Abstract
Soluble antigens diffuse out of the brain and can thus stimulate a systemic immune response, whereas particulate antigens (from infectious agents or tumor cells) remain within brain tissue, thus failing to stimulate a systemic immune response. Immune privilege describes how the immune system responds to particulate antigens localized selectively within the brain parenchyma. We believe this immune privilege is caused by the absence of antigen presenting dendritic cells from the brain. We tested the prediction that expression of fms-like tyrosine kinase ligand 3 (Flt3L) in the brain will recruit dendritic cells and induce a systemic immune response against exogenous influenza hemagglutinin in BALB/c mice. Coexpression of Flt3L with HA in the brain parenchyma induced a robust systemic anti-HA immune response, and a small response against myelin basic protein and proteolipid protein epitopes. Depletion of CD4+CD25+ regulatory T cells (Tregs) enhanced both responses. To investigate the autoimmune impact of these immune responses, we characterized the neuropathological and behavioral consequences of intraparenchymal injections of Flt3L and HA in BALB/c and C57BL/6 mice. T cell infiltration in the forebrain was time and strain dependent, and increased in animals treated with Flt3L and depleted of Tregs; however, we failed to detect widespread defects in myelination throughout the forebrain or spinal cord. Results of behavioral tests were all normal. These results demonstrate that Flt3L overcomes the brain's immune privilege, and supports the clinical development of Flt3L as an adjuvant to stimulate clinically effective immune responses against brain neo-antigens, for example, those associated with brain tumors.
Keywords: immune response, dendritic cells, influenza hemagglutinin, regulatory T cells, perivascular cuffs
The CNS, the anterior chamber of the eye, and the testis are classified as immune-privileged organs (1). Immune privilege is thought to protect organs with limited regenerative capacity from immune attack (1–4), but has the consequence of reduced immune surveillance of the brain for neo-antigens associated with viruses or tumors. In the brain, the absence of conventional lymphatic drainage, low levels of constitutive expression of MHC class I and class II molecules (5), production of powerful immunosuppressive factors such as TGF-β (6), or factors that activate regulatory T cells (Tregs) (7), and, perhaps most importantly, scarcity of resident professional antigen presenting cells, i.e., dendritic cells (DCs) (8), all contribute to the particular immune responsiveness of the brain (3). Although the blood–brain barrier is important in regulating the traffic of ions and large molecules into the brain, it plays a limited role in determining the brain's immune responsiveness (9).
Importantly, the immune privilege of the brain is restricted to the parenchyma, whereas the ventricles, choroid plexus, meninges, and cerebrospinal fluid display the full range of innate and adaptive immune responses, because they contain the requisite immune structures and cells (3, 9–11). Soluble antigen will diffuse from the brain parenchyma to the ventricles, will be taken up by DCs, which migrate to the cervical lymph nodes, and will initiate an immune response (12–14). However, large particulate antigens are unable to diffuse out of the brain parenchyma. For example, the immune response to live influenza virus depends on the injection site (15); injection into the ventricles immediately causes immune priming, but injections into the brain parenchyma do not, until the infection breaks into the ventricles. Careful injections of bacillus Calmette–Guérin, a particulate nonsoluble antigen (16, 17), or adenoviral vectors, directly into the brain parenchyma do not stimulate specific systemic immune responses, whereas injections into the ventricles do (18, 19). Nevertheless, injections into the brain parenchyma will induce innate immune responses (e.g., cytokine release, TLR activation) restricted to the brain (20–23).
Once the systemic immune system has been primed through peripheral exposure to antigen, effector lymphocytes have few restrictions in entering the brain and eliminating cells expressing their cognate antigens (24, 25). The possibility therefore arises that if the population of DCs in the brain could be increased, immune surveillance of the brain would be improved. One candidate for increasing numbers of DCs is the cytokine fms-like tyrosine kinase ligand 3 (Flt3L), which induces the development of DCs from monocyte precursors (26). We have previously shown that injection of Flt3L results in the recruitment of DCs into normal rat brain tissue (27). When combined with cytotoxic approaches such as herpes simplex type 1 thymidine kinase (TK) and gancyclovir (GCV), intratumoral expression of Flt3L elicits an anti–brain-tumor immune response in mice (28, 29).
In this study, we explored the mechanistic basis of this treatment, by testing whether Flt3L can overcome the brain's immune privilege and enable a systemic T cell immune response against an antigen expressed exclusively in the brain parenchyma. Expression of the influenza glycoprotein hemagglutinin within the brain parenchyma induced only a systemic HA-specific immune response when coexpressed with Flt3L.
Regulatory T cells are another component of the brain's immune privilege, acting to prevent excessive damage of healthy tissue, but also potentially suppressing the antiviral and antitumor immune responses (30). We compared the effect of depletion of CD4+CD25+ Tregs independently, or combined with Flt3L administration, and found that the two treatments synergized in augmenting the immune response against HA.
We also found that expression of Flt3L in the brain parenchyma elicited a small systemic immune response against myelin basic protein (MBP) and proteolipid protein 1 (PLP) epitopes. However, such immune responses, which were increased by Treg depletion, were insufficient to induce behavioral deficits typical of experimental allergic encephalomyelitis, even though brain inflammation was increased. These results suggest that Flt3L can be used to break the immune privilege of the brain parenchyma, and support its use to stimulate immune responses against novel antigens, such as are expressed by brain tumors, or virally infected cells. Furthermore, the restricted CNS inflammation and absence of behavioral deficits support the continued development of combined cytotoxic/immunostimulatory anti-glioma therapy that will begin phase I clinical testing in the upcoming year.
Results
Striatal Expression of HA Alone Fails to Elicit a Substantial Immune Response.
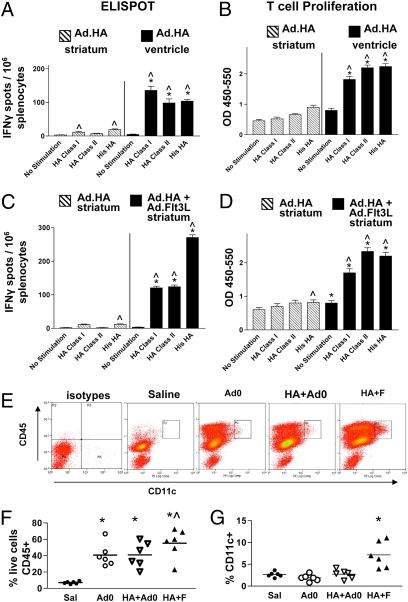
We first tested the prediction that expression of a neo-antigen in the brain parenchyma does not induce a systemic immune response. Ad.HA was injected into the brain striatum, or into the cerebral ventricles. Seven days later, animals were killed, and splenocytes were stimulated with the HA class I peptide, the HA class II peptide, or His-HA protein and analyzed by ELISPOT (Fig. 1A) and T cell proliferation assays (Fig. 1B). Animals injected with Ad.HA in the striatum revealed a minimal increase in the frequency of HA-specific IFNγ-secreting T lymphocytes (Fig. 1A), and no HA-specific T cell proliferation (Fig. 1B). As predicted, ventricular administration of Ad.HA resulted in the induction of a massive HA-specific immune response.
Fig. 1.
Immune privilege of brain parenchyma can be overcome by Flt3L. (A) Seven days after injection into striatum (hatched bars) or into ventricle (solid bars), splenocytes were stimulated with either the HA class I peptide, HA class II peptide, or His-HA protein or unstimulated before quantification of IFNγ production by ELIspot. ^P < 0.05 vs. corresponding no stimulation group; *P < 0.05 vs. splenocytes from intrastriatally injected animals incubated with same stimuli; n = 5/group. (B) Splenocytes were stimulated with either the HA class I peptide, HA class II peptide, or His-HA protein, or as controls, unstimulated. BrdU incorporation into nascent DNA strands was measured by ELISA to determine relative proliferation of T lymphocytes (vertical axis represents optical density). ^P < 0.05 vs. corresponding no stimulation group; *P < 0.05 vs. splenocytes from intrastriatally injected animals incubated with same stimuli; n = 5/group. (C and D) HA-specific immune response in animals injected in striatal parenchyma with Ad.HA alone (hatched bars), or with coinjection of Ad.FLt3L (solid bars). Seven days after injection, cells were isolated from spleens and characterized by ELIspot (C) or T cell proliferation assay (D). Layout and statistics for these figures are identical to A and B. Error bars indicate SEM. (E) Representative flow cytometry dot plots showing effect of Flt3L injection on numbers of CD45 and CD11c immunopositive cells infiltrating the brain. Intact cells were gated on forward and side scatter. First panel shows cells incubated with control antibodies; remaining four panels show, from left to right, cells from animals injected with saline, 6 × 107 Ad.0, 1 × 107 Ad.HA + 5 × 107 Ad.0, and 1 × 107 Ad.HA + 5 × 107 Ad.Flt3L. Numbers of cells in rectangle marked R2 were used to prepare column scatter graph in G. (F) Percentage of live cells extracted from brains immunopositive for CD45, by group. *P < 0.05 vs. saline; ^P < 0.05 vs. Ad.0 or HA+Ad.0. (G) Percentage of CD45+ cells also CD11c positive. *P < 0.05 vs. saline. Data from individuals closest to mean value were chosen for representative dot plots in E.
Intrastriatal Flt3L Induces a Systemic HA-Specific Immune Response.
In the experimental autoimmune encephalomyelitis model (EAE) model of multiple sclerosis, systemic delivery of Flt3L results in an increase of circulating and meningeal DCs, and a more severe disease phenotype (31) (32). In a brain tumor model, we have previously demonstrated that Ad.Flt3L recruits DCs to the brain and, together with Ad.TK, induces an antiglioma immune response. One possible way in which Flt3L might accomplish this is by overcoming the brain's immune privilege. To investigate this possibility, we assessed whether striatal expression of Flt3L would enable recognition of HA expressed in the brain striatum. We injected BALB/c mice intrastriatally with Ad.HA with or without coadministration of Ad.Flt3L. Seven days later, animals were killed, and splenocytes were stimulated with either the HA class I peptide, the HA class II peptide, or His-HA protein and then analyzed by ELISPOT (Fig. 1C) and T cell proliferation (Fig. 1D). Coadministration of Ad.Flt3L with Ad.HA into the brain striatum caused a large increase in the frequency of HA-specific, IFNγ-secreting T lymphocytes (Fig. 1C) and HA-specific T cell proliferation (Fig. 1D). To examine the possibility that the effect of FLt3L is mediated by recruitment of DCs, we assessed by flow cytometry the frequency of CD11c+ cells infiltrating the striata of animals injected with Ad.HA and either Ad.Flt3L, or a control vector with no transgene (Ad.0) (Fig. 1 E–G). Injection of either combination of vectors resulted in a large increase in CD45+ cells in the brain compared with that in animals injected with saline (Fig. 1F), but only in the animals injected with Ad.FLt3L was the fraction of these cells expressing CD11c (DCs) increased compared with controls (injected with saline or Ad.0) (Fig. 1G).
Expression of Flt3L in Striatum Induces Immune Response against Brain Antigens.
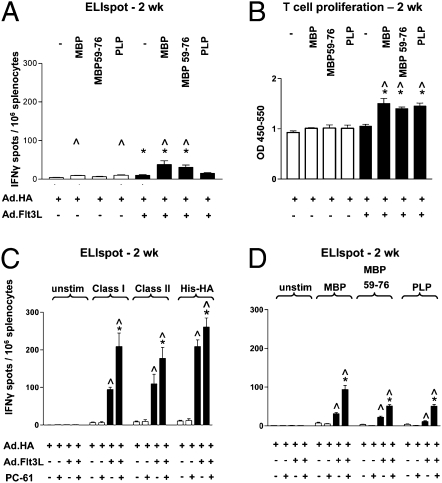
As Flt3L induced an immune response against a neo-antigen restricted to the brain parenchyma, we asked whether the immune response would also target brain protein self-epitopes. Splenocytes from BALB/c mice injected intrastriatally with either Ad.HA alone or Ad.HA + Ad.Flt3L and killed at 1 or 2 wk were analyzed by ELISPOT and T cell proliferation assays using self-antigens MBP and PLP as stimuli. At 1 wk, the response to self-epitopes was minimal and not influenced by Flt3L in an antigen-specific way (SI Appendix, Fig. S3). At 2 wk, Flt3L treatment had increased the number of T lymphocytes secreting IFNγ (Fig. 2A) in response to MBP or MBP peptide, but not PLP, and T cell proliferation (Fig. 2B) in response to MBP or PLP. There was also a small increase in IFNγ spots in the unstimulated group (Fig. 2A), suggesting that Flt3L administration increases the nonspecific immune reactivity, not just the antigen-specific response, although the interaction between stimulus and Flt3L treatment in a two-way ANOVA (P < 0.017) implies antigen dependence, reflected in the lack of response to PLP.
Fig. 2.
Minor immune response against self-brain antigen is induced by injection of Ad.Flt3L and HA, and augmented by Treg depletion. (A and B) Ad.HA alone (open bars) or Ad.HA + Ad.Flt3L (filled bars) were injected in the left striata of BALB/c mice. (A) Fourteen days after vector injection, splenocytes were stimulated with MBP pure protein, the MBP 59–67 peptide, or the PLP 139 peptide, or unstimulated before quantification of IFNγ production by ELIspot. *P < 0.05 vs. splenocytes from Ad.HA-injected animals (i.e., no Flt3L) incubated with same stimulus; ^P < 0.05 vs. corresponding no stimulation group; n = 5/group. (B) Splenocytes were stimulated with MBP pure protein, MBP 59–67 peptide, or PLP 139 peptide, or as controls, unstimulated. BrdU incorporation into nascent DNA strands was measured to determine relative proliferation of T lymphocytes lymphocytes (vertical axis represents optical density). *P < 0.05 vs. splenocytes from Ad.HA-injected animals (i.e., no Flt3L) incubated with same stimuli; ^P < 0.05 vs. corresponding no stimulation group; n = 5/group. (C and D) Effect of Treg depletion. Ad.HA alone (open bars) or Ad.HA + Ad.Flt3L (filled bars) was injected into the left striata of BALB/c mice. Tregs were depleted by systemic administration of PC-61 or with saline as a control. Two weeks later, animals were euthanized and splenocytes were isolated for ELISPOT assays. (C) The frequency of HA-specific IFNγ secreting T lymphocytes was quantified after stimulation with either the HA class I peptide, HA class II peptide, or His-HA protein, or as controls, unstimulated. *P < 0.05 vs. saline depletion of the same vector treatment and stimulation group; ^P < 0.05 vs. splenocytes from Ad.HA-injected (i.e., no FLt3L) animals in the same depletion and stimulation group; n = 5. (D) Frequency of myelin or PLP-specific, IFNγ-secreting T lymphocytes was quantified following stimulation with MBP pure protein, MBP 59–67 peptide, or PLP 139 peptide, or as controls, unstimulated. Data were analyzed by two-way ANOVA followed by Tukey–Kramer multiple comparison test. *P < 0.05 vs. saline depletion of same vector treatment and stimulation group; ^P < 0.05 vs. splenocytes from Ad.HA-injected (i.e., no Flt3L) animals in the same depletion and stimulation group; n = 5/group. Error bars indicate SEM.
Depletion of Regulatory T Cells Enhances Immune Responses Against Neo- and Self-Antigens following Delivery of Flt3L.
The contribution of Tregs to the initiation and resolution of the EAE disease phenotype has been widely studied (33–35), and depletion of CD4+CD25+ Tregs exacerbates clinical symptoms of EAE in mice (36, 37). We wished to assess the effect of Treg depletion on the induction of neo-antigen or self-antigen specific immune responses in Flt3L-treated animals. Depletion of CD4+CD25+ Tregs with the rat monoclonal anti-CD25 antibody PC-61 significantly increased the systemic immune response against a neo-antigen only in animals receiving a striatal injection of Ad.Flt3L (Fig. 2C). When splenocytes from these animals were stimulated with peptides derived from MBP or PLP, an increase in the frequency of IFNγ secreting T lymphocytes specific for self-brain antigens was also observed (Fig. 2D).
Flt3l Treatment and Treg Depletion Do Not Induce Overt Demyelination or Behavioral Abnormalities.
In view of the discovery of elevated levels of self-reactive T cells in Flt3L-treated mice, especially in animals depleted of Tregs, the possibility of auto-immune damage was investigated in detail. First, in addition to the 2-wk time point, another cohort of animals was maintained until 2 mo after vector injection and Treg depletion, to enable any autoimmune effects to become more obvious. Second, behavior of animals was tested systematically at the 2-mo time point. Third, the experiment was repeated using C57BL/6 mice in addition to BALB/c, as C57BL/6 mice are reported to be more susceptible to EAE (38).
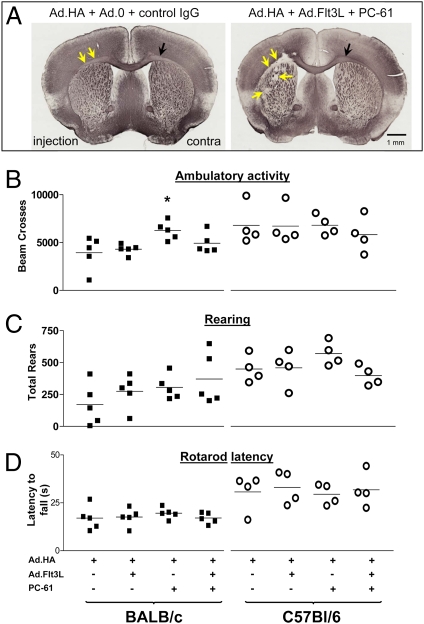
Myelin morphology was examined by MBP immunohistochemistry (Fig. 3A; taken from the 2-mo time point). The noninjected hemispheres did not show any damage in any of the groups. The injected hemispheres, however, displayed some signs of localized damage at the 2-wk and 2-mo time points, being of a significant nature in animals treated with both Flt3L and Treg depletion.
Fig. 3.
Myelin lesions in C57BL/6and BALB/c mice injected with Flt3L and depleted of Tregs are restricted to injected hemisphere and do not affect behavior. (A) Coronal brain sections immunolabeled for MBP from C57BL/6 mice injected with 1 × 107 Ad.HA + 5 × 107 Ad.0 and treated with a control antibody (Left), or 1 × 107 Ad.HA + 5 × 107 Ad.Flt3L and PC-61 (Right). Yellow arrows on right image indicate destruction of white matter in injected hemisphere (injection); black arrow shows intact white matter contralaterally (contra). Little damage is visible in the animal not treated with FLt3L or PC61 (Left). (B) Ambulatory activity of mice in open field trials. Vertical axis shows number of times that mice crossed light beams in a 60-min trial. Left four columns show data from BALB/c mice; right four columns show data from C57BL/6 mice. *P < 0.05 vs. group treated with Ad.HA only (i.e., no Flt3L or PC-61). (C). Rearing data do not show any behavioral effects of treatments. (D) Mice were placed on a rod that was then rotated at slowly increasing speed until the mouse fell. Latency to fall was recorded (vertical axis). There were no statistically significant differences between groups.
At 2 mo, animals were subjected to a battery of psychomotor tests. In both strains in all four groups, righting response, eye blink, ear twitch, startle response, and olfactory and visual orienting were all normal at 2 mo, and every animal was able to hang onto a wire for 30 s without falling, with the single exception of one BALB/c mouse treated with Flt3L alone, which fell from the wire after 8 s. This mouse was unremarkable in any other measure. Immediately before being killed at 2 mo, all mice were able to hold up their tails with no sign of flaccidity, and were able to grip a wire cage with each of their hind feet. In open field trials, all mice, regardless of group, were similarly active (Fig. 3 B and C), although the BALB/c group treated with PC-61 alone was slightly more active (Fig. 3B, Left). All animals were able to remain on a rotating rod for a similar length of time (Fig. 3D). No other significant differences among groups were found.
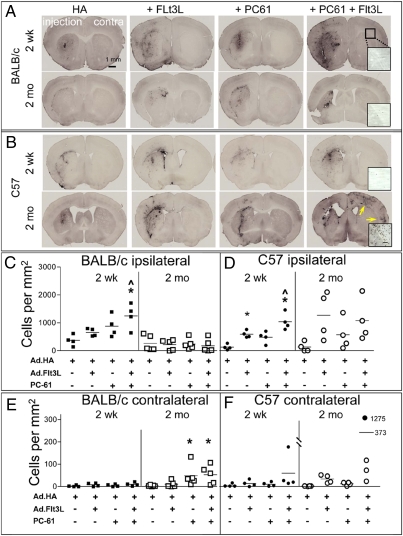
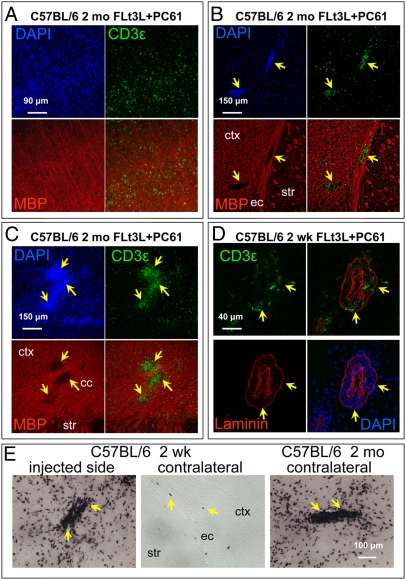
Brains from all animals were immunolabeled for CD3ε (a pan-T cell marker) to reveal infiltrating T cells as a measure of inflammation (Fig. 4), and T cells counted in the injected striatum (Fig. 4 C and D) and the contralateral cortex and corpus callosum (Fig. 4 E and F). At 2 wk, the pattern of T cell infiltration was similar between strains, being minimal in animals receiving HA only, and maximal in animals depleted of Tregs and given Flt3L (Fig. 4 A and B, Top, and C and D, Left). At 2 mo, in the injected hemispheres, the patterns differed dramatically between strains; in the BALB/c mice, T cell infiltration was substantially less than at 2 wk, whereas in the C57BL/6 it had increased. In the contralateral hemispheres there were essentially no T cells present at 2 wk, but they were increased at 2 mo in both strains in animals treated with Flt3L and depleted of Tregs. One animal in particular, from the Flt3L-treated, Treg depleted group had very extensive T cell infiltration in both hemispheres, and this animal was characterized further by immunofluorescent labeling for MBP and CD3ε. Numerous T cells were found scattered diffusely throughout the cortex (Fig. 5A), or concentrated in clusters (Fig. 5 B and C). The relationship between these clusters and blood vessels was investigated with double immunolabeling for CD3ε and laminin; clusters of T cells were localized to perivascular cuffs (Fig. 5D). At 2 wk, these perivascular cuffs were found only on the injected side (Fig. 5 E, Left), whereas in the contralateral hemisphere T cells were very sparse and mostly restricted to the region of the external capsule (Fig. 5E, Middle). However, in the animal with the greatest T cell infiltration at 2 mo, these clusters could also be seen in the contralateral cortex (Fig. 5E, Right). As T cells usually target the spinal cord in EAE, we examined segments of thoracic and cervical spinal cords from all mice from the group that displayed the most contralateral inflammation, i.e., C57BL/6, 2 mo postinjection of Flt3L and Treg depletion. T cells were only found in the cervical spinal cord of the one animal with the highest contralateral brain inflammation, within an area of the dorsal spinal cord corresponding to the cortico-spinal tract (SI Appendix, Fig. S4). We saw no T cell infiltration in the anterior horn, as is usually seen in EAE. We conclude that the T cells in the spinal cord of this animal are most likely infiltrating in response to inflammation caused by degenerating cortico-spinal axons following forebrain inflammation and potential tissue destruction.
Fig. 4.
T cell infiltration at 2 wk and 2 mo following administration of Ad.HA and Ad.Flt3L, and depletion of Tregs with PC61. (A) Coronal sections of BALB/c mice injected with combinations of vectors and antibodies indicated at top of figure and perfused at time point indicated at left of figure and immunolabeled for CD3ε (pan-T cell marker). Images are from a representative section from the brain most extensively infiltrated with T cells from each group. Injected hemisphere (injection), noninjected hemisphere (contra), and scale bar representing 1 mm are all indicated (Top Left) and are the same throughout. (B) Sections from C57BL/6 mice. Layout is identical to that in A. Yellow arrows (Bottom Right) indicate infiltration in the contralateral hemisphere. Insets, Top Right in rightmost panels, are higher-ower images of contralateral external capsule, showing degree of T cell infiltration. (Scale bar, 100 μm.) (C) Numbers of CD3ε immunoreactive T cells in injected striatum of each BALB/c mouse by time point and treatment group. Cells were counted in the most extensively infiltrated section and expressed as number per square millimeter of area of striatum in that section. *P < 0.05 vs. Ad.HA (i.e., no Flt3L or PC-61) group at same time point; ^P < 0.05 vs. equivalent group at 2-mo time point. (D) Corresponding counts for C57BL/6 mice. *P < 0.05 vs. Ad.HA group at same time point; ^P < 0.05 vs. Ad.HA+PC-61 group. (E and F) Similar counts for contralateral cortex + corpus callosum. This area was chosen because it contained noticeably more T cells than the contralateral striatum. *P < 0.05 vs. Ad.HA group at same time point. One C57BL/6 mouse had very extensive T cell infiltration in the contralateral cortex (B, Bottom Right), and the count for this animal is plotted in F with a filled circle at an arbitrary location, with the numerical value indicated beside the plotted point to facilitate graphing; similarly, as the mean for this group is off-scale, the mean is represented with a bar in an arbitrary location with its numerical value beside the bar. Disparate intergroup variance in this dataset, even following log or other transformation, precluded analysis of variance.
Fig. 5.
T cells are scattered through cortex and cluster in perivascular cuffs. (A–C) Immunofluorescent labeling of MBP (red) and CD3ε (green) in coronal sections of C57BL/6 mouse brains from Flt3L-treated, Treg-depleted, 2-mo group. All images are maximum projections of confocal stacks from the hemisphere contralateral to the injection. A is from the dorsal cortex; B is from the lateral striatum (str), external capsule (ec), and adjacent cortex (ctx); and C is from the dorsal aspect of the corpus callosum (cc). (D) immunofluorescent labeling of CD3ε (green) and laminin (red) in a coronal section of a C57BL/6 mouse from Flt3L-treated, Treg-depleted, 2-wk group. Image is a single confocal section taken from a ventromedial location within the injected striatum. Notice the accumulation of T cells within perivascular cuffs. Yellow arrows indicate same regions in different channels. (E) Immunoperoxidase labeling of CD3ε. (Left) Image from a C57BL/6 mouse from Flt3L-treated, Treg-depleted, 2-wk group; image is taken from dorsal striatum (str) of injected hemisphere. (Middle) Image from same section, illustrating contralateral external capsule (ec) and adjacent cortex (ctx). (Right) Image from a C57 mouse from the Flt3L-treated, Treg-depleted, 2-mo group, captured in a location similar to that of the Middle image. (Scale bar, 100 μm.) Yellow arrows indicate perivascular cuffs.
Discussion
The data described demonstrate that expression of Flt3L in the brain overcomes the brain's immune privilege, i.e., enables a systemic immune response specifically against an antigen the expression of which is restricted to the brain parenchyma. This result is significant because the brain's immune privilege is likely to limit effective anti–brain-tumor immune therapies. We have previously described a treatment in which Ad.Flt3L and Ad.TK are injected into brain tumors in rodents (28, 29). In several model systems, this treatment induces a potent, systemic, clinically effective, and specific antiglioma immune response, without untoward autoimmune effects, but the mechanism by which Flt3L asserts its effect is still an active research question. The experiments described in this paper were designed to answer three questions: (i) whether Flt3L expression enables a systemic immune response specifically against an antigen expressed solely within the brain parenchyma; (ii) if so, how this effect compares with the effect of depleting Tregs; and (iii) whether either of these treatments results in harmful autoimmune responses.
The model antigen HA was chosen because of the well-characterized class-I– and class-II–restricted immune responses in BALB/c mice, and was expressed from a recombinant adenovirus. As expected, injection of Ad.HA into the striatum did not cause systemic anti-HA immune responses. As a positive control, Ad.HA was injected into the ventricles and induced a robust systemic anti–HA-specific immune response. Using this paradigm of brain immune privilege, we tested the effect of coadministration of Ad.Flt3L, and found that it enables a strong systemic immune response against HA expressed within the brain parenchyma, i.e., overcomes immune privilege. To test the hypothesis that this effect is mediated by recruitment of DCs to the brain, we assessed numbers of DCs in animals injected with Ad.Flt3L and confirmed that the DC numbers were increased. This finding does not prove that DCs are causally involved in the breakdown of immune privilege but is consistent with this possibility.
The fact that a robust systemic anti-HA immune response can be induced by coadministration with Flt3L raised the question of whether it also induces an autoimmune response against endogenous brain antigens. Analysis of the frequency of IFNγ secreting T lymphocytes and T cell proliferation in response to stimulation with MBP and PLP revealed a small immune response against these antigens, and brain infiltration by CD3ε + T cells. The effect of Treg depletion by itself was generally minor but, in combination with Flt3L, resulted in a stronger immune response to both exogenous and self-antigens. However, Flt3L and depletion of Tregs failed to induce the kind of motor deficits, demyelination, or spinal cord inflammation normally associated with EAE. These results strongly suggest that Flt3L administration increases immune reactivity against antigens in the brain parenchyma, whether endogenous or exogenous, and that in combination with Treg depletion results in long-term inflammation. However, the evidence for deleterious autoimmunity is not definitive, as any of the effects that we observed could be due to the combination of enhanced immune reactivity and the anti-HA immune response. Resolving this issue beyond reasonable doubt may require experiments involving adoptive transfer of immune cells from Flt3L-treated, Treg-depleted donors into naive, syngeneic hosts.
Overall, our data indicate that an immune response against antigens localized to the brain, such as those expressed by brain tumors or virus, could be stimulated by administration of Flt3L intracranially and enhanced by depletion of Tregs. These data are consistent with previous work from our group indicating that combined use of Flt3L + TK (+ ganciclovir) to treat unilateral intracranial brain tumors induces an effective anti–brain-tumor immune response without overt autoimmunity (37–42), and the data strongly support the addition of Flt3L as an adjuvant to cytotoxic and immunostimulatory approaches currently under evaluation for the treatment of human malignant brain tumors.
Methods
Adenoviral Vectors.
Ad.Flt3L and Ad.0 have been described elsewhere (28, 43). The HA cDNA plasmid provided by A. Caton (Wistar Instute, Philadelphia, PA) was cloned into the adenoviral vector shuttle plasmid pΔE1sp1a (Microbix). The first-generation adenoviral vector Ad.HA was rescued by cotranfection with pJM17 and amplified and purified as described previously (39). Cloning details and characterization of the vector are described in SI Appendix, SI Methods, and the vector is depicted schematically in SI Appendix, Fig. S1.
Stereotactic Injection of Adenoviral Vectors and Treg Depletion.
All animal experiments were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center. Mice were injected with vectors or saline into the left striatum (+0.5 mm AP, –2.2 mm ML, –3.0 mm DV from the bregma) or ventricle (+0.8 mm AP, +1 mm ML, –2.0 mm DV from the bregma), as described previously (40). To deplete CD25+ Tregs, mice were treated with a single i.p. injection of 1 mg ascites fluid (600 μL) from the PC61 hybridoma (41). Results of a pilot experiment confirming depletion of Foxp3+, CD25+ Tregs after PC-61 treatment are shown in SI Appendix, Fig. S2. At 7, 14, or 60 d postinjection, animals were perfused for IHC, or brains and spleens were collected for analysis of immune cell responses. IHC and tissue processing are described in SI Appendix, SI Methods.
IFNγ ELISPOT and T-Cell Proliferation Assays.
Splenocytes were assessed for IFNγ secreting T lymphocyte frequency by ELISPOT, or T-cell proliferation by BrdU incorporation with the following stimuli: His-HA protein (5 μg/mL), HA518 class I peptide (IYSTVASSL, 1 μg/mL), the HA class II peptide (HNTNGVTAACSHE, 1 μg/mL), MBP peptide (HTRTTHYGSLPQKSQHGR, 0.1 μg/mL), or PLP139 peptide (HSLGKWLGHPDKF, 0.1 μg/mL).
Behavior and Statistical Analysis.
Animals were tested in open-field, rotating rod paradigms, and subjected to a battery of tests collectively known as neuroscreen. Procedures are described in SI Appendix, SI Methods.
Quantitative data were subjected to one- or two-way ANOVA followed by a Tukey–Kramer multiple comparison test using NCSS 2007 software. When data failed normality or variance homogeneity tests, the data were square root or log transformed before ANOVA.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant 1UO1 NS052465.01. The brain tumor program in our institute is funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants 1RO1-NS 057711 and 1R21-NSO54143 (to M.G.C.) and National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants 1 RO1 NS 054193 and RO1 NS 42893 (to P.R.L). The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics (to P.R.L. and M.G.C., respectively). The Drown Foundation, the Linda Tallen & David Paul Kane Foundation Annual Fellowship and the Board of Governors at CSMC also provided support. M.C. is supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke 1F32 NS058156.01. D.L. was supported by a postdoctoral fellowship from the Human Frontier Science Program. Ali Zadmehr helped with tissue sectioning.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913496107/-/DCSupplemental.
References
- 1.Mrass P, Weninger W. Immune cell migration as a means to control immune privilege: Lessons from the CNS and tumors. Immunol Rev. 2006;213:195–212. doi: 10.1111/j.1600-065X.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 2.Arck PC, Gilhar A, Bienenstock J, Paus R. The alchemy of immune privilege explored from a neuroimmunological perspective. Curr Opin Pharmacol. 2008;8:480–489. doi: 10.1016/j.coph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: An evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 5.Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- 6.Logan A, Frautschy SA, Gonzalez AM, Sporn MB, Baird A. Enhanced expression of transforming growth factor beta 1 in the rat brain after a localized cerebral injury. Brain Res. 1992;587:216–225. doi: 10.1016/0006-8993(92)91000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boche D, Cunningham C, Docagne F, Scott H, Perry VH. TGFbeta1 regulates the inflammatory response during chronic neurodegeneration. Neurobiol Dis. 2006;22:638–650. doi: 10.1016/j.nbd.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74:599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 9.Wekerle H. Breaking ignorance: The case of the brain. Curr Top Microbiol Immunol. 2006;305:25–50. doi: 10.1007/3-540-29714-6_2. [DOI] [PubMed] [Google Scholar]
- 10.Cartmell T, et al. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J Neurosci. 1999;19:1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Yamada S, DePasquale M, Patlak CS, Cserr HF. Albumin outflow into deep cervical lymph from different regions of rabbit brain. Am J Physiol. 1991;261:H1197–H1204. doi: 10.1152/ajpheart.1991.261.4.H1197. [DOI] [PubMed] [Google Scholar]
- 13.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545:103–113. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 14.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matyszak MK, Perry VH. A comparison of leucocyte responses to heat-killed bacillus Calmette-Guérin in different CNS compartments. Neuropathol Appl Neurobiol. 1996;22:44–53. [PubMed] [Google Scholar]
- 17.Matyszak MK, Perry VH. Bacillus Calmette-Guérin sequestered in the brain parenchyma escapes immune recognition. J Neuroimmunol. 1998;82:73–80. doi: 10.1016/S0165-5728(97)00190-2. [DOI] [PubMed] [Google Scholar]
- 18.Barcia C, et al. Immunological thresholds in neurological gene therapy: Highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2006;2:309–322. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcia C, et al. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: Implications for clinical trials. Mol Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zirger JM, et al. Rapid upregulation of interferon-regulated and chemokine mRNAs upon injection of 108 international units, but not lower doses, of adenoviral vectors into the brain. J Virol. 2006;80:5655–5659. doi: 10.1128/JVI.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CE, Schiedner G, Kochanek S, Castro MG, Löwenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: Toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: The role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7:347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barcia C, et al. Immunological thresholds in neurological gene therapy: Highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2006;2:309–322. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcia C, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 27.Curtin JF, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtin JF, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candolfi M, et al. Release of HMGB1 in response to pro-apoptotic glioma killing strategies: Efficacy and neurotoxicity. Clin Cancer Res. 2009;15:15–21. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol. 2008;69:760–770. doi: 10.1016/j.humimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 32.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: Contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 36.Kohm AP, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 37.Akirav EM, Bergman CM, Hill M,, Ruddle NH. Depletion of CD4(+)CD25(+) T cells exacerbates experimental autoimmune encephalomyelitis induced by mouse, but not rat, antigens. J Neurosci Res. 2009;87:3511–3519. doi: 10.1002/jnr.21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(-/-) mice: Susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Southgate T, Kroeger KM, Liu C, Lowenstein PR, Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors. In: Gerfen C, Holmes H, Sibley D, Skolnick P, Wray S, editors. Curr Prot Neurosci. New York: Wiley; 2008. Chapter 4, Unit 4.23.21–4.23.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puntel M, et al. Gene transfer into rat brain using adenoviral vectors. In: Gerfen C, Holmes H, Sibley D, Skolnick P, Wray S, editors. Curr Prot Neurosci. New York: Wiley; 2010. Chapter 4, Unit 4.24.1–4.24.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtin JF, et al. Treg depletion inhibits efficacy of cancer immunotherapy: Implications for clinical trials. PLoS ONE. 2008;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caton AJ, Cerasoli DM, Shih FF. Immune recognition of influenza hemagglutinin as a viral and a neo-self-antigen. Immunol Res. 1998;17:23–32. doi: 10.1007/BF02786427. [DOI] [PubMed] [Google Scholar]
- 43.Ali S, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): Treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali S, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King GD, et al. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther. 2008;16:682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King GD, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro-oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mozdzanowska K, Feng J, Gerhard W. Virus-neutralizing activity mediated by the Fab fragment of a hemagglutinin-specific antibody is sufficient for the resolution of influenza virus infection in SCID mice. J Virol. 2003;77:8322–8328. doi: 10.1128/JVI.77.15.8322-8328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.