Abstract
T helper type 17 (TH17) cells are highly proinflammatory effector T cells that are characterized by the production of high amounts of IL-17A, IL-17F, IL-21, and IL-22. Furthermore, TH17 cells have been associated with a number of autoimmune diseases. However, it is not clear whether TH17 cells can also serve as effective helper cells. Here we show that TH17 cells can function as B-cell helpers in that they not only induce a strong proliferative response of B cells in vitro but also trigger antibody production with class switch recombination in vivo. Transfer of TH17 cells into WT or T-cell receptor α–deficient mice, which lack endogenous T cells, induces a pronounced antibody response with preferential isotype class switching to IgG1, IgG2a, IgG2b, and IgG3, as well as the formation of germinal centers. Conversely, blockade of IL-17 signaling results in a significant reduction in both number and size of germinal centers. Whereas IL-21 is known to help B cells, IL-17 on its own drives B cells to undergo preferential isotype class switching to IgG2a and IgG3 subtypes. These observations provide insights into the unappreciated role of TH17 cells and their signature cytokines in mediating B-cell differentiation and class switch recombination.
Keywords: germinal center, IL-17, immunoglobulin, isotype class switching, T helper cell
During the course of infections, professional antigen presenting cells (APCs) such as dendritic cells (DCs) pick up antigen and migrate to the draining lymph nodes, where they induce clonal expansion and differentiation of naïve T cells. Thus far, three different effector T helper subsets have been identified: T helper type 1 (TH1), TH2, and TH17 (1). Each subset is distinguished on the basis of their unique expression pattern of cytokines and transcription factors. TH1 cells are characterized by the production of IFN-γ, whereas TH2 cells produce IL-4, IL-5, and IL-13. TH17 cells, on the other hand, produce IL-21 and IL-22, as well as their signature cytokines IL-17A and IL-17F (2). In addition to these effector T-cell subsets, a specialized T helper cell subset, called follicular B helper T cells (TFH), has been identified, which plays an important role in helping B cells to induce germinal centers (GCs) and isotype class switching (3). TFH express C-X-C chemokine receptor type 5 (CXCR5) and the induced costimulatory molecule ICOS on the surface and secrete IL-21 (3). Expression of the chemokine receptor CXCR5 enables TFH to migrate into CXCL13-rich areas of B-cell follicles and GCs, where they stimulate activated B cells to become long-lived antibody-secreting plasma cells or memory B cells. Recent studies suggested that TFH are an independent T-cell lineage because none of the known transcription factors required for TH1, TH2, TH17, or Treg cells is needed for their development (4, 5). In addition, Bcl-6 was identified as one of the key transcription factors required for their generation (6–8). TFH predominantly produce IL-21, which is known to promote isotype switching to IgG1. However, IL-21 cannot account for the whole spectrum of antibody classes produced in response to various pathogens (3). Therefore, it was postulated that specific cytokines produced by other effector T helper subsets might also play a role in T–B collaboration. TH1 and TH2 cells are known to provide B-cell help, and their signature cytokines IFN-γ and IL-4 induce class switch recombination to IgG2a or IgG1 and IgE, respectively (9, 10). Using IFN-γ and IL-4 reporter mice, Reinhardt et al. (11) demonstrated that B cells form conjugates with antigen-activated, cytokine-producing effector T cells and that the profile of cytokine production defines isotype class switching in conjugated B cells. However, it is not clear whether TH17 cells can provide help to B cells and if so whether they induce class switch recombination to a specific antibody subtype.
TH17 cells are highly proinflammatory T cells that are involved in the clearance of extracellular bacteria and fungi (2). In addition, they also induce chronic inflammatory and autoimmune diseases (2). A recent study in lupus- and arthritis-prone BXD2 mice suggested that IL-17 had a role in the development of systemic autoimmunity in this mouse model (12). Furthermore, it was shown that the combination of IL-17 plus B cell-activating factor protected B cells from apoptosis and promoted their proliferation and differentiation into plasma cells (13). In this context, it is of particular interest that TH17 cells and TFH share several features, including the expression of ICOS, IL-21, and the transcription factor cmaf (14). These shared properties of TH17 cells and TFH prompted us to study the potential of TH17 cells to help B cells. Here, we show that TH17 cells not only trigger B-cell proliferation but also promote the formation of GCs together with isotype switching to IgG1, IgG2a, IgG2b, and IgG3. Interestingly, IL-17 on its own drove class switch recombination to IgG2a and IgG3, whereas IL-21 in addition promoted the switch to IgG2b and IgG1. Thus, the cytokines produced by TH17 cells seem to induce effective B-cell differentiation that is distinct from help elicited by TH1 and TH2 subtypes.
Results and Discussion
TH17 Cells Induce B-Cell Proliferation and Isotype Class Switching in Vitro.
TH17 cells have so far been considered as proinflammatory T cells driving autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and psoriasis, but their potential to affect the fate of B cells has not been addressed. First, we wanted to evaluate the effect of TH17 cells on the proliferation of B cells. Therefore, we differentiated CD4+ T cells from myelin oligodendrocyte glycoprotein (MOG)-specific T-cell receptor (TCR) transgenic mice (2D2) into TH0 or TH17 cells and subsequently cocultured them with purified, carboxyfluorescein succinimidyl ester (CFSE)-labeled CD19+ B cells and MOG35-55 peptide. TH0 cells produced small amounts of IFN-γ and IL-10 and very little IL-4 and IL-17, whereas TH17 cells predominantly produced IL-17 (Fig. S1A). Three days later, overall survival of cultured cells and B-cell proliferation was assessed by flow cytometry. Coculture of TH17 cells with B cells exhibited a significantly higher percentage of live cells compared with cultures with B cells alone or coculture of TH0 cells with B cells (Fig. 1A). Analysis of CFSE dilution revealed an increased proliferation of B cells in the presence of TH17 cells compared with B cells cultured with TH0 cells (93.8% dividing B cells in the TH17 condition vs. 70.6% in the TH0 condition) (Fig. 1A). It is well known that TH17 cells produce large amounts of their signature cytokine IL-17 but also feature the production of IL-21 and IL-22. So far, IL-17 has mainly been described as a potent proinflammatory cytokine that acts on fibroblasts, stromal, epithelial, and endothelial cells, and some monocytes to stimulate the secretion of other proinflammatory mediators, such as IL-1, IL-6, chemokine ligand CXCL8 (also known as IL-8), CXCL1, TNF-α, and granulocyte colony-stimulating factor (15, 16). IL-17RA, which is necessary for signal transduction of IL-17, is expressed ubiquitously, with particularly high levels in hematopoietic tissues (16, 17). This expression pattern is interesting, because the main responses to IL-17A were suggested to occur in epithelial cells, endothelial cells, and fibroblasts, although macrophages and DCs are also responsive to IL-17A (18). Only a limited number of IL-17–induced genes have been documented in lymphocytes, and these genes are distinct from those induced by IL-17 in other cell types (12, 17). We could show here that B cells cultured in the presence of differentiated TH17 cells and antigen displayed a markedly increased proliferation. However, it has recently been reported that IL-17 on its own does not induce B-cell proliferation (12, 13). By contrast, IL-21 is known to be critically involved in different aspects of B-cell activation and differentiation (19–23). In particular, it has been shown that IL-21 induces the expansion of B cells and the generation of plasma cells during the collaboration of CD4+ T cells and B cells (24). Therefore, it is tempting to speculate that the observed increase of B-cell proliferation in the presence of TH17 cells is due to the abundant production of IL-21 by TH17 cells.
Fig. 1.
TH17 cells induce B-cell proliferation and isotype class switching in vitro. (A) TH0 or TH17 cells from 2D2 MOG35-55-specific TCR transgenic mice were cocultured with purified, CFSE-labeled CD19+ B cells in the presence of MOG35-55 (2 μg/mL). Overall survival of cultured cells was determined 3 d later by flow cytometry on the basis of their location in the forward and side scatter (Upper). Numbers in the lower right corners represent percentage of cells located within the live gate. Proliferation of B cells (CD19+CD4−7AAD−) was assessed determining CFSE dilution on day 3 (Lower). Numbers represent percentage of divided B cells. (B) Mitomycin C–treated TH0 or TH17 cells were cocultured with isolated B cells at various ratios in the presence of MOG35-55 (2 μg/mL). All conditions were plated in triplicate. On day 10 supernatant was analyzed for antibody production and Ig class switching. Graphs show mean concentration (in nanograms per milliliter) ± SEM. *P < 0.05; **P < 0.01.
The observation that TH17 cells stimulate proliferation of B cells prompted us to determine whether TH17 cells are also able to influence other aspects of B-cell biology. Therefore, we cocultured purified CD19+ B cells with either TH0 or TH17 cells in various ratios and in the presence of MOG35-55 peptide. After 10 d, the supernatant of these cultures was analyzed for isotype class switching by Ig ELISA. Interestingly, TH17 cells significantly increased class switch recombination to IgG1, IgG2a, IgG2b, and IgG3 when compared with TH0 cells (Fig. 1B). It is well known that antigen-specific T cells provide soluble and cognate help to B cells for the development of T cell–dependent humoral immune responses. Previous studies have reported that both TH1 and TH2 cells can provide efficient help to B cells, and their signature cytokines IFN-γ and IL-4 promote isotype switching to IgG2a or IgG1 and IgE, respectively (9). In vitro TH17 cells act as excellent helper cells, mediating B-cell proliferation and Ig class switching to IgG1, IgG2a, IgG2b, and IgG3 (Fig. 1B), an isotype pattern distinct from TH1 and TH2 cells. However, because IgM levels were also increased in the presence of TH17 cells, we could not exclude in this in vitro system that increased proliferation or survival of B cells contributed to these effects.
TH17 Cells Help B Cells to Class Switch in Vivo.
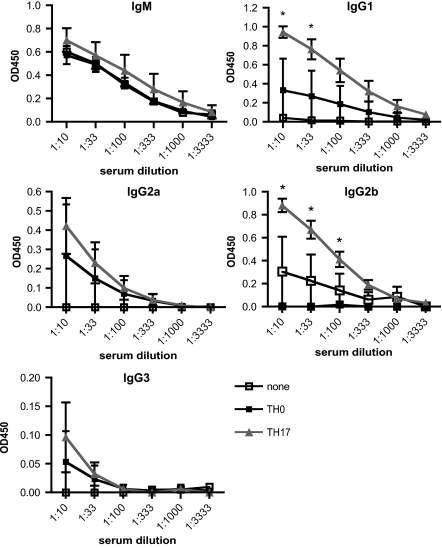
On the basis of these in vitro data, we wanted to determine whether TH17 cells can also provide help to B cells in vivo and mediate B-cell differentiation with class switch recombination. Therefore, we transferred MOG-specific TH17 cells from 2D2 mice into C57BL/6 WT mice, followed by immunization with recombinant MOG (rMOG) protein. T cells stimulated under neutral conditions (TH0) were used as a control. TH17 cells promoted a significantly stronger MOG-specific antibody response compared with TH0 cells or when no cells were transferred (Fig. 2). In some experiments, TH1 or TH2 cells were used as positive controls. Interestingly, the predominant antibody isotype produced differed depending on the cell type transferred. Previous reports have shown that TH1 cells drive a dominant IgG2a response and to a lesser but significant extent isotype switching to IgG1 and IgG2b (25), whereas TH2 cells induce Ig isotype switching to IgG1 (9). In contrast, when TH17 cells were transferred, we observed high levels of IgG1 and IgG2b and a modest increase in the levels of IgG2a and IgG3. The profile of Ig induced by TH17 cell transfer was surprising and somehow reminiscent of the profile observed with TH1 cells. However, whereas TH1 cells induced the strongest IgG2a response compared with all other groups, the IgG1, IgG2b, and IgG3 response was more pronounced in TH17 recipients than in mice that received TH1 cells. To confirm that TH17 cells had maintained their phenotype in vivo, we recovered transferred T cells from the draining lymph nodes based on TCR Vα3.2 expression and analyzed them for cytokine production by intracellular staining. Transferred T cells showed a stable production of IL-17 with little IFN-γ (Fig. S1B). Interestingly, transfer of TH17 cells into IL-17RA KO mice resulted in a significant decrease in the production of IgG2b and a slight decrease of IgG2a, whereas levels of IgG1 and IgG3 were unaltered (Fig. S2).
Fig. 2.
TH17 cells transferred into WT mice help B cells to class switch in vivo. TH0 or TH17 cells from 2D2 MOG35-55-specific TCR transgenic mice were transferred i.v. into WT B6 mice. At the same time mice were immunized with 50 μg rMOG emulsified in incomplete Freund's adjuvant (IFA) s.c. in both flanks. Serum was obtained on day 7 after transfer/immunization, and MOG-specific antibody response and isotype switch was determined. Serum was applied in serial dilutions starting at 1:10. Each group represents three to five mice, and graphs show mean OD (at 450 nm) ± SEM. *P < 0.05, TH17 recipients vs. TH0.
TH17 Cells Induce GC Formation.
Production of antigen-specific, isotype-switched antibodies clearly indicated that TH17 cells are able to help B cells after antigen challenge. However, it is unclear whether the interaction between TFH and B cells on the one hand and TH1, TH2, or TH17 and B cells on the other hand occur at the same place and have the same character. Odegard et al. (26) suggested the existence of an extrafollicular T helper cell activity that might be particularly relevant for controlling self-reactive IgG production and that is associated with CXCR4 expression and the loss of P-selectin ligand 1 binding on these T helper cells. To shed more light on the TH17-driven B-cell response, we performed immunofluorescence stainings in draining lymph nodes and spleen of TH0 and TH17 recipients to determine the extent of GC formation. GCs were identified by double staining for GL-7, a marker of GC B cells (27), and B220. Interestingly, we observed a strong induction of GCs in TH17 recipients in the draining lymph nodes and spleen 7 d after immunization/transfer (Fig. 3 A and B). Mice that received no T cells exhibited only very small GCs, whereas TH0 recipients featured an intermediate pattern with rather small but clearly defined GCs. Quantification of GCs in the lymph nodes and spleens of the recipient mice revealed that TH17 recipients formed a substantially higher number of GCs than control mice that received either TH0 cells or no T cells (Fig. 3B). Analysis of pixels per lymph node, representing the area of GL-7–positive cells and therefore the size of the GC, showed even more obvious differences, with TH17 recipients exhibiting the biggest GCs both in draining lymph nodes and the spleen (Fig. 3B). TH17 cells produce a number of cytokines, such as IL-17A, IL-17F, IL-21, and IL-22, and their signature cytokine IL-17A has been predominantly associated with the induction of tissue inflammation. Interestingly, transfer of TH17 cells into IL-17RA–deficient mice resulted in a reduction of both number and size of GCs (Fig. 3 A and B) resembling the “no T-cell transfer” group. These data are in line with the observation that BXD2 mice, which show high levels of IL-17, develop spontaneous GCs in the spleen (12). Similarly, BXD2 mice lacking IL-17RA revealed smaller GCs and inhibition of systemic autoimmunity. IL-21, which is also produced by TH17 cells, has previously been associated with the formation of GCs. To address the role of IL-21 in our system we transferred TH17 cells into IL-21R KO recipients. Interestingly, IL-21R–deficient mice showed a complete absence of GCs both in the draining lymph nodes and spleen, which was paralleled by a severe defect in their humoral immune response, with complete lack of isotype switching (Fig. S2). Our observation in IL-21R KO mice is consistent with recent reports that IL-21 regulates GC responses in a B cell–intrinsic manner (28, 29). These findings complement earlier studies, which suggested that IL-21 influenced B-cell responses mainly in an indirect manner via the generation of follicular T helper cells (4, 5).
Fig. 3.
TH17 cells transferred into WT mice induce GC formation. TH0 or TH17 cells from 2D2 MOG35-55-specific TCR transgenic mice were transferred i.v. into WT or IL-17RA–deficient B6 mice. At the same time mice were immunized with 50 μg rMOG emulsified in IFA s.c. in both flanks. On day 7, formation of GCs was analyzed by staining of lymph node sections for GL-7-FITC and B220-PE. Transferred T cells were detected with a Vα3.2 antibody. (A) Lymph node sections were analyzed with a ×10 objective (Upper) and ×20 objective (Lower). (B) The number of GCs was calculated by evaluation of two sections of two to four draining lymph nodes or the spleen of each mouse. Size of GCs was analyzed by determining total pixels of the GL-7–positive area per two sections of draining lymph nodes or spleen using ImageJ software. Graphs represent mean ± SEM. Each group consisted of three to five mice. *P < 0.05; **P < 0.01.
To analyze the spatial relationship between transferred T cells and host B cells, we stained for TCR Vα3.2 in addition to B220 and GL-7. Most of the transferred TH17 could be detected in the T cell–rich zone. However, our data clearly show that some T cells also infiltrated the B-cell follicles, allowing a close interaction with antigen-specific GC B cells (Fig. 3A). Our data complement earlier studies demonstrating the potential of both TH1 and TH2 cells to migrate to the B-cell follicles and support B-cell responses (25, 30). However, there is currently much controversy about how T cells that provide help to B cells develop and whether they are phenotypically different from effector T cells.
TH17 Cells Are Sufficient for Isotype Class Switch in TCRα KO Mice.
Transfer of TH17 cells into WT mice followed by immunization with antigen showed that TH17 cells provoke a pronounced B-cell response with class switching. However, transferred TH17 cells could act in an indirect manner, mediating the differentiation of endogenous T cells into TFH cells via their production of IL-21. To address this possibility, we transferred differentiated T cells from 2D2 mice into TCRα chain KO mice, which lack endogenous T cells, followed by immunization with rMOG. One week later, we collected sera and harvested draining lymph nodes and spleens from these mice for further analysis. Interestingly, transfer of TH17 cells was sufficient to induce a strong B-cell response with class switching to IgG1, IgG2a, IgG2b, and IgG3 (Fig. 4). Mice that received TH0 cells or no T cells could not mount a sufficient immune response and only produced MOG-specific IgM antibodies but no other antibody subclasses (Fig. 4). T cells recovered from TH17 recipients showed a stable phenotype with robust IL-17 production and no significant IFN-γ production (Fig. S3A).
Fig. 4.
TH17 cells are sufficient to induce class switching in TCRα KO mice. TH0 or TH17 cells from 2D2 MOG35-55-specific TCR transgenic mice were transferred i.v. into TCRα KO B6 mice. At the same time mice were immunized with 50 μg rMOG emulsified in IFA s.c. in the flanks. Serum was obtained on day 7 after transfer/immunization, and MOG-specific antibody response and isotype switch was determined using a MOG-specific Ig ELISA. Serum was applied in serial dilutions starting at 1:10. Each group represents three to five mice, and graphs show mean OD (at 450 nm) ± SEM. *P < 0.05; **P < 0.01, TH17 condition vs. TH0.
Immunofluorescence staining revealed the induction of small GCs in the draining lymph nodes and spleens of TH17 recipient TCRα chain-deficient mice, whereas control mice that received either no T cells or TH0 cells showed complete absence of GCs (Fig. S3B). Transferred TH17 cells were predominantly located within the T-cell zone but could also be found in B-cell follicles in close relationship with GCs, as we had observed in WT mice. Our observations in TCRα chain KO mice that lack endogenous T cells and thus do not have any TFH cells clearly demonstrated that TH17 cells themselves are sufficient to induce a strong antigen-specific antibody response. Recovery of transferred cells from recipient mice further confirmed that these cells retained their phenotype with preferential production of IL-17 and no significant production of IL-4 or IFN-γ. However, it is known that effector T cells can dedifferentiate to some extent and acquire functions usually associated with another helper T-cell subset (31–33).
IL-17 Induces Ig Class Switching.
TH17 cells produce a wide range of cytokines, including IL-17A, IL-17F, IL-21, and IL-22. Among these, only IL-21 has been shown to help B cells and induce Ig class switching (22–24). However, our observation that IL-17RA–deficient mice develop smaller and fewer GCs after adoptive transfer of TH17 cells suggests that IL-17 itself may also be crucial in class switching and GC formation. To address this question, we purified B cells from WT B6 mice, stimulated them with anti-CD40 and anti-IgM, and added IL-17 in various concentrations. In parallel, we stimulated B cells in the presence of IL-21 to compare the ability of IL-17 vs. IL-21 to induce Ig class switching. After 10 d of culture, we analyzed the supernatant by Ig ELISA. IL-21 induced class switching to IgG1, IgG2a, IgG2b, and IgG3 (Fig. 5). In contrast, IL-17 triggered strong class switching to IgG2a and IgG3, very low class switch recombination to IgG2b, and none to IgG1 (Fig. 5). Therefore, only B cells cultured in the presence of IL-21 showed a strong induction of IgG1 and IgG2b, whereas IL-17 had no effect on class switching to IgG1 and little to IgG2b (Fig. 5). Therefore, the observed class switch to IgG1 and IgG2b in vivo is most likely induced by IL-21, whereas both IL-17 and IL-21 could mediate class switch to IgG2a and IgG3.
Fig. 5.
IL-17 induces class switching in vitro. B cells were purified and activated in the presence of increasing doses of the indicated cytokines. Antibody production and class switch recombination were analyzed in response to stimulation with anti-CD40 (5 μg/mL)/IgM (5 μg/mL) plus IL-17 (0.1/1/10 ng/mL) or IL-21 (10/25/50 ng/mL) or no cytokine as a control. Columns for each cytokine represent increasing concentrations from left to right. Graphs show mean concentration (in nanograms per milliliter) ± SEM, representing 10 cultures per condition. *P < 0.05; **P < 0.01, IL-17 or IL-21 vs. no cytokine.
Taken together, our data suggest that TH17 cells act as excellent B-cell helpers, mediating formation of GCs and isotype class switching. Therefore, we suggest that IL-17 plays an important role in the generation of a humoral immune response because IL-17 on its own drives GC formation and class switch recombination. Therefore, inhibition of TH17 cells or their signature cytokine IL-17 may be an important target not only in organ-specific but also in systemic autoimmune diseases.
Materials and Methods
Mice, Immunization, and Adoptive Transfer.
C57BL/6 WT and TCRα chain KO mice were purchased from Jackson Laboratories. Experiments using IL-17RA KO mice were done in collaboration with Jay K. Kolls (Louisiana State University Health Sciences Center, New Orleans, LA). Animals were kept in a conventional, specific pathogen-free facility at the Harvard Institutes of Medicine, and all experiments were performed in accordance with the guidelines prescribed by the Standing Committee on Animals at Harvard Medical School.
Mice were immunized s.c. with 50 μg recombinant rat MOG emulsified in incomplete Freund's adjuvant oil. Furthermore, mice received 5 × 106 differentiated T cells i.v. at the time of immunization. On day 7 after immunization/transfer mice were killed. Blood was drawn by cardiac puncture and draining lymph nodes and spleen were either frozen in optimum cutting temperature medium for immunohistological analysis (Sakura Finetek) or were further processed into single-cell suspensions and subjected to flow cytometric analysis.
Antigen.
A plasmid construct encoding the extracellular domain of rat MOG protein (MOG amino acids 1–125) was generously provided by C. Linington (University of Aberdeen, Aberdeen, UK), and rMOG protein was purified from inclusion bodies as described previously (34).
In Vitro T-Cell Differentiation.
CD4+ T cells were purified from spleen and lymph node cells of MOG TCR transgenic (2D2) mice (35) using anti-CD4 beads (Miltenyi Biotech) and further sorted into naïve CD4+CD62Lhi T cells. Subsequently, naïve CD4+ T cells were stimulated with irradiated, CD4-depleted spleen and lymph node cells of 2D2 mice and 2.5 μg/mL anti-CD3 (145-2C11) for 4 d in the presence of cytokines (all R&D Systems) and neutralizing antibodies to induce T-cell differentiation. For TH1 10 ng/mL IL-12 plus 20 μg/mL anti-IL-4, for TH2 10 ng/mL IL-4 plus anti-IL-12 (20 μg/mL), and for TH17 2.5 ng/mL TGF-β, 25 ng/mL IL-6, 20 μg/mL anti-IL-4, and 20 μg/mL anti-IFN-γ (XMG1.2) were added into the culture. Cells were supplemented with recombinant IL-2 (10 ng/mL; TH0, TH1, and TH2) or IL-23 (10 ng/mL; TH17) on day 2.
B-Cell Proliferation/CFSE Labeling.
CD19+ B cells were purified from the spleen of WT C57BL/6 mice and subsequently labeled with CFSE (Molecular Probes). Cells (30 × 106) were resuspended in prewarmed PBS + 0.1% BSA, and CFSE was added for a final concentration of 5 μM. After 3 min of incubation at 37 °C, 5 mL of ice-cold clone medium was added, and cells were incubated for another 5 min on ice. Subsequently, cells were washed three times and then cocultured with differentiated 2D2 T cells plus MOG35-55 (2 μg/mL). On day 3, cells were stained for CD4 and CD19. Furthermore, 7-Amino-actinomycin D 7AAD was included as a marker for dead cells.
ELISA.
For the detection of MOG-specific antibodies in the serum of immunized mice, 96-well-plates were coated overnight at 4 °C with rMOG (4 μg/mL) in PBS, blocked for 2 h at room temperature, and incubated with sera (serial dilutions starting at 1:10) for 2 h. After washing, serum antibodies adherent to the plate-bound rMOG were detected by HRP-labeled, isotype-specific anti-Ig antibodies and 3, 3′, 5, 5′-tetramethylbenzidine. For detection of total Ig levels, 96-well-plates were coated overnight at 4 °C with goat-anti-mouse Ig antibody instead of rMOG in carbonate buffer.
Immunofluorescence Staining.
Draining lymph nodes and spleen were harvested and immediately frozen in optimum cutting temperature embedding media. Sections 7 μm in thickness were cut with a Leica cryomicrotome (Leica Microsystems). B220, GL-7, and Vα3.2 were detected on acetone-dehydrated slides using GL-7-FITC, B220-APC, and Vα3.2-biotin antibodies followed by incubation with Streptavidin-PE (all from BD Bioscience). Samples were washed three times with PBS after each staining step. Subsequently, samples were mounted using ProLong mounting medium (Invitrogen). All images were captured using a LSM510 laser scanning microscope (Carl Zeiss). ImageJ software was used for quantification of GCs (National Institutes of Health).
Flow Cytometry.
Cells were labeled at 4 °C with the following monoclonal antibodies: Vα3.2-FITC, GL-7-FITC, CD95-PE, ICOS-PE, and CXCR5-biotin (all from BD Biosciences) and CD19-APC-Cy7 and CD4-Pacific Blue (both from BioLegend).
For the detection of intracellular cytokines, cells were restimulated for 4.5 h with PMA (50 ng/mL) and ionomycin (1 μg/mL) (both from Sigma) in the presence of monensin (1 μL/mL; BD Bioscience). Cells were then stained for surface markers and fixed in Cytoperm/Cytofix (BD Biosciences). Subsequently, cells were permeabilized with Perm/Wash Buffer (BD Biosciences) and stained with cytokine antibodies (IFN-γ-PECy7, IL-17-PE, and IL-10-APC) diluted in Perm/Wash buffer as described previously (36). Cells were acquired on a LSRII (BD Biosciences), and data were analyzed using Flow Jo software (Treestar).
In Vitro Ig Isotype Switching.
B cells were isolated using a negative B-cell isolation kit from Miltenyi Biotech. Alternatively, B cells were isolated using CD19 MACS beads, and naïve B cells were sorted by flow cytometry on the basis of IgD expression. Subsequently, B cells were stimulated with anti-CD40 (5 μg/mL) (BioLegend) plus anti-IgM (5 μg/mL) (Jackson Immunoresearch), and IL-17 or IL-22 (all R&D Systems) were added at various concentrations. Alternatively, B cells were cocultured with differentiated, mitomycin C–treated T cells in various ratios in the presence of MOG35-55 (2 μg/mL). The supernatant was analyzed for Ig levels on day 10 using the Ig-specific ELISA described above.
Statistical Analysis.
All data are representative of experiments performed at least three times with similar results. Significance was assessed by an unpaired t test. *P < 0.05; **P < 0.01.
Supplementary Material
Acknowledgments
We thank T. Koeglsperger for technical assistance with the confocal microscope and D. Kozoriz for cell sorting. This work was supported by National Institutes of Health Grants P01AI045757, P01NS038037, R37NS030843, R01NS035685, P01AI056299 (to V.K.K.) and RO1NS059996-01A1 (to E.B.) and National Multiple Sclerosis Society Grants RG2571 (to V.K.K.) and TA3014A1/1 (to E.B.). V.K.K. is a recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health. M.M. (Mi 1221/1-1) and T.K. (KO 2964/2-1) were supported by the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009234107/-/DCSupplemental.
References
- 1.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 3.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 4.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 10.Toellner KM, et al. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 13.Doreau A, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 14.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Z, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 17.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 21.Mehta DS, et al. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki K, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 24.Kuchen S, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 25.Smith KM, et al. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 26.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han S, Zheng B, Takahashi Y, Kelsoe G. Distinctive characteristics of germinal center B cells. Semin Immunol. 1997;9:255–260. doi: 10.1006/smim.1997.0081. [DOI] [PubMed] [Google Scholar]
- 28.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith KM, Brewer JM, Rush CM, Riley J, Garside P. In vivo generated Th1 cells can migrate to B cell follicles to support B cell responses. J Immunol. 2004;173:1640–1646. doi: 10.4049/jimmunol.173.3.1640. [DOI] [PubMed] [Google Scholar]
- 31.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Adelmann M, et al. The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J Neuroimmunol. 1995;63:17–27. doi: 10.1016/0165-5728(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 35.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.