Abstract
The hippocampus is crucial for both spatial navigation and episodic memory, suggesting that it provides a common function to both. Here we adapt a spatial paradigm, developed for rodents, for use with functional MRI in humans to show that activation of the right hippocampus predicts the use of an allocentric spatial representation, and activation of the left hippocampus predicts the use of a sequential egocentric representation. Both representations can be identified in hippocampal activity before their effect on behavior at subsequent choice-points. Our results suggest that, rather than providing a single common function, the two hippocampi provide complementary representations for navigation, concerning places on the right and temporal sequences on the left, both of which likely contribute to different aspects of episodic memory.
Keywords: allocentric, decision making, egocentric, episodic memory, cognitive control
The hippocampus plays a crucial role in both spatial navigation and episodic memory (1–6). However, the nature of the fundamental hippocampal process or representation that might underlie both functions remains the subject of intense speculation, including suggestions that it is best characterized as associative (7), sequential (8), flexible relational (2), allocentric (1, 5, 9), or spatial contextual (4, 5). Similar speculation surrounds the nature of any lateralization of these representations (1, 5, 10), and whether the firing of hippocampal neurons in freely moving rodents reflects allocentric position, spatial context, or sequential position along a route (5, 11, 12). Here we show that the hippocampus predicts and supports navigation via sequential representations in the left hippocampus and allocentric spatial representations in the right hippocampus. These complementary lateralized representations suggest an explanation for the multiple hippocampal contributions to different aspects of spatial and episodic memory.
Within spatial memory a distinction has been made between “allocentric” (world-centered) and “egocentric” (body-centered) representations, with allocentric (or place-learning) and simple egocentric (stimulus response-like) navigation shown to depend on the hippocampus and dorsal striatum, respectively, in rodents (5, 13). Rondi-Reig et al. (14) recently demonstrated that an additional sequential egocentric representation depends on the rodent hippocampus. The human hippocampus has likewise been associated with allocentric representations of location, allowing accurate navigation from new starting locations (15) based on the configuration of environmental cues (16, 17) or recognition of locations from a new viewpoint (18, 19). Similarly, navigation via a fixed route (15, 17) or relative to a single landmark (16), consistent with simple egocentric representations, has been associated with the dorsal striatum. However, to our knowledge, the neural bases of the sequential egocentric representation have not yet been identified in humans, and could provide a link between spatial navigation and episodic memory.
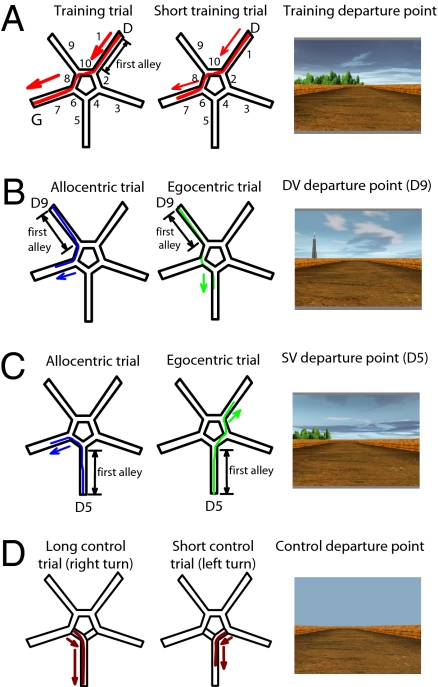
Here we adapt the Starmaze task developed for mice (14, 20) to investigate the neural bases of sequential egocentric representations and allocentric representations in humans. The task allows the parallel use of both types of representation during learning and performance of training trials, but also allows the use of one or other representation to be detected by the response made in probe trials. We used functional MRI (fMRI) during the navigation of the start alley of probe trials to determine the neural bases of the type of representation used as defined by the subsequent behavioral response. Our task is based on the virtual reality Starmaze (21), composed of 10 alleys, 5 central ones forming a pentagon and 5 alleys radiating from the angles of the central pentagon (Fig. 1A). Participants used a keypad to move their viewpoint forward or backward or to turn left or right; they could move around and perform rotations freely in all of the alleys. Distant environmental cues surrounded the maze for orientation. Participants were told to find a fixed goal that had no visible identifiers. When this goal was reached, fireworks went off to indicate the successful end of the trial (positive reinforcement). The experiment consisted of interleaved training trials, probe trials, and control trials (see Table S1 for complete trial order and SI Text for details). In training trials, successful navigation might be supported by either type of representation: sequential egocentric (sequence of body-turns) or allocentric (location relative to environmental cues). In probe trials, which were not distinguished from training trials in the instructions, participants had to find the goal from one of two different departure points, which allowed us to dissociate the use of either type of representation according to the path chosen by the participant (Fig. 1 B and C). The two probe-trial departure points were designed to differentially bias the participant toward use of an allocentric representation (departure point “DV,” which has a very different view to that from the training-trial departure point) or toward use of a sequential egocentric representation (departure point “SV,” which has a more similar view to that from the training-trial departure point). Use of either representation in a probe trial was considered correct; therefore, probe trials ended once the participant had made a response consistent with the use of a specific representation, without reinforcement. Control trials consisted of a navigation task in the same maze, but without environmental features, where participants had to move down two alleys joined by a turn (to the right or left). Half of the control trials ended midway down the final alley (“short control trials,” like 25% of training trials and all probe trials) (Fig. 1D).
Fig. 1.
Trial-types performed in the “Starmaze,” a virtual maze comprising a central pentagonal ring (alleys 2, 4, 6, 8, and 10), five radiating alleys (alleys 1, 3, 5, 7, and 9), and surrounding environmental cues. (A) Training trials: Participants start in alley 1 (see initial view, Right) and must navigate to the goal at the end of alley 7, which triggers fireworks (Left; arrow shows ideal path). Participants can use allocentric or sequential egocentric representations to solve the task. Twenty-five percent of training trials finish in the middle of alley 7, without fireworks (Center). (B and C) Probe trials use a different departure point to identify which type of representation is being used to solve the task. Half of the probe trials depart from alley 9, which has a very different view to alley 1 (B, departure point “DV”), half from alley 5, which has a more similar view to alley 1 (C, departure point “SV”). Participants using an allocentric representation including the distal environmental cues will go to alley 7 (paths shown in blue). Participants using a sequential egocentric representation of body-movements (right-left-right) will take the paths shown in green. The main fMRI analyses concern activation in the first alley. Probe trials always end in the middle of the goal alley, without fireworks. (D) Control trials: Control trials are composed of one single turn (to the right or to the left). No external landmarks are present in the environment. Half of the control trials end in the middle of the goal alley, without fireworks.
We focus on activations before the first choice-point of the maze, so that activation patterns can be analyzed according to the strategy of the participant on that run, as determined by their subsequent choices, but without any of the differences in behavior or stimuli resulting from the different paths following the choice-point. We hypothesized that we would identify, before the first choice-point, areas of the hippocampal prefrontal striatal loop involved in supporting the allocentric and sequential egocentric representations. In addition, we would expect to see some changes in activation over the time-course of the experiment, including increased hippocampal involvement early in the task, when novelty and learning is maximal (16, 22), and increased retrosplenial/medial parietal regions later in the task, as a detailed internal representation of the environment is formed that can support mental imagery (9, 17, 21–25).
Results
Behavioral Results.
All subjects learned the task quickly; a plateau in performance is reached after five training trials (Fig. S1 and SI Text). Sixteen probe trials were performed during the experiment, inserted regularly after training trial 3 (Table S1). Therefore, probe trials, except probe trial 1, were performed at stable performance. Probe trials were considered reliable for strategy identification if the learning criterion was reached (i.e., if the participant had performed two correct training trials before the probe trial) and the subject got to one of the two goal locations during the probe trial (Fig. 1 B and C). Probe trials that did not meet these criteria were considered failed (Fig. S1B).
As in Igloi et al. (21), three response types were observed during probe trials from both DV and SV departure points: (i) Allocentric responses: Participants navigated based on the environmental cues directly reached the goal in alley 7 (Fig. 1 B and C, Left). (ii) Sequential egocentric responses: Participants reproduced the same sequence of body-turns during the probe trials as during training trials, and reached the goal in alley 5 from departure-point DV (Fig. 1B, Middle) or alley 1 from departure-point SV (Fig. 1C, Center). (iii) Mixed responses: Participants started the trial by performing the previously realized body-turns (i.e., using a sequential egocentric representation) but reoriented themselves later on the path by using environmental cues (i.e., using an allocentric representation) and ended up in alley 7. These responses were only observed during probe trials from departure-point SV. For analyses of activations in the first alley, mixed responses were counted as sequential egocentric responses. For probe trials from both departure points, a mixture of sequential egocentric and allocentric responses were seen across participants (Fig. S1B). For probe trials from departure-point SV, participants mainly made sequential egocentric responses (Fig. S1B, Left) and for probe trials from departure-point DV, participants made mainly allocentric responses (Fig. S1B, Right). Over the 17 participants tested, 15 had made both allocentric and sequential egocentric responses during the experiment. The remaining two subjects made sequential egocentric responses in all probe trials. After the fMRI session, participants were debriefed. We asked them what kind of information they had used to find the goal. Out of 17 participants, 16 reported having used landmarks for orientation and 14 spontaneously described the geographical location of at least two landmarks around the maze. The one remaining participant, who was always responding egocentrically, only reported having used the right-left-right sequence of turns. None reported the use of verbal elements in their strategy (SI Text).
Imaging Results.
Functional MRI data were fitted by a general linear model containing separate regressors for the first alley, the middle part, and the last alley separately for training trials, probe trials (from either departure point) in which a sequential egocentric response was made (“sequential egocentric probe trials”), and probe trials in which an allocentric response was made (“allocentric probe trials”). Control trials, which contained only two alleys, were modeled by two separate regressors. We focus on activations in the first navigated alley, as noted above. For all trial types, we compared activation during navigation of the first alley (when the path is identical for both types of response) to that in the first alley of the control trial.
Hippocampal activations.
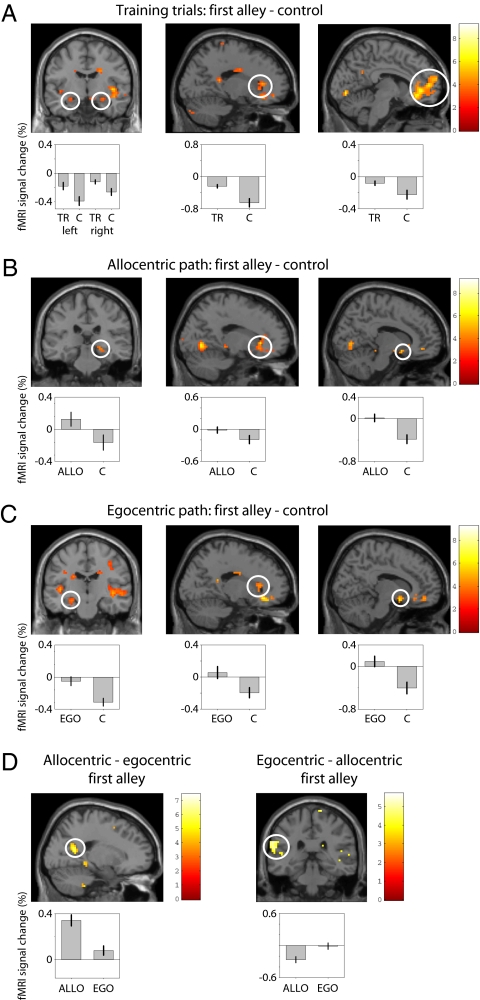
Training trials showed increased hippocampal activation bilaterally (right peak at MNI coordinates 30 −6 −15; left peak at −21 −15 −15) (Fig. 2A). For the probe trials, a lateralized hippocampal response was observed. Allocentric probe trials showed right hippocampal activation, peaked at 24 −24 −9 (Fig. 2B), whereas sequential egocentric probe trials showed left hippocampal activation (Fig. 2C) (peak: −21 −15 15). To examine the observed hippocampal lateralization in more detail, we extracted the fMRI data from 8-mm diameter regions of interest in left and right hippocampus centered on the peak activations for the sequential-egocentric and allocentric contrasts. A 2 × 2 ANOVA with factors laterality (left or right) and representation (sequential egocentric or allocentric) was performed on the average activation in the two regions of interest for sequential-egocentric—control and for allocentric—control contrasts. There were no main effects and a significant interaction (F = 4.4, P < 0.05), showing that the lateralization of hippocampal activity (i.e., difference in activation between left and right) predicts whether navigation is based on sequence or place memories (Fig. S2).
Fig. 2.
Functional MRI results. (A) Training trials. (Left) Bilateral hippocampal activation (Right peak: 30 −6 −15; Left peak: −2 −15 −15) during navigation of the first alley of training trials (TR), relative to control trials (C). (Center) Caudate nucleus activation (peak: 21 27 0). (Right) Medial prefrontal activation (peak: −3 42 0). (B) Probe trials showing allocentric responses (ALLO). (Left) Right hippocampal activation (24 −24 −9) in the first alley relative to control trials. (Center) Caudate nucleus activation (18 27 0). (Right) Ventral striatal activation (12 9 −12). (C) Probe trials showing sequential egocentric responses (EGO). (Left) Left hippocampal activation (−21 −15 −15) in the first alley relative to control trials. (Center) Right caudate activation (18 27 9). (Right) Ventral striatal activation (12 6 −15). (D) (Left) Parieto-occipital sulcus activation (18 −58 20) in the first alley of probe trials showing allocentric versus sequential egocentric responses. (Right) Posterior insula and left postcentral gyrus (−51 −19 29) activation in the first alley of probe trials showing sequential egocentric versus allocentric responses. For display purposes, hippocampal activations are thresholded at P < 0.005. Mean percentage BOLD signal change for each trial type is shown beneath activation plots.
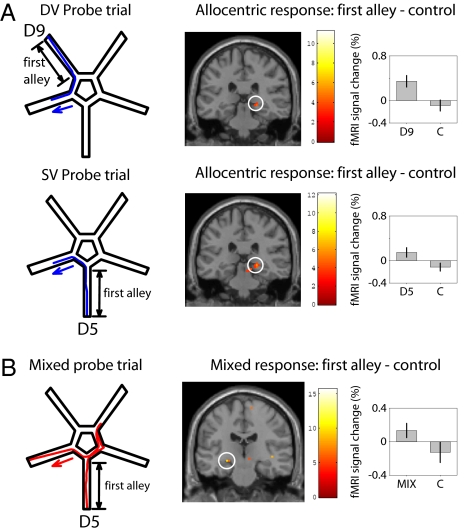
We also analyzed activation for similar responses separately for probe trials from the two departure points (DV and SV). Allocentric responses from either start alley correspond to right hippocampal activation (Fig. 3A). There were very few egocentric responses from the DV start alley, almost entirely produced by the two purely sequential-egocentric responders. However, these two participants do show significant left hippocampal activation in probe trials from the DV start alley. These analyses rule out a simple confound of start alley in explaining our lateralised hippocampal activations. Furthermore, for the first alley of the mixed trials versus control trials, we found left hippocampal activation (Fig. 3B), consistent with our interpretation of the use of a sequential egocentric representation during the first part of a mixed trial.
Fig. 3.
Further analyses of activation during probe trials. (A) Allocentric responses correspond to right hippocampal activation during the first alley of probe trials from either departure point (DV above, peak: 21 −30 −6; SV below, peak: 21 −30 −6). (B) Mixed responses (i.e., when participants start by expressing the sequential egocentric strategy but subsequently reorient using environmental cues to reach alley 7, Left) correspond to left hippocampal activation (peak: −24 −21 −9) during the first alley versus control trials.
Training trials also showed activation in the right dorsal striatum (peak: 18 27 0, extending into the right caudate nucleus), the right ventral striatum (peak: 12 9 −12) and in the anterior cingulate cortex (peak: 18 30 9, extending into the head of the caudate nucleus). See Fig. 2A and Table S2 for a full list of activations.
For both allocentric and egocentric probe trials, compared with control trials, there was activation in the right caudate nucleus (peak: 18 27 9) and nucleus accumbens (12 6 −15) (Fig. 2 B and C, Center and Right). Contrasting the first alley of allocentric versus egocentric probe trials (Fig. 2D, Left) showed an increased bilateral activation of the superior parieto-occipital sulcus (Right: 18 −54 15, Left: −15 −57 18), the right parahippocampus (24 −39 −6), and a subthreshold right hippocampal peak (24 −24 −6). Contrasting the first alley of egocentric versus allocentric probe trials (Fig. 2D, Right) revealed left-side lateralized activation of the parietal cortex (−51 −15 24), left posterior insula (−51 −33 6), superior medial frontal gyrus (−3 60 18), and a subthreshold left hippocampal peak (27 −15 −18). See Table S3 for a full list of activations.
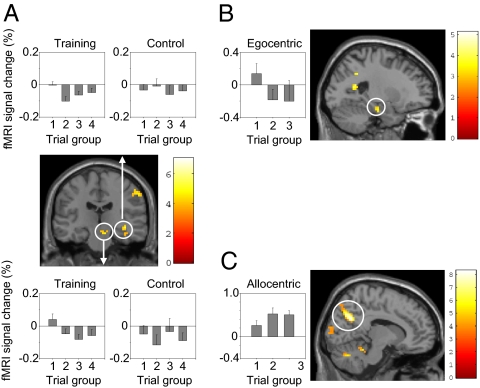
We also looked for regions showing decaying or increasing activation across the time-course of the experiment, using a separate model in which a regressor for the entire trials of each condition was parametrically modulated by an exponential function reflecting the position of that trial within the experiment [e.g., for trial i of n trials of a given type, the decreasing exponential parameter would be proportional to exp(− i/n)]. (Fig. 4 and Table S4). Both training trials and egocentric probe trials showed decreasing activation over the time-course of the experiment in the hippocampus. For training trials, decreasing activation was found in the right hippocampus (peak: 3 −12 −9) and also in the substantia nigra (9 −21 −18). Activation in both structures show higher values during the first quarter of the experiment than during the last three quarters (Fig. 4A). These decreasing activation patterns appeared to be related: the two time series (deconvolved with respect to the hrf) from the voxels in the hippocampus and the midbrain showing the maximal decrease were significantly correlated with each other in each participant (mean correlation, R = 0.312) (Table S5). For sequential egocentric probe trials, decreasing activation was found in the left hippocampus (−21 −15 −21), with higher activation in the first third of the experiment (Fig. 4B). Finally, allocentric probe trials showed increasing activation of the parieto-occipital sulcus (peak: 12 −63 30), with greater activation in the first third of the experiment (Fig. 4C).
Fig. 4.
Changes in activation over the duration of the experiment. (A) Exponentially decreasing activation during training trials in the right hippocampus (peak: 30 −12 −9; see Upper for signal change in training and control trials grouped into quartiles across the experiment) and substantia nigra (9 −21 −18; see Upper for signal-change plots). (B) Exponentially decreasing activation during egocentric probe trials in the left hippocampus (−21 −15 −15; see Left for signal change for egocentric probe trials grouped into tertiles across the experiment). (C) Exponentially increasing activation during allocentric probe trials in the parieto-occipital sulcus (12 −66 24; see Left for signal change for allocentric probe trials across the experiment).
Discussion
We investigated the neural bases of allocentric and sequential egocentric representations. Activation in the initial alley of a probe trial provided a well-controlled way to investigate the neural bases supporting these two types of representations, as revealed by the subsequent choice of destination alley. Our findings support the idea of lateralized hippocampal involvement during spatial navigation. The right hippocampus is involved in allocentric or map-based navigation, whereas the left hippocampus is involved in the sequential organization of successive choices. Both representations are active in parallel during the training phase of the task.
Striatal and Medial Prefrontal Activations.
The role of the dorsal striatum in simple stimulus-response (S-R) learning is well established (13, 26). Dorsal striatal activations (centered on the caudate) suggest that simpler S-R associations form part of both of the more cognitive strategies (allocentric and sequential egocentric), consistent with a striatal role in learning skilled responses and with an interaction between hippocampus and striatum in route recognition (27). The ventral striatal activation observed in probe trials and training trials is consistent with suggestions that this area acts in concert with the hippocampus in spatial memory consolidation and learning (28). The medial prefrontal activation seen in training, egocentric, and allocentric trials is consistent with its role as a coordinator between striatal and hippocampal systems, as suggested by studies in rodents (29) and humans (16).
Laterality and Representations in the Hippocampus.
We focused on the brain network activated during the first alley of the navigated path. The representation used to direct behavior (sequential egocentric or allocentric) is chosen during this first alley, before its expression in behavior (Fig. 1 B and C). Focusing on activations in the first alley also allows us to avoid potential confounds caused by differences in behavior, such as the specific turns and scenes viewed along the paths following the first choice-point. The lateralized hippocampal activations were not simply related to differences in the visual scene from the start alleys, as allocentric responses activated the right hippocampus during probe trials from either departure point (Fig. 3A). The interaction between laterality and representation shows that the difference between activity in left and right hippocampi in the start alley of the maze predicted the subsequent choice of path between those based on sequence or place memories (Fig. S2).
The left hippocampus has long been implicated in verbal tasks, such as the learning of narrative prose (30) or the learning and retrieval of word lists (31, see also ref. 5). In addition, successful encoding of verbal materials into episodic memory is associated with activation of the left medial temporal lobe (32). Could the activation associated with the sequential egocentric representation reflect a verbalized memory, such as “right turn-left turn-right turn”? During the debriefing, all our participants where asked if they had used a verbal strategy. The answers were negative, making explicit use of a verbal strategy unlikely. In addition, participants who used a spatial strategy to solve an eight-arm radial maze activated their hippocampus, whereas those who used a verbal strategy (i.e. counting the alleys) activated the caudate nucleus (17). Equally, left hippocampal activation is often associated with episodic memory for nonverbal stimuli (1, 33).
Could hippocampal lateralization reflect a more general hemispheric lateralization of perceptual processing? Our results are consistent with suggestions of lateralization of serial or local processing (on the left) versus parallel, global or holistic processing (on the right) (34, 35). However, the lateralized activations in our study are relatively specific to the hippocampus, and so are unlikely to simply follow from a more general hemispheric lateralization of function. As such, our results may be more closely related to findings of functional lateralization in the hippocampi of rodents (36) and birds (37). Overall, we suggest that involvement of the left human hippocampus in remembering narrative prose (30), learning novel sequences (38–40), and in supporting sequential egocentric representations in our study, could reflect a more general role in associative processing of sequential elements of an episode.
Relation to Episodic Memory.
The sequential egocentric representation defined in the Starmaze refers to a spatiotemporal association of different choice-points and requires a sequential organization of the information which may relate to findings that novel explicit (38–40) or implicit (39) sequence learning recruits the hippocampus. Left-lateralized hippocampal activation for sequential egocentric representations also supports the idea that the left hippocampus mediates spatiotemporal associations between the multiple events that constitute the elements of an episodic memory (2, 41). This finding is consistent with hippocampal involvement in sequential route-based navigation in rodents (14) and the planning of complex routes in human (15, 42).
The activity in the left or right hippocampus corresponding to the use of one or other representation, which predicts the participant's subsequent choice of route, may relate to two different characteristics of the firing of hippocampal place cells. Place-cell firing provides an allocentric representation of the animal's current location within its environment (5), but is also influenced by the past and future locations along the animal's current trajectory, as seen in the phase of firing relative to the theta rhythm (43, 44), modulations of firing rate (11), and sequential patterns of firing (12), which may comprise a sequential egocentric representation. Firing patterns of place cells predicting the animals’ trajectory have been shown in the start arm of a maze preceding the first choice-point (11), consistent with distinct hippocampal representations we report in the start alley. In addition, both place cells and cells encoding specific goal locations during navigation have been reported in humans (45). Equally, the right hippocampal representation of allocentric spatial location has long been argued to be an important component of the representation of context within episodic memory (1, 3–5, 9). This representation is also consistent with studies implicating the hippocampus [often specifically on the right (10, 41)] in flexible representation of environmental topography (3, 46), accurate large-scale navigation (41), and memory for arrays of locations when containing large numbers of objects (10, 19) or tested from a shifted viewpoint (19).
Our finding of lateralized hippocampal activation corresponding to place or sequence representations provides unique evidence for distinct roles of the two hippocampi within the same subject. These results imply that, rather than supporting a single representation common to both navigation and episodic memory, the left and right hippocampi supply complementary representations that can be combined to support different aspects of navigation and episodic memory.
Parallel Learning of Both Representations.
The Starmaze task gives the possibility to assess the free choice of two spatial navigation representations: an allocentric and a sequential egocentric representation (21). The probe trials indicated that subjects use both allocentric and sequential egocentric representations throughout the task, which supports the idea of coexisting strategies supported by multiple parallel memory systems (5, 21, 26, 47–49). Correspondingly, the pattern of activation in the training trials (bilateral hippocampus, medial prefrontal, and caudate) overlaps with both the allocentric and the sequential egocentric activations during probe trials. Activations that distinguished allocentric from sequential egocentric strategies included the bilateral parieto-occipital sulcus: a region previously shown to be related to allocentric spatial processing (22, 25, 50), retrieval of spatial memories into imagery (24), and navigation (15, 51). The reverse pattern (sequential egocentric vs. allocentric strategies) shows activations of the posterior insula, which corresponds to the human analog of the primate vestibular cortex (52) and posterior parietal operculum, which might be related to movement processing (53).
Activations Reflecting Learning, Novelty, and Imagery.
The right hippocampus and the midbrain showed a time-dependent decrease of activity during learning trials, consistent with previous studies showing an important role of both of these regions in novelty detection (54), and with the involvement of the hippocampus in spatial-change detection (16) and learning (22). Furthermore, in agreement with a recent report (55), the time-courses of hippocampal and midbrain activation were highly correlated throughout the experiment. The left hippocampal activity during egocentric probe trials also decreased over successive trials, again consistent with reducing hippocampal involvement as sequential tasks become familiar (39) and learning reduces (22). These findings may relate to previous fMRI studies of novelty, in that left hippocampal activation is associated with sequential novelty (40) and right hippocampal activation is associated with spatial novelty (16).
In sum, our results show that the human hippocampus separates two distinct memory representations already a few seconds before they are expressed in behavioral decisions. We provide identification of one of these, the sequential egocentric representation, in the human hippocampus. Furthermore, the lateralized involvement of the hippocampus in representing both place and sequence memories suggest that the two hippocampi provide complementary representations for navigation, both of which likely contribute to different aspects of episodic memory.
Methods
Virtual Reality Design.
The virtual reality design (SI Text) comprised five central alleys forming a pentagon and five alleys radiating from the angles of the central pentagon (Fig. 1A). Participants used a keypad to move their viewpoint forward or backward or to turn left or right; they could move around and perform rotations freely in all of the alleys. Distant environmental cues surround the maze for orientation. These cues were placed between the ends of adjacent alleys and every cue was present twice around the maze, so that solving the task requires knowledge of the spatial configuration of cues rather than a guidance strategy based on a single cue. Participants were told to find a goal that would always be at the same place in the environment. The goal had no visible identifiers but, when it was reached, fireworks went off to indicate the successful end of the trial (feedback). Participants knew that the environment would not change during the experiment and that some trials would be terminated before feedback occurred. The experiment consisted of interleaved training trials, probe trials, and control trials (see Table S1 for complete trial order and SI Text for details). In training trials, successful navigation might be supported by either type of representation: sequential egocentric (sequence of body-turns) or allocentric (location relative to environmental cues). In probe trials, which were not distinguished from training trials in the instructions, participants had to find the goal from one of two different departure points (DV and SV), which allow dissociation of the use of either type of representation according to the path chosen (Fig. 1 B and C). Use of either representation in a probe trial was considered correct, so that a probe trial ended once the participant had made a response consistent with either an allocentric or a sequential egocentric representation. To avoid positive reinforcement (or absence of expected reinforcement) of one strategy or another during probe trials (from a new starting position), which would produce a bias toward subsequent expression of that strategy, probe trials ended before the point at which reinforcement would be delivered. To get subjects used to these prematurely ending trials, and not to alert them to the difference between probe and training trials, some of the training trials (25%) and control trials (50%) also ended early (Fig. 1 A and D, Center).
We focused on activations before the first choice-point of the maze, so that activation patterns could be analyzed according to the strategy of the participant on that run, as determined by their subsequent choices, but without any of the differences in behavior or stimuli which might result from making those choices (which lead to different paths).
Participants and Analysis of Behavioral Data.
Nineteen male participants (aged 19–38, mean age 24.3) gave written consent and were paid for participating as approved by the UCLH Research Ethics Committee. All were right-handed, with normal or corrected-to-normal vision, and reported to be in good health with no history of neurological disease. Two participants were excluded from scanning or further analysis because of failure to understand the task instructions. Every 200 ms, the exact position of the participant and his moving direction was registered in a Cartesian coordinate system. These records were analyzed to obtain for each trial the number of visited alleys and the distance error, measuring the accuracy of the path (Fig. S1).
Acquisition and Analysis of fMRI Time Series.
Functional MRI time series were modeled using two separate general linear models. The first model included separate regressors for the start alley, middle part, and last alley of every type of trial (i.e., training trials, egocentric responses for probe trials and allocentric responses for probe trials). Two additional regressors modeled the first and second alleys of the control trials, which corresponds to 11 regressors [i.e., 3 × 3 (training egocentric and allocentric trials) and 2 (control trials)].The model also included regressors based on estimates of head movement obtained from the realignment procedure. At the single-subject level, we contrasted the first alley of training, egocentric responses, and allocentric responses versus first alley of control trials. Furthermore, we calculated the contrast between first alley of egocentric versus allocentric responses. The corresponding contrast images were then entered into second level analyses. In a second model we investigated modulation of brain activity over the time-course of the experiment. This model included the training trials, egocentric responses for probe trials, and allocentric responses for probe trials (collapsed across the different within-trial phases as time effects across trials are more likely reflected in the whole trial than only in alley one) and further parametric regressors for all trial types. The parametric regressors modeled the time-course of the experiment, through an exponential function reflecting the stage of the task [i.e., for the training trial i out of 48 trials the exponential parameter is: exp(−i/48); similarly, for the egocentric response j out of 8 the exponential parameter is exp(−j/8)]. We entered into second level analyses the effects of the parametric modulation on training trials, egocentric responses, and allocentric responses. We also investigated correlation between activation in the right hippocampus and the midbrain using a Spearman correlation analysis for each subject on the deconvolved timeseries of the peak activation voxel defined by the exponential model in the two regions (Table S5). See SI Text for further details.
Supplementary Material
Acknowledgments
We thank Eric Deléglise for help in programming the virtual environment and the Wellcome Trust Centre for Neuroimaging at University College London for providing help and scanning facilities. This work was supported by European Union New and Emerging Science and Technology Fp6Grant 12959 – (Wayfinding), the Medical Research Council, United Kingdom, the Fondation pour la Recherche Médicale (DLC 20060206428-FRM), France, and National Agency for Research Young Researcher Program, France, 07-JCJC-0108-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004243107/-/DCSupplemental.
References
- 1.Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 2.Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaffan D. What is a memory system? Horel's critique revisited. Behav Brain Res. 2001;127:5–11. doi: 10.1016/s0166-4328(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 6.Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- 7.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Morris RG. Episodic-like memory in animals: Psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:1453–1465. doi: 10.1098/rstb.2001.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- 11.Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J Neurosci. 2007;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 14.Rondi-Reig L, et al. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci. 2006;26:4071–4081. doi: 10.1523/JNEUROSCI.3408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 16.Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrey S, et al. Distinct visual perspective-taking strategies involve the left and right medial temporal lobe structures differently. Brain. 2008;131:523–534. doi: 10.1093/brain/awm317. [DOI] [PubMed] [Google Scholar]
- 19.King JA, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 2002;12:811–820. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- 20.Burguière E, et al. Spatial navigation impairment in mice lacking cerebellar LTD: A motor adaptation deficit? Nat Neurosci. 2005;8:1292–1294. doi: 10.1038/nn1532. [DOI] [PubMed] [Google Scholar]
- 21.Igloi K, Zaoui M, Berthoz A, Rondi-Reig L. Sequential egocentric strategy is acquired as early as allocentric strategy: Parallel acquisition of these two navigation strategies. Hippocampus. 2009;19:1199–1211. doi: 10.1002/hipo.20595. [DOI] [PubMed] [Google Scholar]
- 22.Wolbers T, Büchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess N. Spatial cognition and the brain. Ann N Y Acad Sci. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- 24.Burgess N, Becker S, King JA, O'Keefe J. Memory for events and their spatial context: Models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ino T, et al. Mental navigation in humans is processed in the anterior bank of the parieto-occipital sulcus. Neurosci Lett. 2002;322:182–186. doi: 10.1016/s0304-3940(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 26.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 27.Voermans NC, et al. Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28:6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 30.Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28:349–359. doi: 10.1016/0028-3932(90)90061-r. [DOI] [PubMed] [Google Scholar]
- 31.Milner B, Kimura T. Dissociable visual learning defects after unilateral temporal lobectomy in man. 35th Annual Meeting of the Eastern Psychological Association. 1964 [Google Scholar]
- 32.Wagner AD, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 33.Stern CE, et al. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jager G, Postma A. On the hemispheric specialization for categorical and coordinate spatial relations: A review of the current evidence. Neuropsychologia. 2003;41:504–515. doi: 10.1016/s0028-3932(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 35.Kosslyn SM, Thompson WL, Gitelman DR, Alpert NM. Neural systems that encode categorical versus coordinate spatial relations representations: PET investigations. Psychobiology. 1998;26:333–347. [Google Scholar]
- 36.Klur S, et al. Hippocampal-dependent spatial memory functions might be lateralized in rats: An approach combining gene expression profiling and reversible inactivation. Hippocampus. 2009;19:800–816. doi: 10.1002/hipo.20562. [DOI] [PubMed] [Google Scholar]
- 37.Bingman VP, Gagliardo A. Of birds and men: Convergent evolution in hippocampal lateralization and spatial cognition. Cortex. 2006;42:99–100. doi: 10.1016/s0010-9452(08)70329-0. [DOI] [PubMed] [Google Scholar]
- 38.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 40.Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiers HJ, et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- 42.Ghaem O, et al. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 1997;8:739–744. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- 43.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 44.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 45.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 46.Hartley T, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection. New York: Oxford University Press; 2001. [Google Scholar]
- 48.Devan BD, White NM. Parallel information processing in the dorsal striatum: Relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iaria G, Chen JK, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: Complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25:890–899. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- 51.Maguire EA, et al. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 52.Gunny R, Yousry TA. Imaging anatomy of the vestibular and visual systems. Curr Opin Neurol. 2007;20:3–11. doi: 10.1097/WCO.0b013e328013f458. [DOI] [PubMed] [Google Scholar]
- 53.Eickhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K. Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp. 2006;27:611–621. doi: 10.1002/hbm.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 55.Shohamy D, Wagner AD. Integrating memories in the human brain: Hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.