 0.11 | 5.23 ± 0.25 | 8.34 ± 0.63 |
| E. coli + D. radiodurans | ||||||
| Nondialyzed | 0.99 ± 0.17 | 1.95 ± 0.29 | 7.15 ± 0.38 | 1.54 ± 0.17 | 1.92 ± 0.11 | 8.02 ± 0.61 |
| Dialyzed | 1.03 ± 0.14 | 9.15 ± 0.61 | 8.96 ± 0.44 | 1.36 ± 0.11 | 6.12 ± 0.39 | 9.23 ± 0.56 |
Cell-free extracts were prepared as described in Materials and Methods and irradiated either separately or in a 1:1 (E. coli + D. radiodurans) mixture (with or without previous dialysis by filtration), and the respective protein carbonylation was quantified. E. coli and D. radiodurans protein carbonylation levels in extracts are similar to those obtained with irradiated cells. In nonirradiated mixed (E. coli + D. radiodurans) extracts, high constitutive protein carbonylation levels of E. coli predominate, irrespective of dialysis. In irradiated mixed (E. coli + D. radiodurans) extracts, low levels of D. radiodurans protein carbonylation are dominant, showing the D. radiodurans protective effect on the E. coli proteome in vitro. This protection is lost with dialysis. The results presented are the mean plus SD of four measurements.
Experiments similar to those in Fig. 1 and Fig. 3 were conducted with two radiation-resistant variants of the E. coli strains CB1000 and CB2000 independently derived by directed evolutionary selection for resistance to increasing levels of ionizing radiation (7). Fig. S3 A and B shows a correlation between cell killing and PC induced by γ radiation in both sensitive and resistant E. coli strains. Additionally, the decay of the capacity of these strains to generate viable phage λ correlates with the ionizing radiation-induced PC in host cells before infection (Fig. S3C).
Discussion
D. radiodurans is a robust bacterium that is extremely resistant to ionizing radiation, UV light, toxic chemicals, and prolonged periods of desiccation (1). After ionizing radiation with ~7 kGy, the genome of this organism is shattered into more than 150 fragments without affecting cell survival (6, 9). This dose, highly lethal to other bacterial species, causes similar DNA DSB (per Mbase) as in D. radiodurans (10). The D. radiodurans genome is not endowed with a repertoire of genes involved in biological responses to DNA damage distinct from those in naturally occurring ionizing radiation-sensitive bacteria such as Shewanella oneidensis and Pseudomonas putida (6, 11). This observation has long awaited a cogent explanation and prompted us to hypothesize that the profound resistance to ionizing radiation in D. radiodurans involves novel mechanisms.
In the present studies we explored the possibility that organisms such as D. radiodurans and ionizing radiation-resistant strains of E. coli generated by directed evolution in the laboratory are endowed with mechanisms that provide protection against radiation-induced oxidative damage to proteins (PC) and/or efficiently repair such damage. This notion is supported by the results of the experiments described here. In particular, quantitative observations on the dose–response of cell survival after exposure to ionizing radiation and on dose-dependent PC support this hypothesis. However, we cannot exclude the possibility that oxidative damage to some other cellular component, induced with identical kinetics, would yield the same correlation.
The results of our experiments indicate that the quantitative relationship between PC and cellular lethality is causal rather than consequential (Fig. 2). Notably, inactivation of the recA gene of D. radiodurans markedly enhances sensitivity to ionizing radiation while retaining wild-type levels of PC (Fig. 4). Additionally, exposure of E. coli to ionizing radiation or UVC light before infection with undamaged bacteriophage λ results in a progressive loss of phage production, suggesting that PC of host cells by UV light or ionizing radiation reduces the efficiency of protein-dependent events required for the biosynthesis of viable phage particles (Fig. 3 and Fig. S2 A and B). Because the phage genome is intact, the loss of phage viability is caused by oxidative damage to the host cell. Therefore, death of the intracellular virus can ensue by oxidative inactivation of cellular functions even without damage to the viral genome.
The spectra of DNA damage in cells exposed to ionizing radiation and UV light are distinct. Exposure to ionizing radiation primarily generates DNA strand breaks, whereas exposure to UV light primarily generates photoproducts (12). The cellular responses to these types of DNA damage are correspondingly distinctive. Hence, the observation that saturation levels of PC are indistinguishable in both D. radiodurans and E. coli exposed to either source of damage (Fig. 1 A and B) is consistent with the notion that the susceptibility of the proteomes of these two bacterial species to PC is essentially indistinguishable. Additionally, regardless of large differences in the sensitivity of various bacteria to a variety of lethal agents, the results presented in Fig. 2 show little interspecies variation in the tolerance of PC over the entire range of cell killing examined. Thus, efficient biological responses to genomic insult ultimately depend on the integrity of the proteome, including proteins required for genomic repair and maintenance, supporting the conclusion that protein oxidation is a fundamental determinant of cell death (2, 6). Taking into account the limitations of PC quantification (Results), the present study shows that two or three carbonyls per average protein, or ~5 million carbonyls per E. coli cell, are lethal upon exposure to two distinct sources of cellular damage.
The nature of the cellular component(s) that affords resistance to radiation remains to be established, as does its mechanisms of action. Previous studies identified a fraction of D. radiodurans with a molecular weight cutoff of ~15 kDa that promoted modestly enhanced survival of E. coli exposed to ionizing radiation when added to the medium (13). Importantly, the present studies demonstrate that the addition of cell-free extracts of D. radiodurans afforded protection of the proteome of irradiated E. coli extracts (Table 1). Our results show that this radioprotective effect is contained in molecular species of less than 3 kDa (Table 1). Hence, the active component apparently is a low molecular weight entity that does not discriminate between the proteome of E. coli and D. radiodurans. The radioresistance of several bacteria has been shown to correlate with intracellular Mn++/Fe++ concentration ratios (10, 13–15). Hence, the protective components in extracts of D. radiodurans may include manganese complexes that attenuate the iron-catalyzed Fenton reaction, a major source of *OH radicals (13, 14).
Antioxidant enzyme systems in D. radiodurans can be inactivated without significant effects on its extreme radiation resistance, an observation that supports the existence of efficient nonenzymatic ROS-scavenging systems (14). Overexpression of the deinococcal pigment deinoxanthin (16) or the cytosolic scavenger pyrroloquinoline-quinone promotes radioprotection of E. coli (17). However, inactivation of deinoxanthin synthesis had only a minor effect on the survival of D. radiodurans exposed to ionizing radiation (16). Thus, it is likely that multiple distinct molecular mechanisms contribute to various degrees to the resistance of bacteria to ionizing (and UV) radiation (and other toxic agents) by protecting the proteome from oxidative damage. Indeed, sequencing the genomes of several independent isolates of E. coli obtained by directed evolution to ionizing radiation resistance revealed more than 60 nonoverlapping mutations (7) that do not reflect known genes involved in a single radioprotective or repair mechanism.
Hence, there may be multiple routes to ionizing radiation resistance mediated by proteome protection. Oxidative damage to proteins also may contribute substantially to the killing of cells exposed to other toxic conditions, including desiccation (18) and solar (UVA) radiation (19). Therefore, in the study of cytotoxic agents, it is important to quantify protein oxidation in parallel with damage to nucleic acids, lipoproteins, or lipids in membranous structures.
Materials and Methods
Bacterial Strains, Growth Conditions, and Irradiation.
The following bacterial strains were used: E. coli MG1655 wild type, E. coli ΔrecA::kan, E. coli CB1000, E. coli CB2000, E. coli CB founder strain (MG1655), D. radiodurans R1 (ATCC 13939) wild type, and D. radiodurans ΔrecA::tet. E. coli strains denoted “CB” (for “Cox-Battista”) were obtained from John Battista (Louisiana State University, Baton Rouge, LA) (10).
Bacteria were grown in rich media: E. coli strains were grown in LB, and D. radiodurans were grown in tryptone-glucose-yeast extract (TGY) broth at 30 °C to the exponential phase (OD600 = 0.2–0.4), washed in 0.01 M MgSO4, and concentrated 25 times for γ irradiation and five times for UVC (254-nm) irradiation. All radiation experiments were performed on ice. For E. coli, γ irradiation was performed with a 137Cs source (dose rate of 26 Gy/min), and for D. radiodurans irradiation was performed with a 60Co source (dose rate of 11 Gy/s). UVC irradiation (peak emission 260 nm) of both species was at the dose rate of 4.5 J/m2·s−1. Viable cell counts of E. coli and D. radiodurans were estimated by plating serial dilutions on LB (overnight at 37 °C) and TGY (3–4 days at 30 °C) plates, respectively.
Preparation of Protein Extracts and Protein Carbonylation Measurement.
Exponentially growing bacteria were harvested from a rich medium (TGY for D. radiodurans and LB for E. coli), resusupended in 10−2 M MgSO4, and irradiated on ice by γ rays or by UV light. The PC was quantified immediately to avoid metabolization of the carbonylated proteins. Irradiated cells of E. coli and D. radiodurans strains were pelleted by centrifugation immediately after irradiation and resuspended in the lysis buffer of the OxyElisa protein carbonylation detection kit (Millipore) supplemented with a mixture of protease inhibitors (Roche). Resuspended cells were frozen immediately in liquid nitrogen. Cells were broken by two freeze-thaw cycles, homogenized in a Dounce homogenizer, and centrifuged 20 min at 12,000 × g. The amount of protein in the supernatant was measured by the Lowry method (20) using the OxyElisa kit. Protein extracts diluted to 10 μg/mL were loaded into wells (provided in the kit) and incubated over night at 4 °C to allow proteins to adsorb to the surface, followed by DHR derivatization of adsorbed proteins and detection of derivatized dinitrophenol (DNP)-carbonyl by a mouse DNP-specific monoclonal antibody conjugated to HRP. Subsequent incubation with enzyme substrate 3,3′,5,5′-tetramethylbenzidine resulted in a colored product that was quantified using a microplate reader with maximum absorbance at 450 nm.
To estimate the ED50, PC was plotted against logarithm of the dose. The data were fitted to the equation:
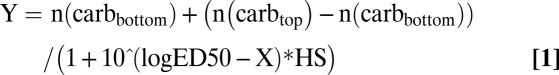 |
where n(carbbottom) is the amount of carbonylation at the bottom plateau, n(carbtop) is the amount of carbonylation at the top plateau, and HS is the Hill slope of the curve.
Measurement of ROS Production.
E. coli MG1655 wild type and D. radiodurans R1 and ΔrecA cells were labeled with 25 μM dihydrorhodamin-123 before irradiation. After irradiation, cells were washed in minimal medium, and their fluorescence was measured with excitation at 500 nm and emission at 530 nm.
Phage λ Production: Infective Centers and Single-Burst Size.
E. coli MG16555 wild type, CBF, CB1000, and CB2000 cultures were grown to log-phase (OD600 = 0.2) in the presence of 1% maltose (to induce high levels of λ receptor) and subjected to UVC and γ irradiation as described above. Irradiated E. coli was pelleted and resuspended in 1 mL of LB broth supplemented with 30 mM MgSO4, 15 mM CaCl2, and 1% maltose (final concentrations). The cells were infected with λ phage at multiplicity of infection (m.o.i.) 0.1 for 10 min at 37 °C. For infective centers, serial dilutions were plated onto LB plates with 4.5% top agar with overnight culture of E. coli MG1655 wild type, 30 mM MgSO4, 15 mM CaCl2 and 1% maltose. Plates were incubated overnight at 37 °C, and plaques were counted.
To count the number of phages produced per single infected cell (E. coli MG16555 wild type), irradiated cells were infected at m.o.i. 0.3 and diluted to obtain one cell per tube in three tubes that were incubated in 0.4 mL LB for 60 min at 37 °C. A drop of chloroform was added to help release viruses produced during the incubation. The content of each tube was mixed with 0.7% top agar supplemented with overnight culture of E. coli MG1655 wild type, 30 mM MgSO4, 15 mM CaCl2, and 1% maltose. Plaques were counted after overnight incubation at 37 °C.
Irradiation of Protein Extracts.
Protein extracts of nonirradiated exponential E. coli MG1655 and D. radiodurans R1 were prepared as described above. Protein amount was determined by the Lowry method. Extracts of both bacteria were adjusted to the same protein amount (1 mg/mL) and divided in two parts. Each extract alone and the 1:1 mixture were irradiated by γ irradiation (0, 800, and 7,000 Gy) and UVC light (0, 270, and 1,900 J/m2). The same irradiation scheme was applied to the E. coli and D. radiodurans extracts that were filtered by centrifugation in a Microcon device with a 3-kDa cutoff. PC was measured as described above.
Supplementary Material
Acknowledgments
We thank Dr. John Battista (Louisiana State University) for the gift of E. coli strains CBF, CB1000, and CB2000, Dr. Ksenija Zahradka for providing help with highest ionizing radiation exposures at the Institute R. Boskovic (Zagreb, Croatia), Dr. Matthew Meselson (Harvard University) and Dr. Errol Friedberg (University of Texas Southwestern) for providing valuable advice. A.K. is a postdoctoral fellow of Institut National de la Santé et de la Recherche Médicale, which funded this research. Part of the work was carried out at and supported by the Mediterranean Institute for Life Sciences, Split, Croatia.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009312107/-/DCSupplemental.
References
- 1.Cox MM, Battista JR. Deinococcus radiodurans—the consummate survivor. Nat Rev Microbiol. 2005;3:882–892. doi: 10.1038/nrmicro1264. [DOI] [PubMed] [Google Scholar]
- 2.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7:237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 3.Gladyshev E, Meselson M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc Natl Acad Sci USA. 2008;105:5139–5144. doi: 10.1073/pnas.0800966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasius M, Sommer S, Hübscher U. Deinococcus radiodurans: What belongs to the survival kit? Crit Rev Biochem Mol Biol. 2008;43:221–238. doi: 10.1080/10409230802122274. [DOI] [PubMed] [Google Scholar]
- 5.Bruce IN, McNally JA, Rea IM, Bell AL. Age-related changes in non-receptor dependent generation of reactive oxygen species from phagocytes of healthy adults. Mech Ageing Dev. 1997;94:135–144. doi: 10.1016/s0047-6374(96)01867-2. [DOI] [PubMed] [Google Scholar]
- 6.Daly MJ, et al. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5:e92. doi: 10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris DR, et al. Directed evolution of ionizing radiation resistance in Escherichia coli. J Bacteriol. 2009;191:5240–5252. doi: 10.1128/JB.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelm J, Vytásek R, Ostádalová I, Vajner L. Evaluation of different methods detecting intracellular generation of free radicals. Mol Cell Biochem. 2009;328:167–176. doi: 10.1007/s11010-009-0086-5. [DOI] [PubMed] [Google Scholar]
- 9.Daly MJ, Minton KW. Interchromosomal recombination in the extremely radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1995;177:5495–5505. doi: 10.1128/jb.177.19.5495-5505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly MJ, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 11.Makarova KS, et al. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol Mol Biol Rev. 2001;65:44–79. doi: 10.1128/MMBR.65.1.44-79.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg EC, et al. DNA Repair and Mutagenesis. Washington, DC: ASM; 2005. [Google Scholar]
- 13.Bruce AK. Extraction of the radioresistant factor of Micrococcus radiodurans. Radiat Res. 1964;22:155–164. [PubMed] [Google Scholar]
- 14.Daly MJ. Modulating radiation resistance: Insights based on defenses against reactive oxygen species in the radioresistant bacterium Deinococcus radiodurans. Clin Lab Med. 2006;26:491–504. doi: 10.1016/j.cll.2006.03.009. x. [DOI] [PubMed] [Google Scholar]
- 15.Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, et al. Knockout of crtB or crtI gene blocks the carotenoid biosynthetic pathway in Deinococcus radiodurans R1 and influences its resistance to oxidative DNA-damaging agents due to change of free radicals scavenging ability. Arch Microbiol. 2007;188:411–419. doi: 10.1007/s00203-007-0262-5. [DOI] [PubMed] [Google Scholar]
- 17.Misra HS, et al. Pyrroloquinoline-quinone: A reactive oxygen species scavenger in bacteria. FEBS Lett. 2004;578:26–30. doi: 10.1016/j.febslet.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Fredrickson JK, et al. Protein oxidation: Key to bacterial desiccation resistance? ISME J. 2008;2:393–403. doi: 10.1038/ismej.2007.116. [DOI] [PubMed] [Google Scholar]
- 19.Bosshard F, et al. Protein oxidation and aggregation in UVA-irradiated Escherichia coli cells as signs of accelerated cellular senescence. Environ Microbiol. 2010 doi: 10.1111/j.1462-2920.2010.02268.x. 10.1111/j.1462-2920.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.