Abstract
Elongation of the digit rays resulting in the formation of a defined number of phalanges is a process poorly understood in mammals, whereas in the chicken distal mesenchymal bone morphogenetic protein (BMP) signaling in the so-called phalanx-forming region (PFR) or digit crescent (DC) seems to be involved. The human brachydactylies (BDs) are inheritable conditions characterized by variable degrees of digit shortening, thus providing an ideal model to analyze the development and elongation of phalanges. We used a mouse model for BDB1 (Ror2W749X/W749X) lacking middle phalanges and show that a signaling center corresponding to the chick PFR exists in the mouse, which is diminished in BDB1 mice. This resulted in a strongly impaired elongation of the digit condensations due to reduced chondrogenic commitment of undifferentiated distal mesenchymal cells. We further show that a similar BMP-based mechanism accounts for digit shortening in a mouse model for the closely related condition BDA1 (IhhE95K/E95K), altogether indicating the functional significance of the PFR in mammals. Genetic interaction experiments as well as pathway analysis in BDB1 mice suggest that Indian hedgehog and WNT/β-catenin signaling, which we show is inhibited by receptor tyrosine kinase-like orphan receptor 2 (ROR2) in distal limb mesenchyme, are acting upstream of BMP signaling in the PFR.
Keywords: bone morphogenetic protein signaling, brachydactyly, cartilage, limb development, Wnt signaling
The appendicular skeleton arises as a continuous cartilaginous condensation in the center of the limb bud that develops in a proximal to distal sequence. Distal outgrowth is under the control of fibroblast growth factor (FGF) signaling from the apical ectodermal ridge (AER), which accounts for proliferation in the subridge mesenchyme and prevents premature differentiation of mesenchymal cells, thus maintaining a progenitor pool. Cells leaving the range of AER-FGF signaling undergo differentiation into the mesenchymal cell lineages of the limb bud (1, 2).
Evidence from the chick indicates that bone morphogenetic protein (BMP)/pSMAD1/5/8 signaling in a population of cells in front of the growing condensation, referred to as the phalanx-forming region (PFR) or digit crescent (3, 4), is involved in the elongation of the digital rays. This work suggests that the PFR acts as a signaling center to drive distal elongation of the digit and thus determines the number of phalanges via commitment of distal mesenchymal cells to the cartilage condensation. However, evidence for such a mechanism in the mouse or human is missing.
If a PFR-like structure exists in mammals, its failure is expected to cause digit malformation phenotypes such as digit shortening and loss of phalanges. This phenotypic spectrum is typical for a family of human inheritable malformations, the brachydactylies (BDs), which are characterized by the absence or reduction of individual phalanges and/or metacarpals (5). Intriguingly, several mutations causing human BDs (BDA2, BDB2, and BDC) affect the BMP pathway (5), which suggests the involvement of a PFR-like structure in digit growth.
BD types A1 and B1 are of particular interest, because they show a generalized reduction defect in specific phalanges. BDA1 is characterized by shortening/absence of all middle phalanges. BDB1 is characterized by an amputation-like phenotype with shortening/absence of distal and often middle phalanges. Human BDA1 and BDB1 are caused by mutations in Indian hedgehog (IHH) or receptor tyrosine kinase-like orphan receptor 2 (ROR2), respectively (5). IHH is a factor required for endochondral ossification. BDA1 mutations change the signaling capacity and range of IHH, thereby altering distal chondrogenesis (6). ROR2 encodes a receptor tyrosine kinase, which is truncated by BDB1 mutations. The function of ROR2 is not fully understood, and there is evidence suggesting that ROR2 functions as a WNT (co)receptor (7). For example, it was shown that WNT5A via ROR2 can inhibit canonical WNT/β-catenin signaling (8).
Mouse models for BDA1 and BDB1 generated by targeted insertion of human mutations into the mouse Ihh and Ror2 loci partially recapitulate these phenotypes. BDA1 mice with a p.E95K mutation in Ihh (6) show shortened middle phalanges, whereas BDB1 mice with a p.W749X mutation in Ror2 (9) exhibit absent middle phalanges; thus the BDB1 and BDA1 mice present a graded digit reduction phenotype.
To address the mechanism controlling the outgrowth of the digital rays in mammals, we first analyzed the BDB1 mouse, demonstrating that a mesenchymal cell population corresponding to the chick PFR exists in the mouse, contributing to mammalian digit elongation. Consistent with the overlapping but milder phenotype of the BDA1 mice, a milder disruption of the PFR was observed, indicating that a graded decrease in BMP/pSMAD1/5/8 signaling in the PFR might account for different BD phenotypes. From further genetic studies, we propose a model in which IHH, ROR2, and WNT signaling regulate PFR activity.
Results
Brachydactyly in Ror2W749X/W749X Mutants Corresponds to Decreased BMP/pSMAD1/5/8 Signaling in the PFR.
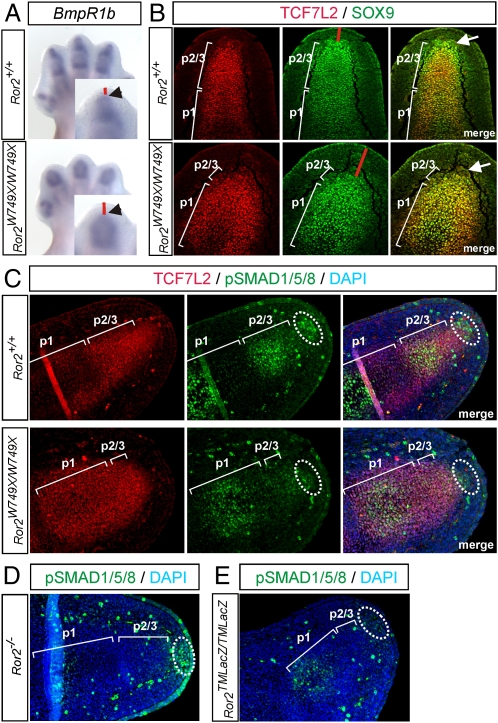
First, we used the Ror2W749X/W749X mice (BDB1 model) to test for the existence and function of a PFR in mammals. The avian PFR has been characterized by the expression of BmpR1b, Sox9, and a high activity of the BMP signal mediators phospho-SMAD1/5/8 (pSMAD1/5/8) (3, 4). Analysis of BmpR1b expression in Ror2W749X/W749X mice by whole-mount in situ hybridization (ISH) showed a reduced distal signal and an increased distance between the most distal BmpR1b expression and the ectoderm (Fig. 1A). Immunostaining for SOX9 colabeled for TCF7L2, which stains the nascent condensation, revealed a population of cells expressing SOX9 distal to the cartilaginous condensation at embryonic day 13.5 (E13.5) in WT mice (Fig. 1B). In Ror2W749X/W749X mice, this population of SOX9 positive cells was absent, and the distance between the distal SOX9 expression region and the ectoderm was increased (Fig. 1B). Furthermore, immunostaining for pSMAD1/5/8 revealed a population of mesenchymal cells distal to the definitive cartilage that was strongly positive for active BMP signaling in WT mice (Fig. 1C), overlapping the population of Sox9-positive cells described above. These findings indicate that a region similar to the PFR described in the chick is also present in the mouse. In Ror2W749X/W749X mice, vastly decreased pSMAD1/5/8 staining in the distal mesenchymal population indicates a breakdown of BMP/SMAD signaling in the distal mesenchyme (Fig. 1C).
Fig. 1.
Existence of the PFR in the mouse and its disruption in Ror2W749X/W749X mutants. (A) Whole-mount ISH showing missing expression of BmpR1b in distal-most mesenchyme (arrowheads) and expanded distance between BmpR1b expression and ectoderm (red bars) in the Ror2W749X/W749X mutant. (B) Immunostaining for TCF7L2 marking the cartilage condensation (red) and SOX9 (green) marking chondrogenic progenitors shows a domain of SOX9 expressing cells distal to definitive cartilage in the WT, which is absent in the Ror2W749X/W749X mutant (arrows). Note the increased distance between SOX9 expression and ectoderm (red bars) in the Ror2W749X/W749X mutant. (C) Immunostaining for phospho-SMAD1/5/8 (green) revealing the presence of a PFR in WT mouse embryos at E13.5 (dotted circle). This population of pSMAD1/5/8-positive cells is lacking in the Ror2W749X/W749X mutant. (D) Immunolabeling for pSMAD1/5/8 shows normal staining in the distal mesenchyme in Ror2−/− mutants, whereas in Ror2TMLacZ/TMLacZ mutants (E) pSMAD1/5/8 staining is diminished. p1, condensation of phalanx 1; p2/3, unseparated primordium of phalanges 2 and 3.
Ror2−/− mice do not show a reduction of the middle phalanx (p2), indicating that the Ror2W749X allele has a gain-of-function effect, which was supported by crossing one Ror2W749X allele on a Ror2-null background, yielding an intermediate phenotype (Fig. S1). Consistently, analysis of pSMAD1/5/8 in Ror2−/− mice showed a normal PFR staining similar to WT mice (Fig. 1D). To further test the involvement of the PFR in the brachydactyly phenotype, we analyzed Ror2TMLacZ/TMLacZ mice, which display a digit phenotype comparable to the Ror2W749X/W749X mice. As expected, Ror2TMLacZ/TMLacZ mice showed absent pSMAD1/5/8 staining in distal mesenchyme (Fig. 1E). Together, these data suggest that a PFR is driving cartilage condensation during digit formation in the mouse, similar to the chick, and that Ror2W749X interferes with PFR function, thus causing brachydactyly.
PFR Failure in Ror2W749X/W749X Mutants Causes Impaired Elongation of the Digit Condensation.
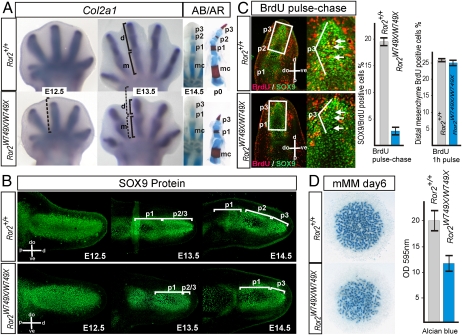
To address the pathomechanism leading to loss of p2 in the Ror2W749X/W749X mouse, we monitored the appearance and differentiation of cartilaginous condensations in the autopod at embryonic day 12.5 (E12.5) to E14.5, the time when the phalanges are formed and become separated by joints. Whole-mount ISH for Collagen type 2 alpha 1 (Col2a1) showed that the initial cartilage elements of the autopod at E12.5 were only slightly shorter in the mutant when compared with the WT (Fig. 2A). At E13.5 the metacarpal arising from the initial condensation showed a normal length, whereas the distal condensations that give rise to the growing phalanges were severely reduced in length (Fig. 2A). Longitudinal sections of the autopod immunolabeled with a SOX9 antibody (Fig. 2B) also showed almost normally sized condensations of the metacarpal at E12.5 and the proximal phalanx (p1) at E13.5. However, the distal-most condensations giving rise to the phalanges 2 and 3 (p2/3) showed severe shortening in mutant mice at E13.5. This led to a striking reduction in distal cartilage size at E14.5, a time at which the activity of the AER ceases and the distal-most condensation in the autopod starts to differentiate into a terminal phalanx (2). This indicates a defect in digit elongation after the establishment of the initial condensations in the autopod in Ror2W749X/W749X mice. Consistently, the phalangeal elongation defect is specific for the Ror2W749X allele, because comparison of distal p2/3 length between WT, Ror2−/−, and Ror2W749X/W749X mice at E13.5 confirmed that the p2/3 condensation is slightly shortened in the Ror2−/− mutant but is markedly reduced in the Ror2W749X/W749X mouse (Fig. S2).
Fig. 2.
Defective distal elongation after establishment of the initial condensation causes digit shortening in the Ror2W749X/W749X mutant via perturbed commitment of mesenchymal cells to cartilage. (A) Cartilage formation in the autopod between E12.5 and E14.5 visualized by whole-mount ISH for Collagen type 2 alpha 1 (Col2a1) and by Alcian blue (AB) staining. At E12.5 the initial condensations in the autopod of the Ror2W749X/W749X mice are only slightly shortened (bracket shows length of WT condensation for comparison). At E13.5 the digit condensations (d) exhibit a marked shortening in the Ror2W749X/W749X mice, resulting in reduced distal phalangeal condensations at E14.5 visualized by Alcian blue (AB) staining. Alcian blue and Alizarin red (AR) staining of a p0 (newborn) digit 3 is shown for comparison; note missing middle phalanx (p2) and terminal phalanx (p3)-like appearance of the most distal element. (B) Anti-SOX9 antibody staining on longitudinal sections through a digit 3 demonstrating a decrease in cartilage formation distal to the first phalanx in the Ror2W749X/W749X mutant. Note that the Ror2W749X/W749X limb buds are wider than WT limb buds but have a normal length at E12.5. (C) BrdU pulse-chase experiment: 1 h pulse of BrdU at E13.5 was used to label mesenchymal cells and then, after blocking further incorporation of BrdU with excess thymidine, their fate was analyzed after 10 h. Sections were stained for BrdU (red) and SOX9 (green). Strong incorporation of mesenchymal cells into the SOX9-positive cartilage condensation was seen in the WT (Ror2+/+), where numerous BrdU positive cells can be seen in the core of the cartilage condensation (arrows). In the Ror2W749X/W749X mutant, no BrdU-positive cells were observed in the core of the distal condensation. Quantification of SOX9/BrdU-positive cells in the distal condensation is shown to the right. Quantification of 1 h BrdU pulse labeling shows normal proliferation in distal mesenchyme. Error bars depict SEs deduced from at least three independent experiments. (D) Micromass cultures derived from E12.5 hand plates stained with Alcian blue for cartilage matrix, showing reduced chondrogenic potential of Ror2W749X/W749X mesenchyme. Colorimetric quantification of Alcian blue staining from at least three independent experiments is shown to the right. m, metacarpal; p1, p2, p3, condensations of phalanges 1, 2 and 3, respectively; p2/3, unseparated primordium of phalanges 2 and 3. Orientation of sections as indicated; dorsal (do), ventral (ve), proximal (p), and distal (d).
Impaired Digit Elongation in Ror2W749X/W749X Mutants Is Caused by Defective Commitment of Mesenchymal Cells to the Cartilage Lineage.
In the chick, the PFR controls digit elongation via cartilaginous commitment of mesenchymal progenitors (3, 4). To determine the rate of cell commitment into the growing cartilage condensation, we quantified the incorporation of mesenchymal cells into the distal condensations using BrdU pulse-chase labeling (6). Pregnant Ror2+/W749X mice were pulse-chase labeled with BrdU at E13.5 and analyzed at E14. Importantly, 1 h pulse labeling with BrdU does not result in a staining in the condensed cartilage but only in the surrounding mesenchyme (6). Coimmunostaining for BrdU and SOX9 ensured that only cartilage cells were counted. The results show a dramatic decrease in mesenchymal cell recruitment into the distal condensation (p2/3), which was reduced to less than 20% of WT values (Fig. 2C). One hour BrdU pulse labeling showed no differences in subridge mesenchyme proliferation rates (Fig. 2C). Compatible with a condensation defect, LacZ staining on Ror2TMLacZ/+ mice, which are phenotypically normal (10), confirmed expression of ROR2 within cartilage condensations and in distal mesenchymal cells undergoing chondrogenesis in the autopod (Fig. S3). Micromass cultures derived from E12.5 hand plate mesenchymal cells stained with Alcian blue for cartilage nodules confirmed a reduced chondrogenic potential of the Ror2W749X/W749X mesenchyme compared with WT (Fig. 2D).
Altogether, these data indicate that a defect in chondrogenesis at a time crucial for the formation of the distal phalanges (between E13.5 and E14.5) is responsible for the digit shortening. Given that we have previously excluded a defect in the proliferation within the cartilage condensations by BrdU pulse labeling (9), the brachydactyly phenotype in the Ror2W749X/W749X mouse is not caused by a defect in the size of the initial condensation or its proliferative expansion but a failure of commitment of mesenchymal cells to the cartilage lineage.
Requirement of Mesenchymal IHH Signaling for the BMP/pSMAD1/5/8 Pathway in the PFR.
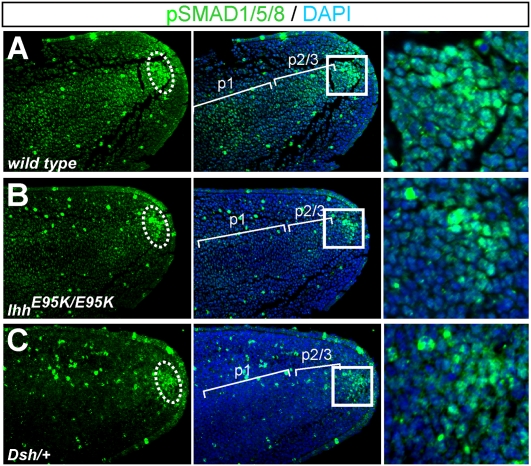
BDB1 and BDA1 share similar digit features, and a mouse model (IhhE95K/E95K) for BDA1 carrying a human mutation (p.E95K) in IHH targeted to the mouse Ihh locus exhibits hypoplastic middle phalanges partially overlapping with the Ror2W749X/W749X mouse. Interestingly, the digit phenotype in the IhhE95K/E95K mutant was caused by a similar, albeit weaker, impairment of chondrogenic cell commitment due to a disruption of the IHH pathway in the distal mesenchyme (6), indicating a common mechanism for both mutant phenotypes. Compared with WT littermates that showed normal pSMAD1/5/8 staining in the PFR and also in the cartilaginous condensations at E13.5 (Fig. 3A), in IhhE95K/E95K mutant mice we observed a reduced pSMAD1/5/8 staining in the PFR and in the cartilage condensations (Fig. 3B). In accordance with the milder phenotype seen in the IhhE95K/E95K mutants, pSMAD1/5/8 staining was reduced to a lesser degree than in the Ror2W749X/W749X mice.
Fig. 3.
BMP/pSMAD1/5/8 signaling in the PFR is decreased in IhhE95K/E95K and Dsh/+ mouse models for BDA1. (A–C) Immunostaining for pSMAD1/5/8 (green) demonstrates down-regulation of distal BMP/SMAD1/5/8 signaling in the PFR of both mutants. Boxed regions are shown as magnifications. Note that pSMAD1/5/8 staining is also decreased within the condensations in IhhE95K/E95K and Dsh/+ mutants compared with WT.
To further substantiate the involvement of IHH signaling in the regulation of BMP signaling in the PFR, we used the short digits mouse mutant (Dsh/+) that also shows a BDA1 phenotype. The Dsh/+ phenotype is due to an up-regulated Pthlh expression from ectopic Shh expression, leading to a suppressed Ihh expression in distal phalanges (11), hence a mechanism comparable to the IhhE95K/E95K mice (6). Immunolabeling for pSMAD1/5/8 also showed a reduced signal in the PFR of Dsh/+ mice (Fig. 3C). Together, these results indicate a critical involvement of the BMP/pSMAD1/5/8 signaling pathway in the pathogenesis of the BDB1 and BDA1 phenotypes, indicating a potential common pathomechanism for BDB1 and BDA1, whereby ROR2 and IHH signaling might interact during digit elongation.
Genetic Interaction of Ror2+/W749X and Ihh+/E95K Mutations Indicate Cooperation of IHH and ROR2 Signaling.
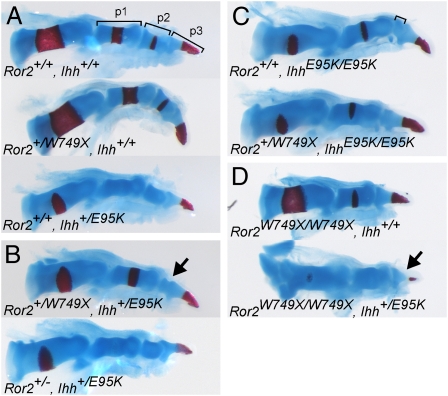
To further test this hypothesis, we crossed the Ror2+/W749X and Ihh+/E95K mice to test for genetic interaction. Ror2+/W749X mice show no digit phenotype, whereas Ihh+/E95K mice exhibit mild shortening of p2 in digits 2 and 5. IhhE95K/E95K mice show a loss of p2 in digit 5 and severely reduced p2 in digits 2–4 (6). Compound Ror2+/W749X and Ihh+/E95K heterozygous mice showed severe reduction of p2 in digits 2 and 3, which was more prominent than the effect of the single IhhE95K allele, indicating a genetic interaction (Fig. 4 A and B and Fig. S4). Again, this effect was specific to the Ror2W749X allele, because Ror2+/−; Ihh+/E95K heterozygous mice showed no compound effect for the digit phenotype (Fig. 4B). When one Ror2W749X allele was crossed to a homozygous IhhE95K/E95K background, we observed a complete loss of the second phalanx in digits 2 and 3 (Fig. 4C and Fig. S4), phenocopying Ror2W749X/W749X mice and being more severe than in IhhE95K/E95K mice. This suggests that both IHH and ROR2 act, at least in part, independently of each other in digit elongation. Loss or severe reduction of the terminal phalanges and nails is a hallmark of human BDB1 that is not recapitulated in the Ror2W749X/W749X mice, probably owing to the high regenerative potential of digit tips in mice. However, when crossing one IhhE95K allele on a Ror2W749X/W749X background, a severely hypoplastic terminal phalanx was observed (Fig. 4D), suggesting an involvement of the IHH pathway in the pathogenesis of BDB1 and a contribution of IHH signaling to the phenotype seen in Ror2W749X/W749X mutants.
Fig. 4.
Genetic interaction of Ror2W749X (BDB1 mutation) and IhhE95K (BDA1 mutation). Skeletal preparations stained for cartilage (Alcian blue) and bone (Alizarin red) of digit 2 from newborn mice of the indicated allelic combinations are shown. (A) Single heterozygous Ror2+/W749X mutants have a WT appearance, whereas Ihh+/E95K mutants show a mild reduction in p2 length. (B) Compound Ror2+/W749X; Ihh+/E95K mutants show severely reduced middle phalanx (arrow). The genetic interaction is specific for the Ror2W749X allele, because a Ror2 null allele does not show genetic interaction with IhhE95K. (C) The phenotype of the homozygous IhhE95K/E95K (reduced p2 size) is enhanced by addition of one Ror2W749X allele, where the middle phalanx is now missing. (D) Similarly, the addition of one IhhE95K allele on the Ror2W749X/W749X background also enhances severity of the phenotype leading to a hypoplastic distal phalanx (arrow).
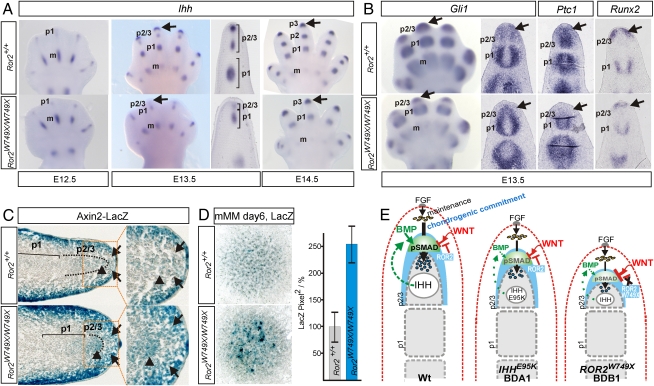
Decreased IHH Signaling in the Distal Mesenchyme of Ror2W749X/W749X Mutants.
To assess the involvement of IHH signaling in the BDB1 phenotype, we analyzed expression of Ihh and IHH downstream targets in Ror2W749X/W749X mice. Whole-mount ISH showed a decrease of Ihh expression in Ror2W749X/W749X mice compared with WT at stage E13.5 (Fig. 5A). Interestingly, distal Ihh expression was restored at E14.5, coinciding with the formation and differentiation of a distal phalanx (tip structure) (Fig. 5A). Analysis of IHH pathway targets Gli1, Ptc1, and Runx2 in Ror2W749X/W749X mice at E13.5 showed a strong down-regulation of the IHH signaling pathway not only in the distal condensations but also in the undifferentiated distal mesenchyme (Fig. 5B), to a level comparable to that in IhhE95K/E95K mice (6).
Fig. 5.
Dysregulation of IHH and canonical WNT pathways in Ror2W749X/W749X mutants. (A) Whole-mount ISH showing a down-regulated distal expression of Ihh in Ror2W749X/W749X mutant hand plates at E13.5 (arrows) that recovered concomitant with the differentiation of the digit tip at E14.5 (arrows). (B) Down-regulation of the IHH pathway in distal mesenchyme shown by whole-mount and section ISH for the IHH targets Gli1, Ptc1, and Runx2. (C) Ror2W749X line crossed to the canonical WNT reporter line Axin2LacZ, demonstrating ectopic activation of the canonical WNT pathway in Ror2W749X/W749X mice. LacZ staining on cryosections shows elevated/ectopic signal in the mutant in distal mesenchyme (arrows) and also in the distal-most condensation (arrowheads). Boxed areas are shown as magnifications. (D) Micromass cultures of E12.5 hand plates derived from Ror2W749X/W749X/Axin2LacZ embryos showing increased LacZ activity in cultures derived from mutant embryos. Right: Histomorphometrical quantification; error bars represent SEs from three independent experiments. (E) Schematic representation of pathway network regulating digit outgrowth in WT and its perturbation in the IhhE95K/E95K and the Ror2W749X/W749X mutants leading to impaired distal elongation of the digit condensations in the BDA1 and BDB1 phenotypes. AER–FGF signaling maintains undifferentiated mesenchymal cells (yellow). BMP/pSMAD1/5/8 signaling in the PFR (green) mediates distal outgrowth of the phalangeal condensation by controlling commitment of mesenchymal cells (arrow) to the condensation. IHH promotes distal outgrowth by enhancing chondrogenic cell commitment, potentially via induction of mesenchymal BMPs. Canonical WNT factors emanating from the ectoderm (red) induce β-catenin signaling in the mesenchyme, which inhibits BMP/pSMAD1/5/8 signaling in the PFR, thus limiting distal growth. The β-catenin signaling in the mesenchyme is negatively regulated by ROR2 (strong expression domain of ROR2 in condensing distal mesenchyme is highlighted in blue). In BDA1, the IHHE95K protein interferes with normal IHH signaling in distal mesenchyme, subsequently leading to a down-regulation of the BMP pathway in the PFR. In BDB1, the expression of a truncated ROR2 molecule leads to an up-regulation of WNT/β-catenin signaling in the mesenchyme concomitant with a down-regulation of Ihh expression and pathway activation, both converging on a drastic down-regulation of BMP/pSMAD1/5/8 signaling in the PFR. m, metacarpal; p1, condensation of phalanx 1; p2/3, unseparated primordium of phalanges 2 and 3.
Ectopic WNT/β-Catenin Signaling in Ror2W749X/W749X Mutants.
Because the Ror2W749X/W749X mutant displays a more pronounced digit phenotype than the IhhE95K/E95K mutant, and human BDB1 exhibits a more severe phenotype than BDA1, a disruption of IHH signaling in the Ror2W749X/W749X mutant cannot be solely responsible for the phenotype. ROR2 is known as an alternative WNT coreceptor involved in the negative regulation of canonical WNT/β-catenin signaling (8). ISH analysis for WNT/β-catenin signaling targets Itf-2 and Nmyc indicated an ectopic activation of canonical WNT signaling in the distal mesenchyme of Ror2W749X/W749X mice (Fig. S5A). Next, we performed immunostaining for dephosphorylated (activated) β-catenin. Sections were costained for SOX9 to ensure that correct planes were compared. Equally strong signals were observed in the muscles of WT and Ror2W749X/W749X mice; however, in the distal limb mesenchyme and also the distal SOX9-positive cell population, the β-catenin signal was significantly stronger in Ror2W749X/W749X mice than in WT mice (Fig. S5B). To definitively demonstrate ectopic WNT/β-catenin signaling in the distal limb, we crossed Ror2+/W749X mice with the Axin2LacZ reporter mice (12). LacZ staining of cryosections at E13.5 showed a strong WNT/β-catenin signal in the ectoderm and in the superficial mesenchyme, as reported previously for the early limb bud (13). However, the distal superficial mesenchyme (subridge mesenchyme) and the cells undergoing chondrogenesis showed absent or low WNT/β-catenin signaling in WT embryos (Fig. 5C, arrows), although these cells are in the range of WNTs emanating from the ectoderm, indicating that β-catenin signaling is suppressed in this area. In Ror2W749X/W749X mice, the distal mesenchyme and the distal condensation showed intense LacZ staining, indicating ectopic activation of the WNT/β-catenin pathway (Fig. 5C, arrows). This finding was also corroborated by whole-mount LacZ staining (Fig. S5C). Finally, to quantify the increase of canonical WNT signaling, we performed micromass cultures of mesenchymal cells from hand plates of E12.5 Ror2+/+/Axin2LacZ and Ror2W749X/W749X/Axin2LacZ embryos. Histomorphometric analysis of LacZ staining as an indicator of WNT/β-catenin signaling showed an increase in cultures derived from Ror2W749X/W749X mice by an average of 2.5-fold compared with WT levels (Fig. 5D).
Discussion
We have shown here that a defect in the PFR underlies digit shortening in mouse models for human BDA1 and BDB1 via a down-regulation of chondrogenic cell commitment, demonstrating that in mammals BMP/SMAD1/5/8 signaling in the PFR is instrumental in driving digit elongation and thus determination of phalanx numbers. Our results indicate that both IHH and ROR2 are acting independently upstream of BMP/SMAD1/5/8 signaling in the mammalian PFR, as summarized in Fig. 5E.
IHH signaling is essential for normal development of the phalanges in mouse and human (6, 14). IHH emanating from the cartilage condensation signals to the distal undifferentiated mesenchyme, regulating chondrogenic commitment of mesenchymal cells via an unknown mechanism (6). We propose that IHH influences distal chondrogenesis via BMP signaling. Hedgehogs regulate Bmps in different organisms from Drosophila to human, and in various developmental contexts including the cartilage growth plate and the limb bud (15, 16). Thus, the positive effect of IHH on distal chondrogenesis required for digit growth might be mediated, at least in part, by induction of prochondrogenic BMPs. In support of this hypothesis, mice with inactivated alleles of Ihh (17) showed a strong decrease of Bmp4 expression in the distal mesenchyme (Fig. S6).
Concomitant with the breakdown of BMP/pSMAD1/5/8 signaling in Ror2W749X/W749X mice, we observed an increase in canonical WNT/β-catenin signaling in the distal limb mesenchyme. WNT/β-catenin signaling can inhibit cartilage differentiation in vitro and in vivo (13, 18, 19) and acts antagonistic to BMP/SMAD signaling in cartilage formation (20, 21). Consistently, our results also point toward a negative role for WNT/β-catenin signaling in the cascade of events leading to the activation of BMP/SMAD signaling during digit elongation, by inhibiting either the formation or the maintenance of the PFR. The hypothesis that up-regulation of canonical WNT signaling might cause a brachydactyly phenotype is supported by mice devoid of the WNT antagonists SFRP1 and SFRP2, which develop a digit phenotype reminiscent of the Ror2W749X/W749X mice (22).
ROR2 was shown to be an alternative WNT receptor, mainly for WNT5A (8, 23), and WNT5A can inhibit the canonical WNT pathway via ROR2 (8). WNT5A has a vital role in promoting digit formation, because Wnt5a−/− mice lack proximal and middle phalanges (24). It has also been shown that WNT5A acts as a negative regulator of WNT/β-catenin signaling in distal limb mesenchyme in vivo (25). In addition to signaling via ROR2, it is likely that WNT5A has further functions because Wnt5a−/− mice have a more severe digit phenotype than Ror2−/− mice (23). WNT5A is known to signal via Frizzled receptors to noncanonical pathways including the WNT/calcium pathway (26), which can also inhibit the β-catenin pathway (27). Thus it is conceivable that the truncated ROR2 protein might act as a scavenger for WNT5A, thus inhibiting WNT5A signaling pathways. Interestingly such a scavenger-like function has been proposed for the Caenorhabditis elegans ROR ortholog CAM-1, which is lacking the C-terminal domain equivalent to mouse/human ROR2 p.W749X (28).
Limb outgrowth and digit elongation also requires intact AER–FGF signaling, which drives subridge mesenchyme proliferation and prevents premature initiation of the terminal phalanx (2). No premature regression of the AER was observed in Ror2W749X/W749X or IhhE95K/E95K mice (6, 9). At E14.5, the remaining distal cartilage is undergoing the program for tip formation in Ror2W749X/W749X mice, at the appropriate time as in WT mice, concordant with a reactivation of Ihh expression. Furthermore, precocious AER ablation is accompanied by apoptosis in the underlying mesoderm (29). We also tested for apoptosis rates by immunostaining for active caspase 3 but found no differences between WT and mutant mice in mesenchyme or in condensations (Fig. S7). This argues against an involvement of AER–FGF signaling in the phenotypes of the Ror2W749X/W749X and IhhE95K/E95K mutants and hence in the pathogenesis of human BDB1 or BDA1.
In the context of the disease mechanism for BDA1 and BDB1 (Fig. 5E), in BDA1 IHHE95K exhibits a negative effect on distal mesenchymal IHH signaling (6), thus resulting in lowered BMP/pSMAD1/5/8 signaling in the PFR. However, chondrogenesis is robust enough to result in a condensation that exceeds the minimal size for the formation of an additional joint, resulting in a shortened p2. In BDB1, Ihh expression and pathway activation is diminished by a yet-unknown mechanism. In addition to that, ROR2W749X interferes with the inhibition of canonical WNT/β-catenin signaling in the distal mesenchyme and the nascent condensation. This altogether results in a drastic reduction of BMP/pSMAD1/5/8 signaling, strongly impairing chondrogenesis and digit elongation. This results in a distal condensation (p2/3) that is too small for the formation of an additional joint. At E14.5 the remaining cartilage undergoes differentiation to a terminal phalanx, and hence the middle phalanx is lost.
In summary, this work demonstrates that a signaling center analogous to the chicken PFR/digit crescent exists in mammals and uncovers genetic mechanisms controlling digit development, with both IHH and ROR2 acting cooperatively to fine-tune BMP signaling in the PFR. In consequence this proposes a pathomechanism for human brachydactylies A1 and B1 via disrupted BMP/SMAD1/5/8 signaling, the pathway affected in brachydactylies A2, B2, and C. This suggests that the distinct yet overlapping phenotypes observed in the brachydactyly disease family can be explained by a unifying molecular network converging on BMP signaling.
Materials and Methods
In Situ Hybridizations.
ISHs on whole-mount embryos as well as on paraffin sections were performed as previously described (30).
Immunohistochemistry.
Immunohistochemistry was done on paraffin sections. Antigen retrieval was performed using citrate buffer or high-pH buffer (Dako). After permeabilization with 0.2% Triton X-100 for 15 min and blocking with 5% normal goat serum, primary antibody incubation was performed at 4 °C overnight and detection with fluorescence-conjugated secondary antibody (Molecular Probes, Invitrogen) at room temperature for 1 h. For phospho-SMAD staining, additional biotinyl tyramid signal amplification was performed according to the manufacturer's protocol (Perkin-Elmer).
BrdU Pulse-Chase Labeling.
Mice were injected i.p. with 200 μg BrdU per gram of body weight. After 1 h, incorporation into DNA was blocked by injection of a 30-fold excess of thymidine, and mice were killed after a further 10 h. Statistical analysis was performed by counting BrdU/SOX9 dual-positive cells relative to SOX9-positive cells on four sections for three of each WT and mutant specimen.
Mouse micromass cultures were prepared from E12.5 hand plates according to standard procedures. Cultures were stained with Alcian blue and quantified photometrically.
All animal experiments were carried out in compliance with legal requirements of the European Union.
Detailed materials and methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Kathrin Seidel and Norbert Brieske for their expert technical assistance and the animal facility of the Max Planck Institute for Molecular Genetics, especially Janine Wetzel, for mouse work assistance. This project was funded by Deutsche Forschungsgemeinschaft Grant SFB 577 (to S.S. and S.M.) and Research Grants Council of Hong Kong Grant HKU760608M (to D.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009314107/-/DCSupplemental.
References
- 1.Zeller R, López-Ríos J, Zuniga A. Vertebrate limb bud development: Moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 2.Casanova JC, Sanz-Ezquerro JJ. Digit morphogenesis: Is the tip different? Dev Growth Differ. 2007;49:479–491. doi: 10.1111/j.1440-169X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Hasso SM, Fallon JF. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci USA. 2008;105:4185–4190. doi: 10.1073/pnas.0707899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montero JA, Lorda-Diez CI, Gañan Y, Macias D, Hurle JM. Activin/TGFbeta and BMP crosstalk determines digit chondrogenesis. Dev Biol. 2008;321:343–356. doi: 10.1016/j.ydbio.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Mundlos S. The brachydactylies: A molecular disease family. Clin Genet. 2009;76:123–136. doi: 10.1111/j.1399-0004.2009.01238.x. [DOI] [PubMed] [Google Scholar]
- 6.Gao B, et al. A mutation in Ihh that causes digit abnormalities alters its signalling capacity and range. Nature. 2009;458:1196–1200. doi: 10.1038/nature07862. [DOI] [PubMed] [Google Scholar]
- 7.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: Orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz R, et al. The mutation ROR2W749X, linked to human BDB, is a recessive mutation in the mouse, causing brachydactyly, mediating patterning of joints and modeling recessive Robinow syndrome. Development. 2008;135:1713–1723. doi: 10.1242/dev.015149. [DOI] [PubMed] [Google Scholar]
- 10.DeChiara TM, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 11.Niedermaier M, et al. An inversion involving the mouse Shh locus results in brachydactyly through dysregulation of Shh expression. J Clin Invest. 2005;115:900–909. doi: 10.1172/JCI200523675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ten Berge D, Brugmann SA, Helms JA, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B, et al. Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat Genet. 2001;28:386–388. doi: 10.1038/ng577. [DOI] [PubMed] [Google Scholar]
- 15.Drossopoulou G, et al. A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signalling and Bmp signalling. Development. 2000;127:1337–1348. doi: 10.1242/dev.127.7.1337. [DOI] [PubMed] [Google Scholar]
- 16.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 17.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- 19.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer L, Boland G, Tuan RS. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J Cell Biochem. 2002;84:816–831. doi: 10.1002/jcb.10091. [DOI] [PubMed] [Google Scholar]
- 22.Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133:989–999. doi: 10.1242/dev.02274. [DOI] [PubMed] [Google Scholar]
- 23.Oishi I, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 25.Topol L, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- 27.Ishitani T, et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. [DOI] [PubMed] [Google Scholar]
- 29.Rowe DA, Cairns JM, Fallon JF. Spatial and temporal patterns of cell death in limb bud mesoderm after apical ectodermal ridge removal. Dev Biol. 1982;93:83–91. doi: 10.1016/0012-1606(82)90241-x. [DOI] [PubMed] [Google Scholar]
- 30.Stricker S, et al. Cloning and expression pattern of chicken Ror2 and functional characterization of truncating mutations in Brachydactyly type B and Robinow syndrome. Dev Dyn. 2006;235:3456–3465. doi: 10.1002/dvdy.20993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.