Abstract
Human platelets were separated by desity-centrifugation into heavy and light populations. Heavy platelets have an average volume approximately twofold greater than light platelets, and have previously been shown to be young platelets.
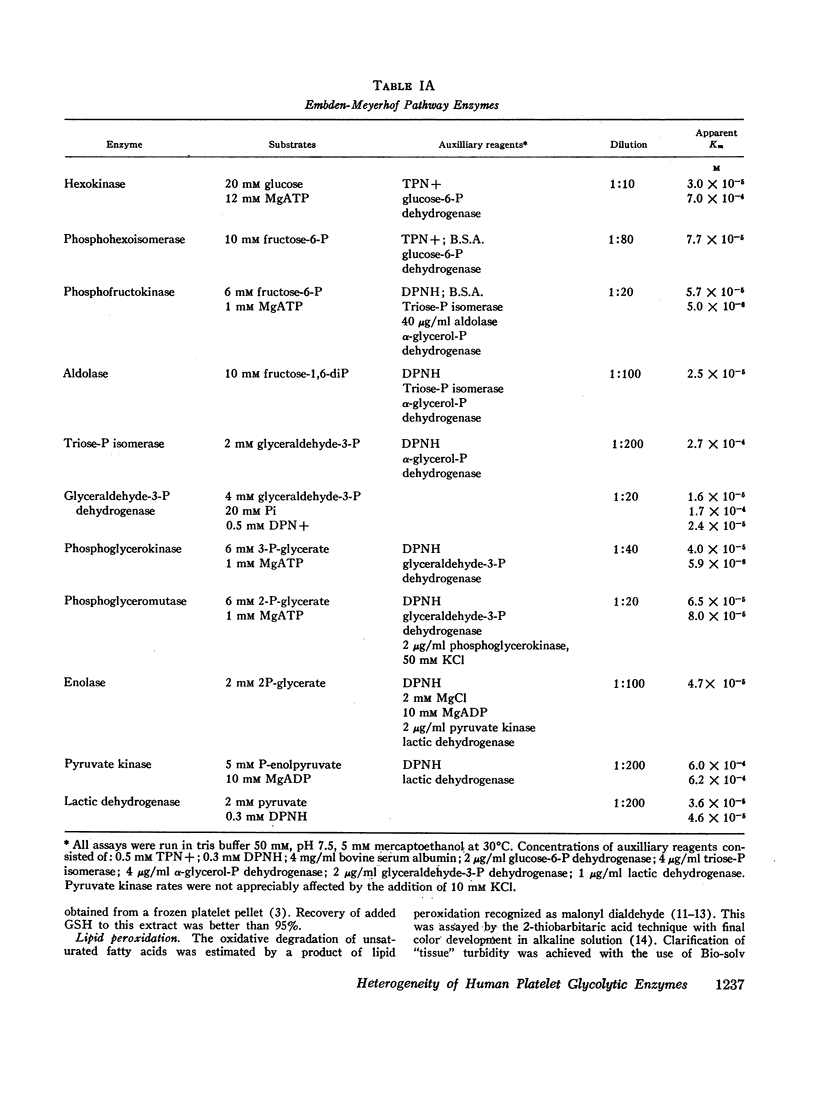
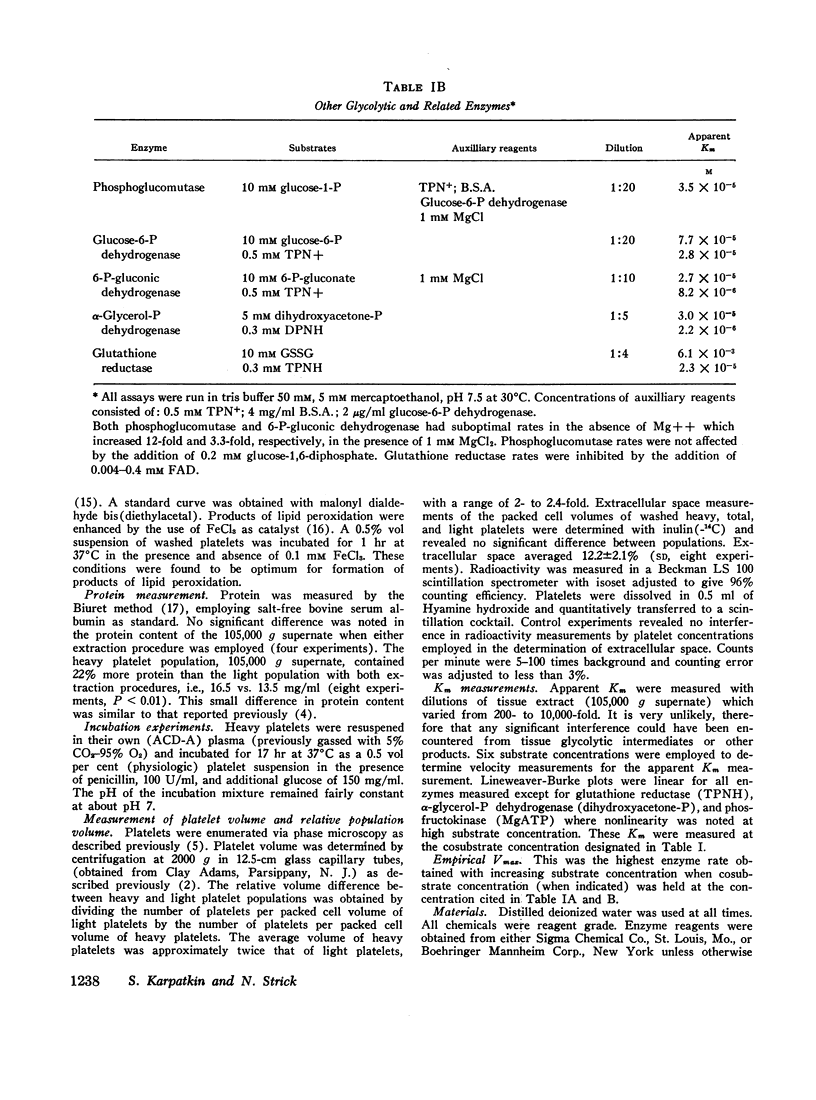
All 11 enzymes of the Embden-Meyerhof pathway plus the five related enzymes: phosphoglucomutase, glucose-6-P dehydrogenase, 6-P-gluconic dehydrogenase, α-glycerol-P dehydrogenase, and glutathione reductase (TPNH) were examined in cell lysates from total, heavy, and light platelet populations. Apparent Km for individual enzymes were measured in a total platelet population. Empirical Vmax of the individual enzymes were measured in total, heavy, and light platelet populations. The three apparent rate-limiting enzymes for glycolysis were hexokinase, phosphofructokinase, and glyceraldehyde-3-P dehydrogenase.
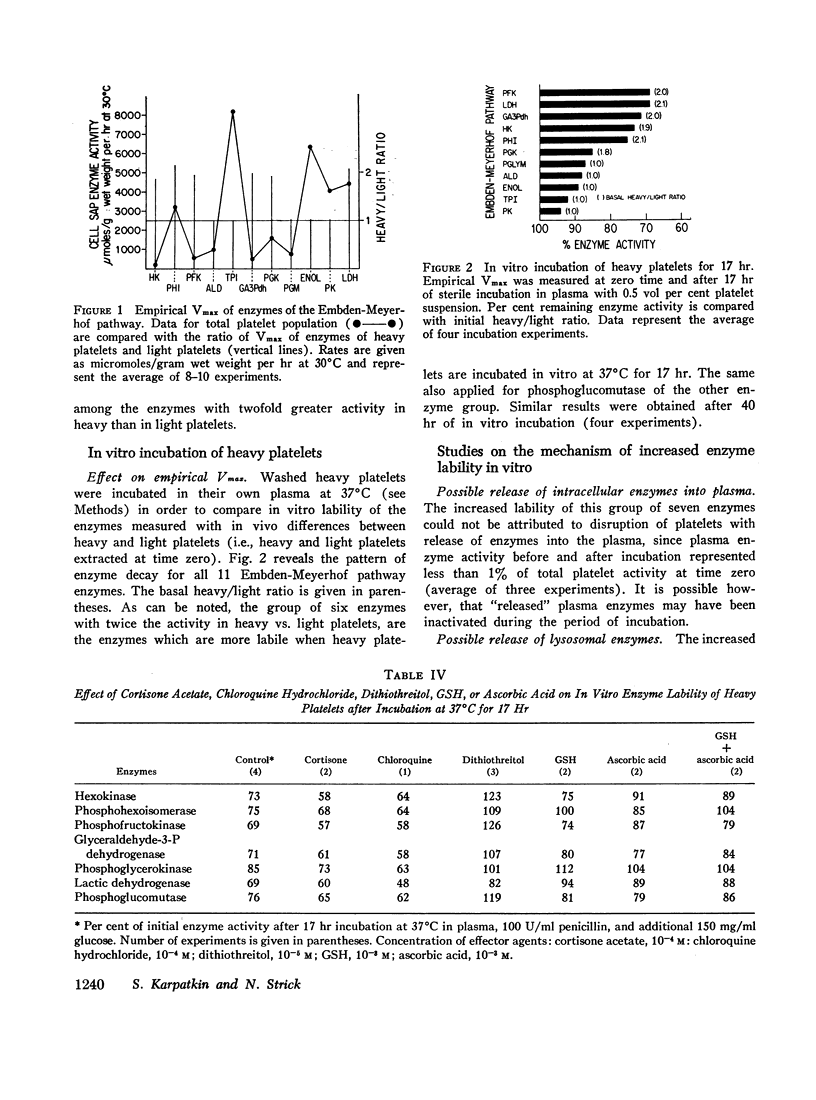
Heavy platelets contained approximately twofold greater enzyme activity (per gram wet weight) than light platelets for 7 of the 16 enzymes measured: hexokinase, phosphohexoisomerase, phosphofructokinase, glyceraldehyde-3-P dehydrogenase, phosphoglycerokinase, lactic dehydrogenase, and phosphoglucomutase. Heavy platelets also contained 1.9-fold greater reduced glutathione (GSH), 1.7-fold greater DPNH, and 1.2-fold greater TPNH than light platelets. Heavy platelets contained 1.8-fold less lipid peroxidation products (malonyl aldehyde equivalents) than light platelets and were 2.4-fold more resistant to lipid peroxidation catalyzed by 0.1 mM FeCl3.
Sterile incubation of heavy platelets, in vitro for 17 hr, resulted in a significant loss of enzyme activity for the “elevated” seven enzymes when compared with the remainder. Reducing agents such as GSH (0.1 mM), ascorbic acid (0.1 mM), and dithiothreitol (0.01 mM), when added to the incubation mixture, significantly reduced the in vitro loss of activity. In vitro incubation was also associated with a significant loss of GSH and DPNH and a 1.8-fold increase in lipid peroxidation products.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIM F., WILBUR K. M., KENASTON C. B. The effect of oxidized fatty acids on the activity of certain oxidative enzymes. Arch Biochem Biophys. 1952 Jul;38:177–184. doi: 10.1016/0003-9861(52)90021-0. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- BORN G. V., GILLSON R. E. Studies on the uptake of 5-hydroxytryptamine by blood platelets. J Physiol. 1959 Jun 11;146(3):472–491. doi: 10.1113/jphysiol.1959.sp006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull B. S., Zucker M. B. Changes in platelet volume produced by temperature, metabolic inhibitors, and aggregating agents. Proc Soc Exp Biol Med. 1965 Nov;120(2):296–301. doi: 10.3181/00379727-120-30516. [DOI] [PubMed] [Google Scholar]
- DESAI I. D., TAPPEL A. L. DAMAGE TO PROTEINS BY PEROXIDIZED LIPIDS. J Lipid Res. 1963 Apr;4:204–207. [PubMed] [Google Scholar]
- Dormandy T. L. Biological rancidification. Lancet. 1969 Sep 27;2(7622):684–688. doi: 10.1016/s0140-6736(69)90390-0. [DOI] [PubMed] [Google Scholar]
- GROSSMAN C. M., KOHN R. ENZYMATIC CHARACTERISTICS OF IN-VITRO INCORPORATION OF P32 ORTHOPHOSPHATE INTO HUMAN PLATELET PHOSPHATIDE. Thromb Diath Haemorrh. 1965 Mar 15;13:126–135. [PubMed] [Google Scholar]
- Gorstein F., Carroll H. J., Puszkin E. Electrolyte concentrations, potassium flux kinetics, and the metabolic dependence of potassium transport in human platelets. J Lab Clin Med. 1967 Dec;70(6):938–950. [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, SCOTT A., HOFFSTEN P. E., GEBICKI J. M., WEINSTEIN J., SCHNEIDER A. STUDIES ON THE MECHANISM OF SWELLING, LYSIS, AND DISTINTEGRATION OF ISOLATED LIVER MITOCHONDRIA EXPOSED TO MIXTURES OF OXIDIZED AND REDUCED GLUTATHIONE. J Biol Chem. 1964 Feb;239:614–621. [PubMed] [Google Scholar]
- Karpatkin S., Charmatz A. Hetrogeneity of human platelets. 3. Glycogen metabolism in platelets of different sizes. Br J Haematol. 1970 Aug;19(2):135–143. doi: 10.1111/j.1365-2141.1970.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J Clin Invest. 1969 Jun;48(6):1073–1082. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969 Jun;48(6):1083–1087. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S., Langer R. M. Biochemical energetics of simulated platelet plug formation. Effect of thrombin, adenosine diphosphate, and epinephrine on intra- and extracellular adenine nucleotide kinetics. J Clin Invest. 1968 Sep;47(9):2158–2168. doi: 10.1172/JCI105902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. J Clin Invest. 1967 Mar;46(3):409–417. doi: 10.1172/JCI105542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N., Majerus P. W. Lipid metabolism in human platelets. II. De novo phospholipid synthesis and the effect of thrombin on the pattern of synthesis. J Clin Invest. 1969 Nov;48(11):2114–2123. doi: 10.1172/JCI106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman B., Rosenberg L., Karpatkin S. Biochemical and biophysical aspects of human platelet adhesion to collagen fibers. J Clin Invest. 1971 Sep;50(9):1854–1863. doi: 10.1172/JCI106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD T. P., ODELL T. T., Jr, GOSSLEE D. G. PLATELET SIZE IN RELATION TO PLATELET AGE. Proc Soc Exp Biol Med. 1964 Mar;115:684–689. doi: 10.3181/00379727-115-29006. [DOI] [PubMed] [Google Scholar]
- Minter F. M., Ingram M. Platelet volume: density relationships in normal and acutely bled dogs. Br J Haematol. 1971 Jan;20(1):55–68. doi: 10.1111/j.1365-2141.1971.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Murphy S., Gardner F. H. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med. 1969 May 15;280(20):1094–1098. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- Mürer E. H., Hellem A. J., Rozenberg M. C. Energy metabolism and platelet function. Scand J Clin Lab Invest. 1967;19(3):280–282. doi: 10.3109/00365516709090638. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Mengel C. E., Meriwether W. D., Zirkle L. G., Jr Inhibition of erythrocyte acetylcholinesterase by peroxides. Biochemistry. 1966 Jan;5(1):40–44. doi: 10.1021/bi00865a006. [DOI] [PubMed] [Google Scholar]
- Okuma M., Steiner M., Baldini M. Lipid peroxidation in aging platelets. Blood. 1969 Nov;34(5):712–716. [PubMed] [Google Scholar]
- Placer Z. A., Cushman L. L., Johnson B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Free radicals in biological systems. Sci Am. 1970 Aug;223(2):70–passim. doi: 10.1038/scientificamerican0870-70. [DOI] [PubMed] [Google Scholar]
- SMITH G. J., DUNKLEY W. L. Initiation of lipid peroxidation by a reduced metal ion. Arch Biochem Biophys. 1962 Jul;98:46–48. doi: 10.1016/0003-9861(62)90142-x. [DOI] [PubMed] [Google Scholar]
- Slater T. F. The inhibitory effects in vitro of phenothiazines and other drugs on lipid-peroxidation systems in rat liver microsomes, and their relationship to the liver necrosis produced by carbon tetrachloride. Biochem J. 1968 Jan;106(1):155–160. doi: 10.1042/bj1060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLS E. D. Effect of unsaturated fatty acids and their peroxides on enzymes. Biochem Pharmacol. 1961 Jul;7:7–16. doi: 10.1016/0006-2952(61)90119-8. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. MECHANISMS OF LIPID PEROXIDE FORMATION IN TISSUES. ROLE OF METALS AND HAEMATIN PROTEINS IN THE CATALYSIS OF THE OXIDATION UNSATURATED FATTY ACIDS. Biochim Biophys Acta. 1965 Apr 5;98:238–251. doi: 10.1016/0005-2760(65)90118-9. [DOI] [PubMed] [Google Scholar]
- Zieve P. D., Solomon H. M. Uptake of amino acids by the human platelet. Am J Physiol. 1968 Jan;214(1):58–61. doi: 10.1152/ajplegacy.1968.214.1.58. [DOI] [PubMed] [Google Scholar]



