Abstract
Although gastrointestinal stromal tumors (GISTs) harboring activating KIT or platelet-derived growth factor receptor A (PDGFRA) mutations respond to treatment with targeted KIT/PDGFRA inhibitors such as imatinib mesylate, these treatments are rarely curative. Most often, a sizeable tumor cell subpopulation survives and remains quiescent for years, eventually resulting in acquired resistance and treatment failure. Here, we report that imatinib induces autophagy as a survival pathway in quiescent GIST cells. Inhibiting autophagy, using RNAi-mediated silencing of autophagy regulators (ATGs) or antimalarial lysosomotrophic agents, promotes the death of GIST cells both in vitro and in vivo. Thus, combining imatinib with autophagy inhibition represents a potentially valuable strategy to promote GIST cytotoxicity and to diminish both cellular quiescence and acquired resistance in GIST patients.
Keywords: imatinib, targeted therapy, quiescence
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract (1). Importantly, GISTs commonly harbor receptor tyrosine kinase mutations resulting in ligand-independent, constitutive activation that drives tumor cell proliferation; 85% have activating KIT mutations and an additional 7% have mutually exclusive platelet-derived growth factor receptor A (PDGFRA) mutations (1). (1). As a result, imatinib mesylate, a small molecule tyrosine kinase inhibitor that abrogates KIT and PDGFR activity, is highly effective as a treatment for metastatic GIST (1). Before imatinib, recurrent or metastatic GIST was uniformly fatal (2).
Although imatinib is quite effective in stabilizing disease progression in GIST, it is generally not curative. Less than 2% of patients experience complete radiographic regression (3). Even upon prolonged imatinib treatment, most are left with a substantial and stable tumor burden consisting of viable nonproliferating tumor cells, indicating that significant numbers of GIST cells can survive imatinib and remain quiescent. Moreover, imatinib withdrawal in such individuals results in rapid disease progression, necessitating lifelong imatinib therapy (4). The inability of imatinib to fully eradicate GIST cells also contributes to acquired imatinib resistance, mostly due to intraallelic second-site KIT mutations that interfere with imatinib binding (5). Hence, from a therapeutic standpoint, it is critical to identify new agents or strategies to kill GIST cells either as single agents or in combination with imatinib.
As a result, we sought to dissect the contributions of macroautophagy (hereafter called autophagy), an evolutionarily conserved lysosomal self-digestion process, to the survival of GIST cells during imanitib-induced quiescence (6). Autophagy is a key mechanism to recycle energy and nutrients during starvation or stress (7). Although the precise role of autophagy in cell survival versus death is highly context dependent (8, 9), growing evidence indicates that autophagy can promote tumor cell survival in response to both cytotoxic and targeted chemotherapies (10). Here, we demonstrate that autophagy is robustly induced in GIST cells upon imatinib treatment; furthermore, inhibiting self-eating, either alone or in combination with imatinib, promotes cell death. Because GISTs are notable for their resistance to cell death, even after targeted KIT/PDGFRA kinase inhibition, these results have significant implications for GIST therapy.
Results
Imatinib-Sensitive GIST Cells Exhibit Reversible Quiescence.
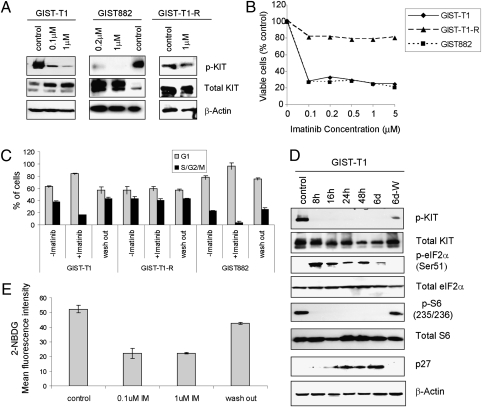
To develop a cell-based model for imatinib-induced quiescence, we evaluated the in vitro response to and recovery from imatinib treatment in three GIST cell lines, all of which contain constitutively active KIT mutations. GIST-T1 and GIST882 are imatinib-sensitive, whereas GIST-T1-R is imatinib resistant. Imatinib blocked KIT (Tyr721) phosphorylation in GIST-T1 and GIST882 (Fig. 1A). After 72 h, significant inhibition of proliferation was observed at doses of 0.1 μM and higher in both sensitive cell lines (Fig. 1B). Flow cytometry for DNA content corroborated a 3- to 5-fold decrease in S+G2/M-phase fraction in GIST-T1 and GIST882 (Fig. 1C and Fig. S1); furthermore, a complementary increase in the G0/G1 fractions was observed, indicative of cell-cycle arrest. In contrast, GIST-T1-R maintained high level KIT phosphorylation in the presence of imatinib and failed to show a proliferative block or G0/G1 arrest (Fig. 1C and Fig. S1). Similar to GIST cells in vivo, which can resume proliferation upon drug withdrawal after prolonged exposure to imatinib, quiescent GIST-T1 and GIST882 cells were capable of resuming proliferation (Fig. S1) and reentering the cell cycle (Fig. 1C) within 24 h of imatinib removal. Consistent with cell-cycle arrest, the cell-cycle inhibitor p27 accumulated during imatinib treatment and dropped significantly after drug withdrawal (Fig. 1D).
Fig. 1.
Imatinib induces reversible quiescence in GIST cells. Experiments were performed in imatinib-sensitive GIST-T1 and GIST882 and imatinib-resistant GIST-T1-R cell lines. (A) Cells were grown in the absence or presence of imatinib for the indicated times, lysed, and immunobloted with antibodies to KIT, pKIT (Y721), and β-actin. (B) Cell proliferation was measured after treatment with the indicated concentrations of imatinib for 72 h. (C) Cell-cycle analysis by flow cytometry after treatment with 1 μM imatinib for 6 d and then 2 d after removal of imatinib. (D) GIST-T1 were grown in the absence (control) or presence of 1 μM imatinib for the indicated times, lysed, and immunoblotted with antibodies to KIT, pKIT (Y721), pEIF2α (Ser51), eIF2α, pS6 (Ser235/236), S6, p27, and β-actin. (E) 2-NBDG uptake measured in GIST-T1 after incubation in imatinib at the indicated doses for 24 h. During washout, cells were incubated in 1 μM imatinib for 24 h, followed by withdrawal of imatinib for 24 h.
In both GIST-T1 and GIST882 cells, imatinib also induced signals associated with diminished protein translation, including increased eIF2α Ser51 phosphorylation, a stress-induced suppressor of global protein translation (11), and decreased phosphorylated S6 ribosomal protein, a downstream reporter of mTOR activation (12) (Fig. 1D and Fig. S1). Again, imatinib withdrawal after 6 d of treatment resulted in rapid reversal of these phosphorylation patterns, correlating with cell cycle reentry (Fig. 1D). Finally, a 2-fold reduction in glucose uptake was observed in sensitive GIST lines upon imatinib treatment that, again, reversed upon removal (Fig. 1E). Collectively, these data demonstrate that imatinib induces quiescence in sensitive GIST cells, which is rapidly reversible. Most importantly, these in vitro results recapitulate key biological features observed in imatinib-treated GIST patients in vivo (4).
Imatinib Is Ineffective at Killing GIST Cells.
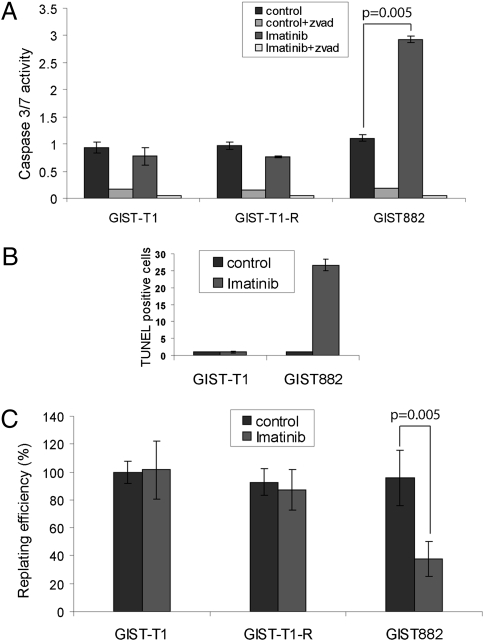
After imatinib therapy in vivo, most GISTs remain stable in size and contain viable cells (13). Thus, we assessed the effects of imatinib on apoptosis induction in vitro. Although GIST-T1 exhibited minimal caspase 3/7 activity after 1 μM imatinib for 72 h, GIST882 showed a 3-fold increase (Fig. 2A). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis confirmed the lack of apoptosis in GIST-T1, whereas ≈25% of GIST882 cells exhibited positive TUNEL staining after treatment (Fig. 2B and Fig. S2). To extend these results, we determined clonogenic recovery after 72 h of imatinib treatment. Replating efficiency was not reduced in imatinib-treated GIST-T1 or GIST-T1-R cells, confirming the lack of apoptosis (Fig. 2C). In contrast, treatment of GIST882 resulted in an ≈60% decrease in clonogenic recovery, consistent with increased caspase 3/7 activation (Fig. 2C). Notably, although a proportion of GIST882 cells died after imatinib exposure, numerous cells survived. Collectively, these results indicate that, even in relatively death-sensitive GIST lines, a significant residual population of cells survives imatinib. Complete or partial resistance to cell death observed in these sensitive cells strikingly resembles the behavior of GIST treated with imatinib in vivo (3, 4).
Fig. 2.
Imatinib induces variable amounts of apoptosis and cell death in different GIST cell lines. (A) Cells were grown for 72 h with or without 1 μM imatinib and assayed for caspase 3/7 activity. (B) Percentage of TUNEL-positive cells for GIST-T1 and GIST882. (C) Clonogenic replating efficiency of cells after treatment with or without 1 μM imatinib for 72 h. Results are the mean ± SD from three independent experiments.
Imatinib Induces Autophagy in GIST Cells.
Because GIST cells persist through imatinib treatment, we hypothesized that autophagy may serve as a survival pathway in GIST cells treated with imatinib. To monitor autophagosome formation, a hallmark of autophagy (14), we created cells stably expressing GFP fused to microtubule-associated protein light chain 3 (GFP-LC3). Untreated GIST-T1 was notable for a small number of GFP-LC3 puncta, indicative of baseline autophagy. Punctate GFP-LC3 rapidly increased in number within 8 h of imatinib treatment, and subsequently decreased at 16 and 24 h (Fig. S3), consistent with the formation and lysosomal turnover of autophagosomes (15, 16). Electron microscopy confirmed increased autophagic vesicles in GIST-T1 cells after treatment with imatinib (Fig. S3).
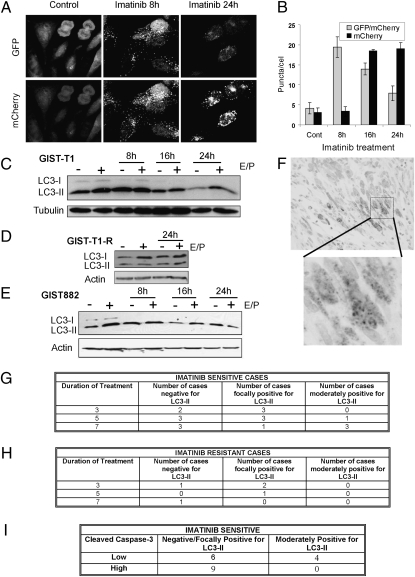
Because the increase in GFP-LC3 puncta at 8 h of treatment was followed by a decrease at later timepoints, we hypothesized that autophagosomes were rapidly maturing into autolysosomes, a hallmark of bona fide autophagic degradation (flux) (6, 14). To test this prediction, we stably expressed a tandem mCherry-GFP-tagged LC3 chimera in GIST-T1, which enabled simultaneous quantification of autophagosome induction and autolysosome maturation. Whereas the GFP signal is sensitive to the acidic and proteolytic conditions of the lysosome, mCherry is stable (14, 16). After 8 h of imatinib treatment, we observed marked induction of double positive (GFP+mCherry+) LC3 puncta, a marker of early autophagosomes, which then decreased at 16 h and 24 h (Fig. 3 A and B). In contrast, mCherry single positive puncta were progressively elevated at later timepoints, indicating autophagosome maturation to autolysosomes.
Fig. 3.
Imatinib induces autophagy in vitro and in vivo. (A) Representative images of GFP and mCherry fluorescent puncta in GIST-T1 cells expressing mCherry-GFP-LC3 grown in complete media with 1 μM imatinib for the indicated times. (B) Quantification of GFP/mCherry double-positive and mCherry single-positive puncta per cell in control or cells treated with 1 μM imatinib for the indicated times. GIST-T1 (C), GIST-T1-R (D), and GIST882 (E) were treated with 1 μM imatinib for the indicated times, lysed, and immunoblotted with antibodies to LC3 or tubulin or actin. (F) α-LC3 immunohistochemistry from a representative spindle cell gastric GIST from a patient treated with neoadjuvant imatinib for 7 d. Note the punctate staining pattern (blowup) consistent with the induction of autophagosomes. (G) Table summarizing results from imatinib-sensitive GISTs. (H) Table summarizing results from imatinib-resistant GISTs. (I) Table summarizing results from CC-3 immunohistochemistry of imatinib-sensitive GISTs.
During autophagy, the lipidation of LC3 results in a faster migrating isoform, LC3-II. Bona fide autophagy (autophagic flux) can be associated with either an increase or decrease in LC3-II, depending on the rate of autophagosome turnover (17). In GIST-T1 cells, we found high baseline LC3-II, which progressively decreased during imatinib treatment (Fig. 3C). However, LC3-II remained stable in the presence of the lysosomal cathepsin inhibitors E64d and pepstatin A, consistent with rapid lysosomal turnover of LC3-II (see “E/P+” lanes). In contrast, GIST-T1R showed high baseline LC3-II that did not change after addition of imatinib (Fig. 3D). In GIST882, we found a similarly high level of baseline LC3-II as seen in GIST-T1, which did not change markedly after addition of imatinib (Fig. 3E). This finding may reflect reduced autophagy in GIST882, which notably demonstrated increased levels of imatinib-induced apoptosis compared with GIST-T1 (Fig. 2 A and B and Fig. S2). Nevertheless, both GIST-T1 and GIST882 treated with imatinib demonstrated the progressive reduction of p62 (SQSTM1), an ubiquitin-binding scaffold protein selectively degraded by autophagy (Fig. S4) (18). In contrast, p62 levels were not affected in GIST-T1-R. Overall, these results indicate that bona fide autophagic degradation is induced in sensitive GIST cells upon imatinib treatment.
Punctate LC3-II Is Observed in Human GISTs Treated with Imatinib.
We next assessed whether imatinib treatment induced autophagosome formation (punctate LC3) in human GIST in vivo by using tissue samples from GIST patients who were randomized to treatment with imatinib (600 mg/d) for either 3, 5, or 7 d before resection (19). KIT and PDGFRA mutation status was known in each case, allowing us to predict imatinib sensitivity based on published in vivo and in vitro sensitivities of each mutant to imatinib (5, 20). Significant viable tumor cell populations were observed in all samples after treatment, indicating that imatinib is minimally cytotoxic in GIST tumor cells at this early timepoint. Using immunohistochemistry to detect the punctate distribution of LC3 within tumor cells (Fig. 3D), cases were scored as negative (0% cells positive), focal (<25% cells positive), and moderate (≥25% cells positive). In the imatinib-sensitive group, increasing amounts of punctate LC3 were seen in the 7-d treatment group compared with patients treated for shorter times (Fig. 3E); in contrast, only negative or focal staining was observed in the imatinib-resistant group (Fig. 3F). Because autophagy induction inversely correlates with cell death in numerous experimental models, we next assessed apoptosis in these samples by using immunohistochemistry for cleaved caspase-3 (CC-3) (8, 9). Cases were scored as low (<20 positive cells/5 high power fields) or high (≥20 positive cells/5 high power fields). Importantly, those imatinib-sensitive cases that exhibited moderate staining for LC3 had low amounts of CC-3, whereas 9 of 15 imatinib-sensitive GIST with low or no staining for autophagy showed high CC-3. These in vivo results are consistent with our in vitro results, demonstrating that imatinib can induce autophagosome formation in GIST; moreover, increased autophagosome formation in these GIST cases treated with imatinib correlates with decreased levels of apoptosis.
ATG Depletion Promotes the Death of Imatinib-Treated GIST Cells.
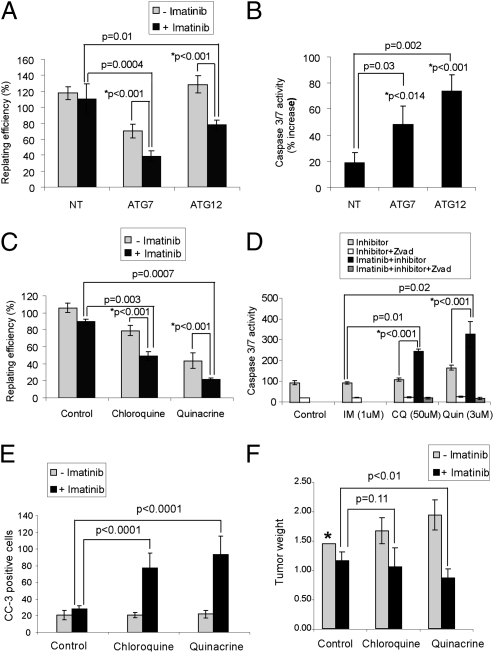
We next asked whether autophagy promotes GIST survival during imatinib treatment. Using RNAi against ATG7 and ATG12, two critical autophagy regulators, we tested whether autophagy inhibition increased GIST cell death in combination with imatinib (21). ATG knockdown significantly reduced GFP-LC3 puncta in imatinib-treated GIST-T1 cells and decreased LC3-II formation by immunoblotting (Fig. S5). Furthermore, ATG7 or ATG12 depletion potently reduced clonogenic recovery by ≈50% in combination with imatinib treatment (Fig. 4A). To determine whether apoptosis was induced, we measured caspase 3/7 activation in imatinib-treated, ATG-depleted GIST cells (Fig. 4B). A 3- to 4-fold increase in caspase 3/7 activity was observed in ATG knockdown cells after treatment with 1 μM imatinib but not in nontargeting controls. Statistical analysis confirmed that ATG knockdown synergized with imatinib to kill GIST cells (Fig. 4 A and B). Collectively, these data support the hypothesis that autophagy contributes to the survival of imatinib-treated GIST cells.
Fig. 4.
ATG depletion and antimalarial lysosomal inhibitors promote cell death in imatinib-treated GIST cells. (A) Clonogenic replating efficiency of cells after 72 h of treatment with or without 1 μM imatinib in ATG7- or ATG12-depleted cells. (B) Caspase 3/7 activity was measured in ATG7- or ATG12-depleted cell lines with administration of 1 μM imatinib for 72 h. (C) Clonogenic replating efficiency of GIST-T1 cells after 48 h of treatment with 1 μM imatinib, 50 μM chloroquine, or 3 μM quinacrine and each autophagy inhibitor in combination with imatinib. (D) Caspase 3/7 activity was measured after treatment with imatinib, chloroquine, or quinacrine and each autophagy inhibitor in combination with imatinib. Results are the mean ± SD from three independent experiments. The percent increase in caspase 3/7 activity in cells treated with imatinib relative to untreated controls is shown. NT, nontargeting siRNA pool. P values indicated with an asterisk are based on statistical analysis of synergy between imatinib and inhibition of autophagy (see details in SI Methods). (E) CC-3 positive cells ± SEM from mouse GIST-T1 xenografts after treatment with imatinib alone or in combination with either chloroquine or quinacrine. (F) Tumor weight ± SEM of mouse GIST-T1 xenografts after treatment with imatinib alone or in combination with either chloroquine or quinacrine. Note that untreated control tumors underestimate tumor size (designated by *) because it is necessary to euthanize all untreated control mice because of excessive tumor growth before the end of the experiment.
Cotreatment with Imatinib and Lysosomotrophic Antimalarial Agents Promotes Cell Death and Abrogates Imatinib Resistance in GIST.
Emerging evidence indicates that cancer cells undergoing autophagy are highly sensitive to treatment with lysosomotrophic agents, such as the antimalarial chloroquine, which inhibits lysosomal acidification and blocks the terminal stages of autophagic proteolysis (22, 23). We confirmed that chloroquine elicited an increase in LC3-II in GIST-T1 cells, as did another antimalarial agent, quinacrine (Fig. S6). Quinacrine also inhibited both baseline and starvation-induced LC3-II turnover in MCF10A mammary cells (Fig. S6), demonstrating that quinacrine functions similarly to other lysosomotrophic agents with regard to its ability to inhibit autophagy.
To assess how these drugs impact GIST cell survival, we treated GIST-T1 cells with chloroquine or quinacrine, both in the presence and absence of imatinib. Indeed, a synergistic decrease in clonogenic survival was observed when GIST-T1 cells were cotreated with imatinib and either chloroquine or quinacrine (Fig. 4C). Importantly, GIST-T1 cells also exhibited significantly reduced viability when treated with either chloroquine or quinacrine as single agents; indeed, quinacrine was highly cytotoxic compared with imatinib alone. Quinacrine also produced the greatest enhancement in apoptosis, eliciting a 2-fold increase in caspase 3/7 activity (Fig. 4D). In addition, a synergistic effect was observed when combining these agents with imatinib, resulting in a 3.5-fold increase in caspase activity compared with imatinib alone (Fig. 4D).
To more thoroughly scrutinize whether imatinib and autophagy inhibitors acted synergisitcally, several concentrations of imatinib (0–5 μM) were combined with several concentrations of either chloroquine (0–200 μM) or quinacrine (0–10 μM), upon which caspase 3/7 activity was assayed. Chou–Talalay analysis demonstrated that imatinib synergized with chloroquine over the entire range of drug concentrations (Fig. S7), whereas imatinib synergized with quinacrine at intermediate doses (Fig. S8) (24, 25).
Furthermore, in a mouse GIST-T1 xenograft model, treatment with imatinib in combination with either chloroquine or quinacrine resulted in a statistically significant increase in apoptosis, evidenced by increased CC-3 staining in tissue sections (Fig. 4E). In addition, mice treated with the combination of imatinib and quinacrine exhibited a statistically significant decrease in tumor size in comparison with those treated with imatinib alone (Fig. 4F). The decrease in tumor size was especially remarkable because treatment lasted only 15 d. Overall, these results corroborate that autophagy inhibition using antimalarials promotes apoptosis and decreases GIST cell viability in vivo.
To support these pharmacological studies, we depleted lysosome-associated membrane glycoprotein-2 (LAMP2), which is required for autophagosome–lysosome fusion (9). LAMP2 knockdown produced an increase in GFP-LC3 puncta and LC3-II and promoted a synergistic increase in the death of imatinib-treated GIST cells as assessed by clonogenic replating and caspase-3/7 activation (Fig. S9).
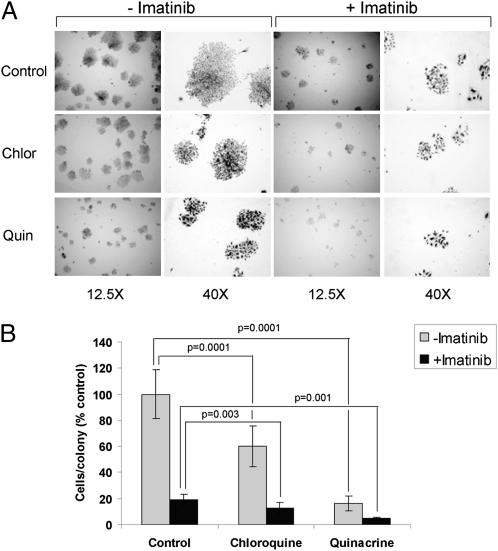
Finally, we tested the ability of chloroquine and quinacrine to attenuate the long-term outgrowth of imatinib-treated GIST cells in vitro. GIST-T1 cells subject to imatinib-induced quiescence exhibit robust cell cycle arrest and metabolic suppression in vitro, consistent with stable disease; nonetheless, upon culture in imatinib for extended periods, these cells do exhibit low-level proliferation (Fig. 5). However, this outgrowth was synergistically reduced when GIST-T1 cells were cultured in the presence of imatinib combined with either quinacrine or chloroquine (Fig. 5). Once again, both chloroquine and quinacrine exhibited significant single agent efficacy in preventing the outgrowth of GIST cells; notably, quinacrine and imatinib demonstrated equivalent results when used as solitary treatments. Overall, these results suggest that chloroquine and quinacrine, two well-tolerated, inexpensive drugs with a long history of use in humans, may be useful to attenuate the expansion of both imatinib-sensitive and -resistant GIST cells.
Fig. 5.
Antimalarials attenuate the outgrowth of imatinib-resistant GIST cells in vitro. (A) Representative images from GIST-T1 cells plated at 100 cells per well and treated for 14 d with 0.1 μM imatinib, 5 μM chloroquine, or 0.5 μM quinacrine either as single agents or in the designated combinations. (B) Quantification of cells per colony under the various conditions; the data are presented as mean ± SEM. P values indicated with an asterisk are based on statistical analysis of synergy between imatinib and inhibition of autophagy (see details in SI Methods).
Discussion
Imatinib has revolutionized the treatment of GIST, resulting in disease stabilization in ≈75% of patients (1); nonetheless, imatinib is associated with two major clinical problems that must be addressed to improve the long-term outcome. First, most, if not all, patients with metastatic disease remain stable or progress; total tumor regression is rare (3). Thus, lifelong therapy with imatinib is standard of care. Indeed, patients with inoperable GIST have remained stable on imatinib for >8 y (3). Moreover, even after extended therapy, GISTs progress rapidly after withdrawal of imatinib (4). The second major problem is the emergence of acquired resistance during therapy, typically the result of second-site intraallelic KIT mutations that inhibit imatinib binding and reactivate oncogenic KIT signaling (5). Because the cell culture models described here recapitulate the key biological features observed in imatinib-treated GIST in vivo, they create the mechanistic platform needed to address these therapeutic barriers.
Growing evidence indicates that autophagy contributes to chemotherapeutic resistance (10). Here, we demonstrate that imatinib induces autophagy in imatinib-sensitive GIST in vitro and in vivo. Imatinib has been shown to induce autophagy in a variety of nonneoplastic and neoplastic cell lines (22, 26, 27). Although imatinib may stimulate autophagy by inhibiting unidentified proteins (off-target effects), our results using GIST-T1-R, an isogenic GIST cell line that has acquired an imatinib-resistant KIT mutation, demonstrate decreased autophagy compared with imatinib-sensitive cells. Thus, autophagy induction by imatinib requires KIT kinase inhibition in GIST.
We also assessed the effects of autophagy inhibition on GIST cell fate and observed that ATG depletion enhanced GIST cell death when combined with imatinib. Recently, the combination of autophagy inhibition and p210BCR/ABL inhibition by imatinib resulted in near complete eradication of CML stem cells, which were otherwise resistant to imatinib alone (22). Little is known about the progenitors that give rise to GIST (28). Whether these precursor cells are susceptible to autophagy inhibitors, either alone or in combination with imatinib, is an important topic for future investigation.
Recent evidence indicates that cancerous cells that induce autophagy in response to targeted therapies exhibit sensitivity to lysomotrophic agents (22, 23). Accordingly, we now demonstrate that lysosomal inhibition sensitizes GIST to die when combined with imatinib both in vitro and in vivo. Furthermore, the outgrowth of GIST cells observed in the presence of imatinib alone is significantly reduced when either chloroquine or quinacrine is combined with imatinib. Remarkably, the inability of imatinib to kill GIST cells may facilitate the proliferation of resistant cells harboring second-site KIT mutations; hence, combination therapy with either chloroquine or quinacrine can attenuate secondary resistance that develops during extended imatinib treatment.
In comparison with chloroquine, our results indicate that quinacrine exhibits more robust cytotoxicity against GIST cells, both as a single agent and in combination with imatinib. Like chloroquine, quinacrine has a long history as an antimalarial, but to our knowledge, it has not been identified as an autophagy inhibitor. However, its structure resembles chloroquine and it is known to accumulate in yeast vacuoles, the equivalent of the mammalian lysosome (29). Importantly, we demonstrate that quinacrine behaves identically to other established lysosomal inhibitors in terms of its ability to inhibit autophagic flux. Based on these results, we hypothesize that the cytotoxic effects of quinacrine in GIST cells are due to autophagy and lysosomal inhibition. Still, we recognize that the cytotoxic effects of quinacrine and chloroquine may involve other processes (30, 31). Because both agents are in clinical trials for cancer, further studies are needed to dissect the precise contributions of autophagy inhibition to their antineoplastic effects.
Overall, we demonstrate that a significant proportion of GIST cells survive imatinib therapy by entering a state of reversible quiescence and activating an autophagy-dependent survival mechanism. Autophagy inhibition, using either ATG knockdown or pharmacological lysosomal inhibition with antimalarials, potentiates imatinib cytotoxicity against GIST cells, both in vitro and in vivo. Based on these results, we predict that combination treatment using imatinib and either chloroquine or quinacrine, two inexpensive antimalarial agents with well-known toxicity profiles, has the potential to greatly improve clinical outcome in GIST patients.
Methods
Autophagy Analysis.
Multiple complementary assays were used to measure autophagosome formation and autophagic flux, including: quantification of punctate LC3 using immunohistochemistry (in tissues) or fluorescence microscopy (in GIST cells stably expressing LC3 fluorescent protein chimeras), the formation and lysosomal turnover of lipidated LC3 (LC3-II), and degradation of the autophagy substrate, p62. All techniques were performed in accordance with established guidelines (14). Details are found in SI Methods.
Cell Colony Outgrowth Assay.
Cells were plated at 100 cells per well in six-well tissue culture plates in various conditions. Colonies were grown out for 14 d in the presence of 0.1 μM imatinib, 5 μM chloroquine, or 0.5 μM quinacrine alone or in combination with imatinib, fixed with methanol, and stained with 0.2% crystal violet. The number of cells per colony was enumerated in 50 randomly oriented colonies. Each experiment was repeated three times.
Details for cell culture, antibodies and chemicals, generation of stable lines, cell-cycle analysis, cell proliferation and apoptosis analyses, glucose uptake analysis, analysis of punctate GFP-LC3 and GFP-mCherry-LC3, analysis of LC3 by immunohistochemistry, clonogenic replating assay, RNA interference, immunoblot analysis, electron microscopy, treatment of GIST-T1 xenografts, human subjects, and statistics are given in SI Methods.
Supplementary Material
Acknowledgments
We thank James Bena, PhD (Department of Quantitative Health Sciences, Cleveland Clinic), for assistance with statistical evaluations and Ms. Paula Carver for excellent technical assistance. GIST 882 was a gift of Dr. Jonathan Fletcher (Brigham and Women's Hospital, Boston). A grant from the Life Raft Group (to B.P.R. and M.D.-R.), National Cancer Institute Grant R01 CA126792 (to J.D.), a Culpeper Medical Scholar Award (Partnership For Cures) (to J.D.), an AACR-Genentech BioOncology Award (to J.D.), and a Howard Hughes Medical Institute Physician-Scientist Early Career Award (to J.D.) supported this work.
Footnotes
Conflict of interest statement: B.P.R. is a member of the Novarits Speakers Bureau, is a consultant for Novartis and has designed educational material for Novartis. A.J.F.L. is a member of the Novartis Speakers Bureau. J.A.T. is a member of the Novartis Speakers Bureau and participates in Novartis-sponsored research and clinical trials. P.S. has received a commercial research grant from Novartis. M.D.-R. has received honoraria from Novartis.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000248107/-/DCSupplemental.
References
- 1.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 2.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: Before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 3.Blanke CD, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 4.Blay JY, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107–1113. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 6.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 8.Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- 9.Thorburn A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 11.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 12.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Agaram NP, et al. Pathologic and molecular heterogeneity in imatinib-stable or imatinib-responsive gastrointestinal stromal tumors. Clin Cancer Res. 2007;13:170–181. doi: 10.1158/1078-0432.CCR-06-1508. [DOI] [PubMed] [Google Scholar]
- 14.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 17.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 18.Bjørkøy G, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAuliffe JC, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: Evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009;16:910–919. doi: 10.1245/s10434-008-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrich MC, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 22.Bellodi C, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119:1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degtyarev M, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou T. The median-effect principle and the combination index for quantitation of synergism and antagonism. In: Chou TR, Rideout DC, editors. Synergism and antagonism in chemotherapy. San Diego: Academic; 1991. pp. 61–102. [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Ertmer A, et al. The anticancer drug imatinib induces cellular autophagy. Leukemia. 2007;21:936–942. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 27.Shingu T, et al. Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. Int J Cancer. 2009;124:1060–1071. doi: 10.1002/ijc.24030. [DOI] [PubMed] [Google Scholar]
- 28.Lorincz A, et al. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts CJ, Raymond CK, Yamashiro CT, Stevens TH. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- 30.Guo C, et al. 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-kappaB and p53 pathways. Oncogene. 2009;28:1151–1161. doi: 10.1038/onc.2008.460. [DOI] [PubMed] [Google Scholar]
- 31.Gurova KV, et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA. 2005;102:17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.