Abstract
Renal ischemia reperfusion injury (IRI) is associated with significant morbidity and mortality. Given the importance of microRNAs (miRNAs) in regulating gene expression, we examined expression profiles of miRNAs following renal IRI. Global miRNA expression profiling on samples prepared from the kidneys of C57BL/6 mice that underwent unilateral warm ischemia revealed nine miRNAs (miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194) that are differentially expressed following IRI when compared with sham controls. These miRNAs were also differently expressed following IRI in immunodeficient RAG-2/common γ-chain double-knockout mice, suggesting that the changes in expression observed are not significantly influenced by lymphocyte infiltration and therefore define a lymphocyte-independent signature of renal IRI. In vitro studies revealed that miR-21 is expressed in proliferating tubular epithelial cells (TEC) and up-regulated by both cell-intrinsic and -extrinsic mechanisms resulting from ischemia and TGF-β signaling, respectively. In vitro, knockdown of miR-21 in TEC resulted in increased cell death, whereas overexpression prevented cell death. However, overexpression of miR-21 alone was not sufficient to prevent TEC death following ischemia. Our findings therefore define a molecular fingerprint of renal injury and suggest miR-21 may play a role in protecting TEC from death.
Keywords: kidney, miR-21, tubular epithelial cells, programmed cell death protein 4
Ischemic injury followed by reperfusion results in the clinical syndrome of acute kidney injury, a common clinical problem (1–4). The initial nonimmune hypoxic injury and subsequent reperfusion leads to activation of innate and adaptive immune responses, resulting in tissue damage (5). Following injury, a repair process involving cellular proliferation must take place to regain renal function (6). Ischemia reperfusion injury (IRI) is also inevitable in kidney transplantation and contributes to delayed graft function and long-term changes in kidney transplants that affect outcome (7–9). Although IRI is clearly a major clinical problem in native kidneys and in the setting of renal transplantation, the pathogenesis of renal IRI is not fully understood.
MicroRNAs (miRNAs) are a class of small, noncoding RNAs ≈21–22 nucleotides in length that regulate gene expression and many disease processes (10, 11). A role for miRNAs in kidney disease is rapidly emerging (12). Numerous hallmarks of IRI (9), such as apoptosis (13), fibrosis (14), epithelial-mesenchymal transition (15), and TLR signaling (16), are regulated by miRNAs in other settings. Recent work has also revealed a role for miRNAs in the regulation of cardiac (13) and hepatic IRI (17).
We hypothesized that miRNA expression patterns may serve as a biomarker of kidney injury. To test this hypothesis we reasoned that it would be necessary to examine miRNA expression patterns in an unbiased manner and examine whether differences in miRNA expression patterns were the result of lymphocyte infiltration or kidney intrinsic responses to injury. Accordingly, we performed global miRNA expression profiling over a 30-d time course on kidneys of C57BL/6 and immunodeficient RAG-2/common γ-chain cytokine receptor double-knockout mice (Ro/cγ0 mice) that underwent 30 min of unilateral warm ischemia as well as sham controls. Our data define a lymphocyte-independent molecular fingerprint of IRI based on differential expression of miRNAs and identify induction of miR-21 through both cell-intrinsic and -extrinsic pathways as important in preventing tubular epithelial cell apoptosis.
Results
To analyze the role of miRNAs in injury and repair responses following renal IRI, groups of C57BL/6 mice underwent 30 min of unilateral warm ischemia. Control mice were also prepared and underwent an identical procedure, except the renal pedicle was not clamped (sham controls). Cohorts of mice in each group (n = 3 per time point) were killed at 1, 3, 5, 7, 14, 21, and 30 d, and their injured kidneys were harvested. H&E-stained tissue sections revealed significant tubular necrosis by day 3 following IRI (Fig. S1). We observed progressive loss of tubules, significant interstitial fibrosis, and disruption of tubular architecture with dilation and cyst formation, and significant loss of glomeruli and contraction of the cortex. By day 30, only small clusters of tubules were observed. We observed progressive infiltration of T cells and macrophages in kidneys that underwent IRI (Fig. S1). B-cell infiltration based on B220 staining (CD45R) was observed at day 3 but then decreased over time. Very few natural killer (NK) cells were observed. Control samples were essentially devoid of lymphocytic or monocytic infiltration, and were comparable to naïve unmanipulated kidneys. We did not observe any significant changes in the histology of kidneys from control mice that underwent a sham procedure.
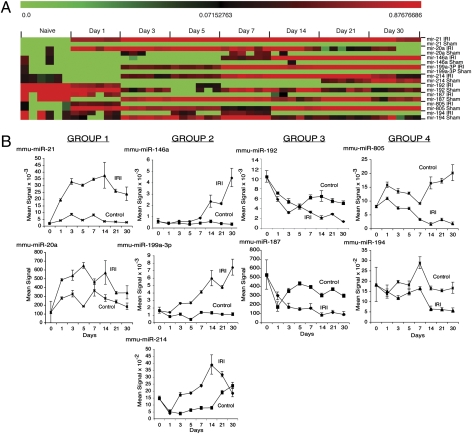
miRNA expression profiling using μParaflo microfluidic biochip array technology was conducted on RNA prepared from IRI, sham, and naive kidneys. Genome-wide miRNA expression profiling of 570 murine miRNAs present in miRBase release 10.0 revealed significant changes in expression of 81 and 84 miRNAs in IRI and sham control samples, respectively (ANOVA P < 0.01; Tables S1 and S2). miRNAs that were differentially expressed in sham controls, apparently as a result of the surgical procedure itself (Table S2), were eliminated from our analysis. Of the remaining miRNAs, 13 were differentially expressed between IRI and sham controls over the time course studied. We validated the 13 differentially expressed miRNAs by performing stem-loop TaqMan real-time PCR assays. Nine miRNAs (miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194) were confirmed to be differentially expressed between IRI and control samples (P < 0.01; Fig. 1 A and B and Fig. S2). These findings were also validated by performing genome-wide miRNA expression profiling using microarray on samples from a second set of mice (Fig. S3). Together, the data suggest that a limited set of miRNAs are differentially regulated following IRI.
Fig. 1.
Identification of a miRNA signature following renal IRI. (A) Heat map of miRNAs differentially expressed in the kidneys of C57BL/6J mice after IRI or sham operation. Mean signal intensities (n = 3) following normalization and z-transformation. (B) Plots of above microarray data expressed as the mean signal intensity ± SE. Data were normalized using a locally weighted regression method. Naïve kidneys serve as day 0. Data grouped based upon similar expression patterns over time.
The differentially expressed miRNAs could be placed into four groups based on their expression patterns (Fig. 1B). mir-21 and miR-20a were rapidly up-regulated following IRI relative to control samples and levels observed in naïve (day 0) kidneys (group 1; Fig. 1B). miR-146a, miR-199a-3p, and miR-214 were also up-regulated relative to control samples and naïve kidney; however, up-regulation occurred after day 3 (group 2). Expression of miR-146a and miR-199a-3p continued to increase over the time course analyzed, whereas expression of miR-214 began to wane by day 21. We also observed that miR-192 and miR-187 were rapidly down-regulated in IRI and control samples relative to the levels observed in naïve kidneys (group 3). Whereas miR-192 and miR-187 expression increased in control samples and then became stable, the level observed in IRI samples continued to decrease. miR-805 and miR-194 (group 4) were down-regulated following IRI, but remained at levels relatively similar to those observed in naïve control kidneys when compared with miRNAs in group 3.
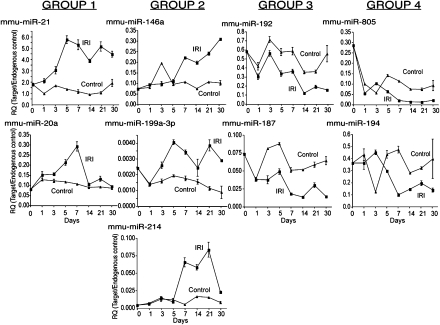
To examine the extent to which miRNA expression levels might be influenced by lymphocytic infiltration, we examined the expression of miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194 by real-time PCR in RAG-2/common γ-chain cytokine receptor double-knockout mice (Ro/cγ0 mice). Ro/cγ0 mice lack NK cells, NKT cells, B cells, and T cells, allowing us to analyze how lymphocytic infiltration may affect miRNA expression. IRI was induced in Ro/cγ0 mice as described previously. The degree of structural injury observed over the time course studied was similar to that observed in wild-type C57BL/6 mice (Fig. S1). As expected, we did not observe lymphocytes infiltrating the kidneys of these mice following IRI. The expression pattern of miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194 was similar in C57BL/6 and Ro/cγ0 mice following IRI (Fig. 2). These findings were confirmed by performing genome-wide miRNA microarray analysis on a second set of mice (Fig. S4). These data strongly suggest that the differential expression of miRNAs observed reflects a lymphocyte-independent profile of IRI.
Fig. 2.
The miRNA fingerprint is lymphocyte independent. The expression of the indicated miRNAs was measured in Ro/cγ0 mice by real-time PCR. The data are expressed as mean ± SE of three independent experiments. Relative quantitation (RQ) was calculated by the ddCT method normalizing miRNA expression to the endogenous control SnoRNA202.
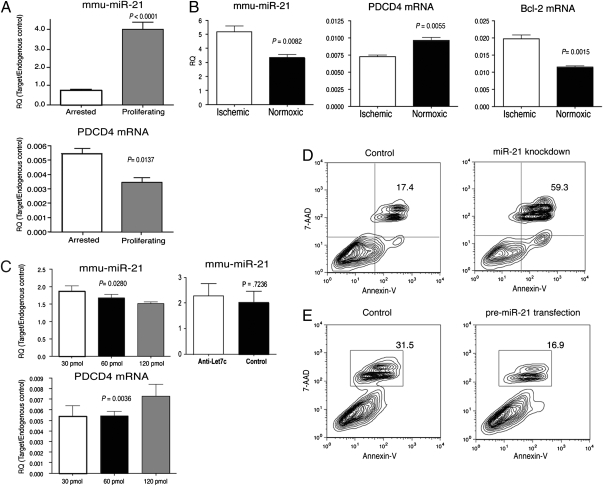
A hallmark of IRI is necrosis and apoptosis of renal tubular epithelial cells (TEC), fibrosis, and proliferation (6, 18, 19). miR-21 expression up-regulates the ERK-MAPK signaling pathway through inhibition of sprouty homolog 1 (Spry1), contributing to interstitial fibrosis after cardiac injury (14). Importantly, ERK-MAPK signaling plays a key role in renal IRI (20). miR-21 also targets the tumor suppressor gene-programmed cell death 4 (PDCD4), resulting in cellular proliferation (21, 22), and is an antiapoptotic factor in various cancers (22–24). Insofar as miR-21 is implicated in proliferation, fibrosis, and apoptosis, all processes that occur as a result of IRI, we hypothesized that miR-21 may play a role in controlling the response of TEC to IRI. We therefore examined whether miR-21 expression may be linked to proliferation of TEC. Primary cultures of murine TEC were established from C57BL/6 mice. miR-21 was expressed at relatively low levels in quiescent cells, but significantly up-regulated in proliferating TEC (Fig. 3A). Up-regulation of miR-21 in proliferating TEC inversely correlated with a decrease in expression of PDCD4 (Fig. 3A). Induction of ischemia in TEC resulted in up-regulation of miR-21 and down-regulation of PDCD4 expression and also correlated with up-regulation of Bcl-2 expression (Fig. 3B).
Fig. 3.
miR-21 regulates proliferation and apoptosis in TEC. (A) Real-time PCR analysis of miR-21 and PDCD4 in arrested and proliferating primary TEC cultures. Cells were growth arrested by growing to confluence. The relative expression of miR-21 was compared in growth-arrested and proliferating cultures by real-time PCR. (B) Expression of miR-21, PDCD4, and Bcl-2 in primary TEC following ischemia. The relative expression of miR-21, PDCD4, and Bcl-2 were compared in ischemic and normoxic TEC cultures at 48 h. (C Left) Knockdown of miR-21 in primary TEC cultures. TEC cultures were transfected with increasing concentrations of anti-miR-21 LNA oligonucleotide and analyzed 48 h later. (Right) Knockdown of an irrelevant miRNA, let-7c, fails to influence miR-21 expression and analyzed 48 h later. TEC were transfected with anti-let-7c LNA oligonucleotides. The levels of miR-21 and PDCD4 were quantitated by real-time PCR. Data in A–C are expressed as relative quantifications (RQ) by normalizing to the endogenous control SnoRNA202 (miR-21) or GAPDH (PDCD4, Bcl-2). (D) Induction of apoptosis in TEC following miR-21 knockdown. Twenty-four hours after transfection with miR-21 or Fugene alone (control), cell death was analyzed by staining for Annexin-V and 7-AAD and quantified by flow cytometry. The percent of dead cells is indicated. (E) Overexpression of miR-21 results in decreased TEC death. Twenty-four hours after transfection with premiR-21 or Fugene alone (control), cell death was analyzed by staining for Annexin-V and 7-AAD and quantified by flow cytometry as above. In all cases, the data shown are representative of at least two experiments.
Knockdown experiments using locked nucleic acid-modified (LNA) miR-21-specific antisense oligonucleotides resulted in a decrease in expression of miR-21 and an increased expression of PDCD4 in a dose-dependent fashion (Fig. 3C). Knockdown of miR-21 also resulted in a significant increase in cell death observed in TEC cultures (Fig. 3D). We also examined the effect of overexpressing miR-21 in TEC cultures exhibiting a relatively high basal level of cell death to assess whether forced expression of miR-21 confers a survival benefit. Overexpression of miR-21 in TEC resulted in a decrease in the level of cell death observed in primary TEC cultures (Fig. 3E). We were unable to effectively knockdown miR-21 in TEC undergoing ischemia, likely because of the apparent up-regulation of miR-21 observed postischemia. Overexpression of miR-21 in TEC did not prevent cell death following ischemia.
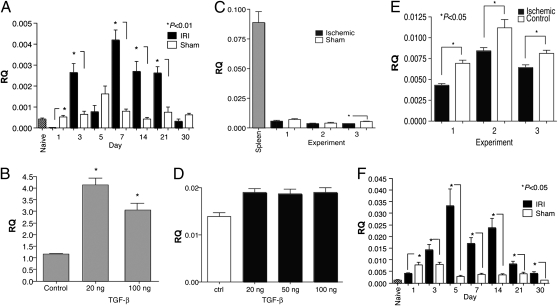
Our findings indicated that miR-21 is up-regulated in TEC and the kidney following ischemic injury or hypoxia (Figs. 1 and 3). We also observed that TGF-β production is increased in the kidney following IRI (Fig. 4A). TGF-β has been suggested to regulate miR-21 expression in muscle cells (25). Moreover, miR-21 expression has been linked to fibrosis (14), a major complication resulting from renal IRI in which TGF-β plays a central role (26). We therefore examined whether the TGF-β signaling pathway played a role in controlling expression of miR-21 following IRI. Stimulation of TEC with TGF-β led to a significant increase in expression of mature miR-21 (Fig. 4B). However, purified TEC did not appear to produce TGF-β following ischemia (Fig. 4C), suggesting that TGF-β stimulation leads to an increase in miR-21 expression via a cell-extrinsic mechanism, whereas ischemia or hypoxia up-regulate miR-21 in a cell-intrinsic fashion. TGF-β stimulation of TEC led to a modest but significant increase in pri-miR-21 levels in TEC (Fig. 4D), whereas ischemia resulted in a decrease in pri-miR-21 levels (Fig. 4E). These data lead us to suggest that in TEC the increase in mature miR-21 observed after TGF-β stimulation or ischemia is most likely due to increased processing of pri-miR-21 rather than transcriptional up-regulation of miR-21. Interestingly, in kidneys undergoing IRI, we observe pri-miR-21 was up-regulated to the greatest extent relative to controls at relatively early time-points (Fig. 4F), although mature miR-21 was up-regulated at all time points observed (Fig. 1). This suggests that cell types other than TEC may also regulate miR-21 expression through distinct mechanisms.
Fig. 4.
TGF-β-induced miR-21 expression in TEC. (A) Analysis of TGF-β expression in the kidneys of C57BL/6 mice undergoing IRI or sham surgery. Kidneys from naïve C57BL/6 mice were used as a control. Shown is expression on various days after injury or sham surgery. TGF-β expression was analyzed by real-time PCR. Pooled RNA from at least three kidneys per time point was analyzed. (B) Cultures of primary TEC were established from C57BL/6 mice and stimulated with either 20 or 100 ng/mL TGF-β for 24 h. The cells were harvested and total RNA prepared. The levels of mature miR-21 were then determined by real-time PCR. Similar results were observed at 72 h. Shown are data from at least three separate experiments. Relative quantitation (RQ) was calculated by the ddCT method normalizing miRNA expression to the endogenous control SnoRNA202. (C) TEC do not produce TGF-β following ischemia. Cultures of primary TEC were established as above and ischemia induced. Sham cells were not overlaid with mineral oil. The cells were harvested and TGF-β expression analyzed by RT-PCR using standard methods. Spleen cells were used as a positive control. Shown are data from at least three separate experiments. RQ was calculated by normalizing expression to the endogenous control GAPDH. (D) TGF-β stimulation leads to a modest increase in pri-miR-21. Cultures of primary TEC were established and stimulated with the indicated amounts of TGF-β for 24 h. The cells were harvested and total RNA prepared. Levels of pri-miR-21 were then quantitated by real-time PCR using the ABI TaqMan mmu-pri-miR-21 assay kit. (E) Ischemia leads to a decrease in pri-miR-21. Cultures of primary TEC were established, ischemia was induced, and the cells were harvested. Levels of pri-miR-21 were then quantitated as indicated for C. (F) Analysis of pri-miR-21 expression in the kidneys of C57BL/6 mice undergoing IRI or sham surgery. Kidneys from naïve C57BL/6 mice were used as a control. Shown is expression on various days after injury or sham surgery. Pooled RNA from at least three kidneys per time point was analyzed.
Discussion
Although IRI is an important clinical problem in many settings, the pathogenesis of IRI is poorly understood. Our data suggest that relatively few miRNAs are differentially regulated following IRI. miRNAs identified as differentially regulated were not derived from nor influenced by lymphocytes or NK cells infiltrating the kidney. Thus, we suggest the miRNA profile identified reflects lymphocyte-independent changes in the kidney following IRI. We focused on miR-21 for further study because it plays a role in several processes that also occur as a result of IRI. Expression of miR-21 was significantly up-regulated in proliferating TEC and correlated with a decrease in expression of PDCD4. Induction of ischemia or hypoxia in TEC also resulted in a significant up-regulation of miR-21, down-regulation of PDCD4 expression, and an up-regulation of Bcl-2. In primary cultures, knockdown of miR-21 resulted in a significant increase in cell death, whereas overexpression of miR-21 led to decreased cell death. We were unable to effectively knock down miR-21 in ischemic TEC cultures apparently because of the robust up-regulation of miR-21 following ischemia. Overexpression of miR-21 did not prevent cell death following ischemia. We suggest that though up-regulation of miR-21 may regulate proliferative responses in TEC by regulating PDCD4 and Bcl-2 and thereby prevent apoptosis, the level to which miR-21 regulates expression of these gene products, as well as others in TEC, is not sufficient to prevent cell death resulting from ischemia. miR-21 may therefore play a role in fine-tuning the cellular responses to injury, although it is not sufficient to prevent cell death resulting from severe injury. We are currently examining whether other miRs identified as differentially regulated are able to synergize with miR-21 to prevent cell death following ischemia.
TGF-β plays a critical role in renal injury, including fibrosis, a hallmark of IRI. Our data suggest that miR-21 expression in TEC is induced as a result of TGF-β signaling. Because TEC do not appear to make TGF-β after hypoxia in vitro (Fig. 4C), we suggest that up-regulation of miR-21 via TGF-β signaling occurs through a cell-extrinsic pathway. In the heart, expression of miR-21 after injury results in interstitial fibrosis (14). We suggest that in the kidney, TGF-β signaling, as well as ischemia or hypoxia, likely mediate fibrosis through their effect on miR-21 expression. In other systems, TGF-β affects miR-21 expression by increased processing of pri-miR-21 (25). In TEC, we observed only a modest increase in pri-miR-21 following TGF-β stimulation, and a decrease in pri-miR-21 following ischemia, even though mature miR-21 levels were increased after either type of stimulation. We therefore suggest that in TEC, TGF-β stimulation and ischemia lead to increased processing of pri-miR-21. In kidneys undergoing IRI, we observed that pri-miR-21 was up-regulated to the greatest extent relative to controls at relatively early time-points, although mature miR-21 was up-regulated at all time points. Because expression of miR-21 in TEC appears to reflect increased processing of primary transcripts, whereas up-regulation of miR-21 in the kidney appears to involve both increased transcription of pri-miR-21 and its processing, we suggest that in the kidney, cell types other than TEC regulate miR-21 expression via distinct mechanisms, including transcriptional up-regulation of miR-21. Though our findings suggest a role for miR-21 in renal injury, the role of miR-21 in tissue injury has been controversial. Work from two groups has revealed fundamentally different roles for miR-21 in regulating apoptosis as a function of cell type and model. Though up-regulation of miR-21 in vascular smooth muscle cells following restenosis has been correlated with increases in Bcl-2 (27), others have reported that miR-21 expression is inversely associated with Bcl-2 expression in breast cancer cells (28). These findings illustrate that the relationship between miRNA expression and function may be tissue- or cell-type dependent as well as mechanistically distinct.
We have not yet systematically examined the role of miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194 in IRI. miR-20a modulates translation of E2F transcription factors that regulate proliferation and apoptosis (29). The up-regulation of miR-21 and miR-20a after IRI suggests that expression of these miRNAs may prevent apoptosis resulting from IRI and induce proliferation to promote repair. miR-199a-3p targets IKKβ, resulting in its down-regulation and the down-regulation of proinflammatory environments (30). miR-199a-3p targets the MET proto-oncogene and ERK-2, thereby inhibiting proliferation and apoptosis (31), and targets hypoxia-inducible factor-1α, thereby reducing apoptosis of cardiac myocytes after hypoxic injury (32). miR-199a-3p may therefore limit kidney injury resulting from hypoxia and may control inflammation and ERK-MAPK signaling, which has been shown to mediate tubular injury after IRI (33). miR-214 is also up-regulated following IRI, and targets PTEN, a negative regulator of the AKT-signaling pathway, thereby promoting cell proliferation and survival (34).
TLR4 signaling plays a critical role in renal IRI (35) and results in NF-κB activation (36). Based on the ability of miR-146a to target the 3′ UTR of TRAF-6 and IRAK-1, it has been hypothesized that NF-κB-dependent induction of miR-146a provides a negative feedback loop that leads to down-regulation of TLR signaling (16). miR-146a expression may lead to down-regulation of TLR signaling which may be required to resolve inflammation and allow for repair following IRI. miR-146a is up-regulated relatively late after IRI, consistent with the notion that it may be involved in resolving inflammation. miR-192 is highly expressed in the kidney (37) and appears to be up-regulated in response to TGF-β signaling, and targets Smad-interacting protein 1 (SIP1), inducing collagen expression (38). We observed a progressive decrease in miR-192 expression following IRI, most likely reflecting contraction of the kidney, which also mirrored changes observed in TGF-β expression. Though little is known about miR-194 and miR-805, miR-805 is down-regulated in LPS-treated macrophages (22). Decreasing expression of miR-805 over time may reflect accumulation of activated macrophages in the injured kidney. Indeed, we observed significant infiltration of macrophages in the kidney by day 14 (Fig. 1B), after which point down-regulation of miR-805 is most apparent (Fig. 2B).
Though we have not yet determined how alterations in the miRNAs observed to be differentially regulated functionally affect the kidney's response to injury, we suggest that the profile of miRNA expression observed after renal IRI reflects a survival response. Indeed, the majority of miRNAs we identified appear to be differentially expressed, perhaps to quell the damage response by regulating apoptosis, proliferation, and inflammation, and may point us toward new pathways that can be targeted to prevent or modulate renal injury. If the miRNAs we identified as being differentially expressed can be shown to play a role in these processes in vivo, they may represent new biomarkers of renal injury.
Materials and Methods
Animals.
Ten- to 12-wk-old male C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) and (C57BL/6J × C57BL/10SgSnAi)-[KO]γc-[KO]Rag2 (Taconic) mice were housed and handled in accordance with institutional policies and procedures.
Induction of Ischemia Reperfusion Injury.
A midline abdominal incision was made, and the right renal pedicle was clamped for 30 min. Mice were maintained at 37 °C, and the abdominal cavity was hydrated with saline-moistened gauze. Following clamp removal, the kidney was visually assessed for reperfusion. Groups of control mice were prepared as above, without clamping of the renal pedicle.
miRNA Microarray Analysis.
Total RNA was extracted from an equal amount of tissue pooled from three kidneys per time point with the miRNeasy Kit (Qiagen) and used for microarray analysis as previously described (39). Microarray assays and statistical analysis were performed by LC Sciences (www.lcsciences.com) using miRNA probe sequences from mmu-miRBase 10.0 (Sanger Institute; http://microrna.sanger.ac.uk/sequences).
Real-Time PCR.
Real-time PCR using miRNA-specific stem-loop primers for reverse transcription and TaqMan probes for mature murine miRNA was performed in accordance with manufacturers’ protocols using an ABI 7900HT Real-Time PCR system (Applied Biosystems). RNA was extracted from an equal amount of tissue pooled from three kidneys per time point using the RNeasy Mini Kit (Qiagen). cDNA synthesis was performed using SuperScript III (Invitrogen). pri-miR-21 levels were determined using the TaqMan Pri-miRNA assays developed by Applied Biosystems. Data analysis was performed using the Applied Biosystems SDS Software package, version 2.2.
Histology and Quantification of Leukocytic Infiltration.
Immunohistochemistry for CD3+ T cells, B220+ B cells, and macrophages was performed on 4-μm sections from formalin-fixed, dehydrated, and paraffin-embedded kidneys. For CD4, CD8, and NK cell staining, grafts were stored snap-frozen in optimum cutting temperature (OCT). Slides were counterstained with haematoxylin using standard techniques. All samples were scored blindly.
Induction of Ischemia in Vitro.
Primary TEC were isolated from C57BL/6 mice and cultured as previously described (40). Cultures were at least 90% cytokeratin positive. Ischemia was induced by mineral oil overlay as previously described (41). For knockdown experiments, primary TEC cultures exhibiting relatively low levels of cell death (less than 20%) were selected and transfected with either hsa-miR-21 or hsa-let-7c antagomirs (Ambion) as recommended by the manufacturer. Apoptosis was then analyzed 24 h later by flow cytometry. Let-7c antagomirs did not affect the levels of cell death observed. To overexpress miR-21, primary TEC cultures exhibiting relatively high levels of cell death (20–25%) were selected and transfected with oligonucleotides encoding pre-miR-21 (Ambion), and apoptosis was then analyzed 24 h later by flow cytometry. Control cultures received transfection vehicle alone. We selected cultures with relatively high background levels of cell death for these experiments to be able to effectively assess whether miR-21 leads to a survival benefit in stressed cultures.
Supplementary Material
Acknowledgments
We thank Dr. Helmut Rennke (Department of Pathology, Brigham and Women's Hospital) and Dr. Joseph V. Bonventre (Renal Division, Brigham and Women's Hospital) for helpful discussions. We also thank Christoph Eicken (LC Sciences, Houston) for suggestions related to microarrays and statistical interpretation of data. K.S. is supported by National Institutes of Health Training Grant T32 AI070085. This work was supported in part by a grant from the Roche Organ Transplantation Research Foundation (to S.G.T. and J.I.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912701107/-/DCSupplemental.
References
- 1.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 4.Koo DD, Welsh KI, Roake JA, Morris PJ, Fuggle SV. Ischemia/reperfusion injury in human kidney transplantation: An immunohistochemical analysis of changes after reperfusion. Am J Pathol. 1998;153:557–566. doi: 10.1016/S0002-9440(10)65598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Cecka M. Clinical outcome of renal transplantation. Factors influencing patient and graft survival. Surg Clin North Am. 1998;78:133–148. doi: 10.1016/s0039-6109(05)70639-3. [DOI] [PubMed] [Google Scholar]
- 8.Tullius SG, Tilney NL. Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation. 1995;59:313–318. [PubMed] [Google Scholar]
- 9.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945–947. doi: 10.1097/00007890-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 11.Erson AE, Petty EM. MicroRNAs in development and disease. Clin Genet. 2008;74:296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 12.Saal S, Harvey SJ. MicroRNAs and the kidney: Coming of age. Curr Opin Nephrol Hypertens. 2009;18:317–323. doi: 10.1097/MNH.0b013e32832c9da2. [DOI] [PubMed] [Google Scholar]
- 13.Ren XP, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 15.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 16.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu CH, Xu CF, Li YM. Association of microRNA-223 expression with hepatic ischemia/reperfusion injury in mice. Dig Dis Sci. 2008;54:2362–2366. doi: 10.1007/s10620-008-0629-8. [DOI] [PubMed] [Google Scholar]
- 18.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277:2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- 21.Frankel LB, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 22.Asangani IA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 23.Seike M, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA. 2009;106:12085–12090. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 25.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creely JJ, DiMari SJ, Howe AM, Haralson MA. Effects of transforming growth factor-beta on collagen synthesis by normal rat kidney epithelial cells. Am J Pathol. 1992;140:45–55. [PMC free article] [PubMed] [Google Scholar]
- 27.Ji R, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 28.Wickramasinghe NS, et al. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sylvestre Y, et al. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, et al. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27:4712–4723. doi: 10.1038/onc.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, et al. MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2) J Biol Chem. 2008;283:18158–18166. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 32.Rane S, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sáenz-Morales D, et al. ERK1/2 mediates cytoskeleton and focal adhesion impairment in proximal epithelial cells after renal ischemia. Cell Physiol Biochem. 2009;23:285–294. doi: 10.1159/000218175. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 35.Si ML, et al. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 36.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ, Palkovits M, Young WS., 3rd miR-7b, a microRNA up-regulated in the hypothalamus after chronic hyperosmolar stimulation, inhibits Fos translation. Proc Natl Acad Sci USA. 2006;103:15669–15674. doi: 10.1073/pnas.0605781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuthrich RP, et al. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- 41.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, Harken AH. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res. 2001;99:288–293. doi: 10.1006/jsre.2001.6201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.