Abstract
High levels of corticosteroids (as circulate after stress) quickly and reversibly enhance hippocampal glutamatergic transmission via nongenomic actions requiring mineralocorticoid receptors. Subsequently, the hormone slowly and long-lastingly normalizes hippocampal cell function, through nuclear glucocorticoid receptors. Here we describe a rapid mineralocorticoid receptor-dependent enhancement of glutamatergic transmission in basolateral amygdala neurons. Contrary to the hippocampus, this rapid enhancement is long-lasting, potentially allowing an extended window for encoding of emotional aspects during stressful events. Importantly, the long-lasting change in state of amygdala neurons greatly affects the responsiveness to subsequent surges of corticosterone, revealing a quick suppression of glutamatergic transmission, which requires the glucocorticoid receptor. Responses of basolateral amygdala neurons to the stress hormone corticosterone can thus switch from excitatory to inhibitory, depending on the recent stress history of the organism.
Keywords: basolateral amygdala, glucocorticoid receptor, glutamate, miniature excitatory postsynaptic current, mineralocorticoid receptor
Shortly after stress, corticosteroid hormones are released in high amounts from the adrenal glands, enter the brain, and predominantly bind to intracellular glucocorticoid receptors (GRs), e.g., in the hippocampal CA1 area (1, 2). Neurons in this area also highly express intracellular mineralocorticoid receptors (MRs), but because of their high affinity these receptor are already largely occupied even under rest. Both receptor types act as transcription factors, altering the expression of responsive genes (3). In this way corticosterone (the prevailing hormone in rodents) changes cellular excitability in the CA1 area, starting 1–2 h after an elevation in corticosteroid level and lasting up to at least several hours (4). Overall, MRs serve to maintain glutamatergic transmission and viability in the CA1 area, whereas GRs cause a delayed suppression of synaptic transmission and plasticity, presumably normalizing stress-dependent rises in activity (4). Thus, the two receptor types exert a slow genomic yin–yang control over hippocampal excitability.
Recently, though, we demonstrated that, in the CA1 area, corticosterone also quickly and reversibly enhances the frequency of miniature excitatory postsynaptic currents (mEPSCs), each of which reflects the spontaneous release of a glutamate-containing vesicle (5). This nongenomic effect critically depends on the presence of MRs thought to reside in the presynaptic terminal membrane, and involves activation of the ERK1/2 pathway (6). The apparent affinity of this membrane-located MR is 10-fold lower than that of the intracellular MR, allowing it to play a prominent role in the behavioral stress response (7). It is thought that corticosterone via membrane MRs (in close interaction with other stress mediators such as noradrenaline and CRH) quickly raises hippocampal excitability, to be normalized 1–2 h later through gene-mediated GR actions (8). This leaves a restricted time-window during which the encoding of stress-related information is facilitated (2, 9, 10).
Exposure to stressful situations, however, will nearly always also activate the amygdala nuclei (10–12). Recent studies suggest that slow GR effects in the basolateral amygdala (BLA) enhance rather than suppress excitatory transmission (13, 14), indicating that corticosterone via its genomic pathway affects CA1 and BLA neurons differently. We wondered whether this is also true for rapid nongenomic effects of corticosterone. Rapid effects are indeed expected to take place, because corticosteroid receptors were observed in the membrane of LA neuron terminals with immunogold electron microscopy (15).
Results
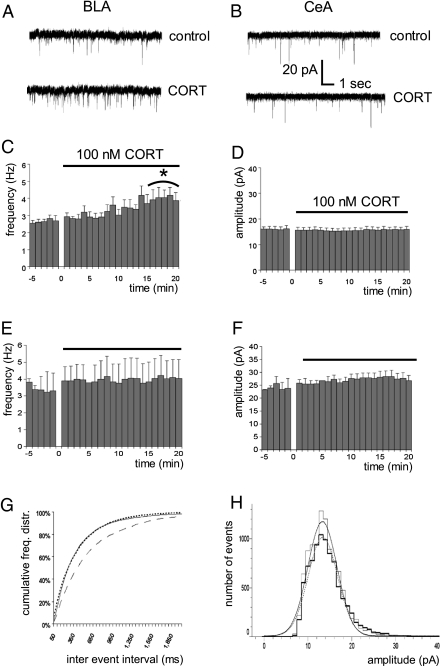
Coronal slices containing the amygdala nuclei were prepared from male young-adult C57/Bl6 mice under rest in the morning, i.e., when corticosteroid levels are very low, as described in ref. 14. Application of 100 nM corticosterone (for 20 min) in vitro increased the mEPSC frequency of principal neurons in the BLA but not the central amygdala (Fig. 1 A–C and E). The mEPSC amplitude in both areas was unaffected (Fig. 1 D, F, and H), as was the mEPSC τ of decay in the BLA (baseline, 8.2 ± 0.7 ms; during corticosterone, 11.9 ± 1.8 ms; n = 7; paired t test, P = 0.063) and the central amygdala (baseline, 15.3 ± 1.7 ms; during corticosterone, 19.2 ± 2.1 ms; n = 9; P = 0.074), although a tendency toward an increase was observed in both areas. Our first conclusion is that, in the BLA, corticosterone induces comparable rapid-onset effects on the mEPSC frequency as in the hippocampal CA1 area but this cannot be generalized to all limbic brain nuclei.
Fig. 1.
Corticosterone enhances mEPSC frequency of BLA but not CeA principal neurons, as shown for typical traces in A and B, respectively. The mEPSC frequency over time is shown for BLA neurons (average of n = 7 cells) before and during application of 100 nM corticosterone in C. Comparison of the averaged frequency during baseline (t = −5–0 min) and the final 5 min during corticosterone administration (t = 15–20 min) revealed a statistically significant difference (paired t test, *P < 0.005). No change was observed during corticosterone administration on mEPSC amplitude (D). In the CeA, neither mEPSC frequency (E; average of seven cells) nor amplitude (F) was affected by corticosterone administration. The cumulative distribution of mEPSC frequencies in BLA neurons (G) showed a significant enhancement during corticosterone administration (solid line; Kolmogorov–Smirnov analysis, P < 0.005) compared with baseline (dashed line), which did not reverse 20 min after washout (dotted line). No change was observed in the frequency distribution of mEPSC amplitudes during compared with before corticosterone application (H; baseline in thin bars, the situation during corticosterone application in bold; dotted and solid lines represent the Gaussian fits before and during corticosterone application, respectively).
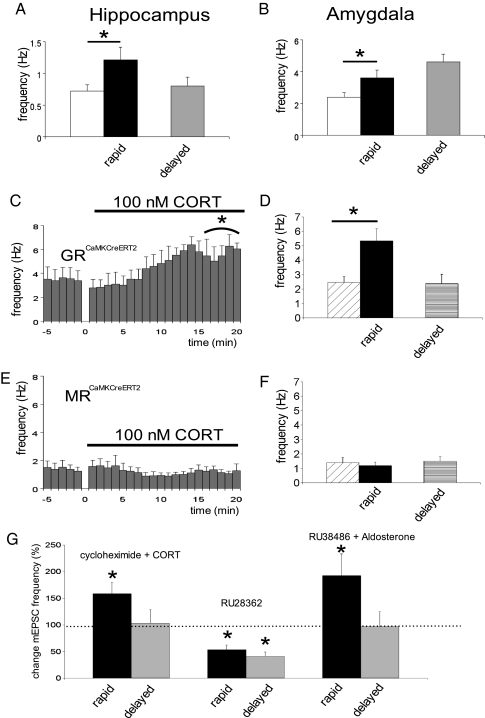
The cumulative histogram (Fig. 1G) suggests that rapid effects of corticosterone on BLA mEPSC frequency are not quickly reversible, in contrast to what was earlier seen in the hippocampal CA1 area. In a subset of cells that were recorded up to 20 min after washout of corticosterone, mEPSC frequency indeed remained high (baseline, 3.2 ± 0.4 Hz; ∼20 min after corticosterone wash-out, 4.0 ± 0.2 Hz; n = 5, P = 0.02 with a paired t test). Because it is difficult to examine effects of corticosterone over even longer periods of time with whole-cell patch-clamping of adult neurons, we compared (in separate groups of cells) mEPSC frequency several hours after a brief pulse of corticosterone with that after vehicle treatment, both in hippocampal CA1 and BLA neurons. A brief pulse of corticosterone (100 nM for 20 min) was indeed sufficient to cause high mEPSC frequency several hours later in principal neurons of the BLA [corticosteroid (n = 7) versus vehicle-treated cells (n = 7), P = 0.002, unpaired t test] but not the CA1 area (n = 10 and 5 cells respectively, P > 0.05; for mean values of corticosterone-treated cells, see Fig. 2 A and B).
Fig. 2.
MR and GR requirement for the development of rapid and delayed corticosteroid effects in the BLA. Application of corticosterone rapidly enhanced mEPSC frequency in CA1 pyramidal neurons (A; black bar, n = 5) compared with pretreatment baseline frequency (open bar; paired t test, P < 0.05). Neurons recorded 1–4 h after slices were treated for 20 min with 100 nM corticosterone displayed a low mEPSC frequency (light gray bar, n = 10). In the BLA, corticosterone treatment also quickly enhanced mEPSC frequency (B; n = 7, P < 0.005). However, 1–4 h after slices were treated for 20 min with 100 nM corticosterone, mEPSC frequency was still elevated (n = 7, see main text). In mice with induced forebrain ablation of GR (GRCaMKCreERT2), application of corticosterone also resulted in rapid enhancement of mEPSC frequency (C and D; n = 8 and 5 cells, respectively) in the BLA. This enhancement, though, was transient (D; n = 4 cells, for recordings 1–4 h after corticosterone application). In mice with induced forebrain ablation of MR (MRCaMKCreERT2), neither rapid (n = 7) nor delayed (n = 6) effects of corticosterone were observed (E and F). Corticosterone in the presence of a protein synthesis inhibitor (cycloheximide) was still able to induce an increase in mEPSC frequency (n = 7, P = 0.03), but the effect was only transient (G). Selective activation of the MR (by aldosterone in the presence of RU 38486; n = 7, P = 0.004) briefly enhanced mEPSC frequency, in contrast to the GR-agonist RU 28362, which even reduced the frequency (n = 5, P = 0.03). Data are expressed as the mean (±SEM) change in mEPSC frequency during the final 5 min of drug application (black bar for each drug) or 20 min after washout (gray bar), in both cases relative to the average frequency at t = −5–0 min (just before drug application, set at 100%). *P < 0.05 compared with baseline (paired t test).
To determine the involvement of the two known corticosteroid receptor types in these effects, we made use of male mutant mice with forebrain ablation of either MR or GR induced at the age of 8–9 wk [MRCaMKCreERT2 and GRCaMKCreERT2, respectively (16); SI Methods] and their respective littermate controls. Corticosteroid effects were clearly visible in GRCaMKCreERT2 mice (Fig. 2 C and D), which do not express GRs but do have MRs in the BLA. By contrast, in MRCaMKCreERT2 mice [i.e., in the absence of BLA MRs (SI Methods)] rapid enhancement of the mEPSC frequency was no longer observed (Fig. 2 E and F), supporting a critical role of MRs in the rapid development of raised mEPSC frequency by corticosterone. Enhanced mEPSC frequency in the presence of corticosterone was observed in all cells (n = 5) from control littermates (MRflox/flox or GRflox/flox), to an extent comparable to that seen in control C57/Bl6 mice (mean frequency ± SEM before corticosterone, 2.28 ± 0.24 Hz; during CORT, 3.84 ± 0.84 Hz). The findings in genetically modified mice were confirmed by pharmacological observations in control C57/Bl6 animals. Thus, selective activation of MRs by application of (10 nM) aldosterone in the presence of a GR antagonist (500 nM RU 38486) resulted in strong enhancement of the mEPSC frequency (Fig. 2G). Conversely, application of the selective GR agonist RU 28362 (100 nM) did not enhance but, rather, decreased the mEPSC frequency (Fig. 2G).
Gradual enhancement of mEPSC frequency was not present after corticosterone administration in MRCaMKCreERT2 mutants (Fig. 2F), nor after RU 28362 application (Fig. 2G), emphasizing that MRs are necessary for the development of both the rapid and lasting enhancement in mEPSC frequency. Interestingly, in the GRCaMKCreERT2 mice (where corticosterone selectively activates MRs) mEPSC frequency did not remain at a high level but returned to baseline values, as is evident from cells recorded 1–4 h after a 20-min pulse of corticosterone (Fig. 2D). In the same vein, application of aldosterone in the presence of RU 38486 induced only a transient response (Fig. 2G). Transient enhancement in mEPSC frequency was also observed when corticosterone was applied in the presence of the protein synthesis inhibitor cycloheximide (Fig. 2G). This suggests that MRs may be necessary but are certainly not sufficient for the maintenance of the high mEPSC frequency; the sustained effect also requires a protein synthesis-dependent process via the GR.
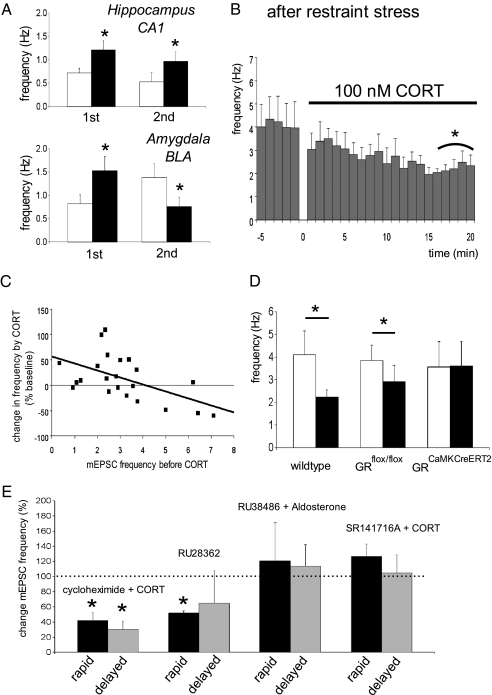
Because one pulse of corticosterone apparently causes prolonged enhancement of mEPSC frequency in BLA neurons, one may wonder whether this makes BLA as opposed to CA1 hippocampal cells refractory to a second pulse, hours later. In the hippocampal CA1 area, mEPSC frequency several hours after corticosterone exposure was low (Fig. 2A), and cells responded to renewed corticosterone exposure with enhanced mEPSC frequency, to a comparable extent as after the first pulse (Fig. 3A). Surprisingly, though, renewed exposure of BLA neurons to corticosterone (in cells that still exhibited increased mEPSC frequency due to the first pulse; Fig. 2B) caused a decrease in mEPSC frequency (Fig. 3A). Like the increase in mEPSC frequency seen after the first pulse, the decrease in response to a second pulse was long lasting; thus, >1 h after washout of the second corticosterone application, mEPSC frequency was at a comparably low level (0.88 ± 0.11 Hz) as seen at the end of the second pulse (Fig. 3A).
Fig. 3.
Exposure to two pulses of corticosterone (10- to 20-min pulse duration, 1- to 3-h pulse interval) induced comparable elevations (P < 0.05) in mEPSC frequency in CA1 pyramidal cells (A; mean ± SEM of n = 6 and 5 cells during the first and second pulse, respectively). By contrast, BLA neurons responded to the first pulse with increased mEPSC frequency (n = 12, P < 0.05), but with a decreased (P < 0.05) mEPSC frequency to a second pulse (n = 6). In BLA cells from animals exposed to restraint stress before slice preparation, corticosterone also rapidly decreased mEPSC frequency (B; n = 8 cells). (C) A significant (P < 0.001) inverse relationship was observed between baseline mEPSC frequency and the percentual change in mEPSC frequency during corticosterone treatment (compared with baseline; no change = 0). For this figure, data obtained in mice with low corticosterone level (under rest, at the circadian nadir) were combined with those obtained 1–4 h after treatment with corticosterone in vitro or restraint stress (in all cases C57/Bl6). Correlation was tested for significance with a Pearson test. (D) In GRflox/flox controls, we observed a significant reduction in mEPSC frequency after stress (n = 9 cells, P < 0.05), similar to what we observed in wild-type C57/Bl6 mice (n = 8). The reduction was not observed in GRCaMKCreERT2 mutants (n = 6 cells). (E) The protein synthesis inhibitor cycloheximide did not interfere with the suppressing effect of corticosterone in slices from stressed mice (n = 5, P = 0.04). The GR-agonist RU 28362 decreased mEPSC frequency after stress (n = 5, P = 0.04), whereas selective activation of the MR (by aldosterone in the presence of RU 38486; n = 6) did not change the frequency. If corticosterone was applied in the presence of the endocannabinoid-R1 antagonist SR141716A (1 μM), mEPSC frequency was not significantly altered (n = 6). Data are expressed as the mean (+SEM) change in mEPSC frequency during the final 5 min of drug application (black bar for each drug) or 20 min after washout (gray bar), relative to the frequency at t = −5–0 min (just before drug application, set at 100%). *P < 0.05 compared with baseline (paired t test).
We probed the physiological significance of the reduced mEPSC frequency in response to a second corticosterone application, by exposing mice to restraint stress 20 min before preparing slices. In accordance with the lasting enhancement in mEPSC frequency observed after in vitro administration of corticosterone, the baseline mEPSC frequency of BLA neurons in slices from stressed animals was high (∼4 Hz; Fig. 3 B and D). Under these conditions, neurons responded to corticosterone exposure in vitro with a rapid and lasting decrease in mEPSC frequency (Fig. 3 B and D). Thus, in slices prepared from animals under rest, corticosterone long lastingly increases mEPSC frequency; once the frequency is enhanced (e.g., by stress), subsequent corticosterone exposure decreases mEPSC frequency. In fact, the mEPSC frequency before corticosterone application predicted the nature of the rapid hormone effects, showing a clear inverse relationship (B = −0.17, P < 0.001; Fig. 3C). A comparable inverse relationship (B = −0.21, P < 0.05) was also observed for a limited number of cells (n = 6) tested in vitro with the membrane-impermeable ligand CORT-BSA.
The rapid decrease in mEPSC frequency was recapitulated in restrained GRflox/flox controls but not in BLA neurons of restrained GRCaMKCreERT2 mice (Fig. 3D), indicating that the rapid decrease critically depends on the local presence of GRs. This was confirmed by experiments in control C57/Bl6 mice where the selective GR agonist RU 28362 significantly decreased mEPSC frequency in BLA neurons recorded after stress (Fig. 3E); mEPSC frequency recorded 20 min after washout of RU 28362 still tended to be low, but these values were more variable. Application of aldosterone in the presence of RU 38486 in slices prepared from stressed animals did not change the mEPSC frequency (Fig. 3E). The nongenomic nature of the mEPSC suppression by corticosterone after stress was supported by the observation that the response persisted in the presence of cycloheximide (Fig. 3E).
Interestingly, if corticosterone was applied to slices from earlier stressed C57/Bl6 mice but now in the presence of the endocannabinoid-R1 antagonist SR141716A (1 μM), we also did not observe a decrease in mEPSC frequency but instead revealed a slight but nonsignificant enhancement (Fig. 3E). This suggests that the rapid suppressive and GR-dependent effects involve endocannabinoids, as was earlier reported for the hypothalamic paraventricular nucleus (17); this differs from the rapid MR-dependent actions observed in the hippocampus (and possibly also in the BLA), which do not seem to depend on retrograde signaling (6). The current data with endocannabinoids also provides a cellular basis for recent behavioral observations implicating endocannabinoids in amygdala-dependent memory formation (18).
Discussion
We here demonstrate that, in BLA neurons of an animal with low circulating corticosteroid levels (i.e., under rest, at the circadian nadir), temporary rises in hormone level—such as may occur during and some time after stress—cause a rapid-onset enhancement of spontaneous glutamate-mediated activity via MRs, similar to what was earlier described for CA1 hippocampal cells (5). In contrast to the transient effect in the CA1 area, though, the enhanced spontaneous glutamate-mediated activity in the BLA is maintained for several hours. For long-lasting effects to occur, the development of rapid effects via MR is necessary, but GR expression is also required for maintaining high mEPSC frequency. These findings suggest that rapid MR-dependent effects are functionally linked to later-developing gene-dependent and GR-requiring processes, along the same line as rapid effects of estrogens were found to influence subsequently developing genomic actions (19). The fact that increased mEPSC frequency in BLA is sustained, as opposed to the transient effects earlier reported for CA1 cells, fits with the slowly developing excitatory effects exerted in the BLA (but not CA1) by corticosteroids on firing frequency accommodation (13, 14). The collective data support that, in the BLA (as opposed to the CA1 area), excitability is raised by corticosterone over a relatively long time-window. One could speculate that this would allow encoding of information via the amygdala over an extended period after stress, a mechanism that might contribute to the preferential facilitation by stress of retention of emotional versus neutral information (10, 20).
Unexpectedly, exposure to a rise in corticosteroid level against a history of stress produced a rapid effect in the opposite direction, via a GR-dependent process. Apparently, the rapid nongenomic effects via MRs and GRs in the BLA serve a similar yin–yang function on cellular excitability as earlier postulated for the genomic actions in the CA1 area, although in a different time-domain. Apparently, one hormone can induce opposite effects, depending on the recent history of the organism and its neurons. Interestingly, in BLA tissue prepared from animals under rest, the enhancement in mEPSC frequency prevails when the endogenous ligand corticosterone is applied, but suppressive effects can still be uncovered by selective activation of GR. By contrast, application of aldosterone/RU 38486 to slices from stressed animals (or corticosterone to slices from stressed GRCaMKCreERT2 mice) did not lead to enhanced mEPSC frequency. These findings, combined with those earlier reported for the hippocampus (6) and PVN (17), suggest that under rest the membrane of presynaptic terminals in the BLA is enriched with MRs, whereas only a limited number of GRs are present postsynaptically. After stress, MRs may internalize while the surface expression of GRs seems enhanced. Electron-microscopic experiments need to verify this.
The “state-dependency” of rapid corticosteroid effects in the BLA is reminiscent of the metaplasticity and sliding-threshold earlier described with regard to synaptic plasticity in the hippocampus after high-frequency stimulation (21). The functional implication could be that in the BLA, renewed exposure to corticosterone some hours after stress helps to reset the spontaneous glutamate transmission, earlier raised via corticosteroids in interaction with other stress mediators like noradrenaline. If so, insufficient levels of corticosterone—as have been hypothesized to occur in individuals vulnerable to posttraumatic stress disorder (22)—might fail to reset the BLA excitability, so that potentially ongoing encoding is not curtailed.
Methods
In the current study, we used male young-adult C57/Bl6 mice (6–9 wk old; Harlan CPB), decapitated under rest in the morning (i.e., when corticosteroid levels are very low) as described in ref. 14. In some experiments, animals were subjected to restraint stress for 20 min immediately before decapitation.
Immediately after decapitation, the brain was removed from the skull and chilled (4 °C) in artificial cerebrospinal fluid (aCSF) containing 120 mmol/L NaCl, 3.5 mmol/L KCl, 5.0 mmol/L MgSO4, 1.25 mmol/L NaH2PO4, 0.2 mmol/L CaCl2, 10 mmol/L D-glucose, and 25.0 mmol/L NaHCO3 (gassed with 95% O2 and 5% CO2). Coronal slices (350 μm thick) containing the BLA and/or dorsal hippocampus were prepared with a vibroslicer (Leica VT 1000S). Slices were stored at room temperature until use in recording aCSF containing 120 mmol/L NaCl, 3.5 mmol/L KCl, 1.3 mmol/L MgSO4, 1.25 mmol/L NaH2PO4, 2.5 mmol/L CaCl2, 10 mmol/L D-glucose, and 25 mmol/L NaHCO3.
One slice at a time was placed in a recording chamber mounted on an upright microscope (Axioskop 2 FS plus; Zeiss) with differential interference contrast, water-immersion objective (63×), and 10× ocular. The slices were continuously perfused with aCSF (flow rate 2–3 mL/min, temperature 32 °C, pH 7.4) consisting of 120 mM NaCl, 3.5 mM KCl, 1.3 mM MgCl2, 2.5 mM CaCl2, 25 mM NaHCO3, 1.25 mM KH2PO4, and 10 mM D-glucose; bicuculline methchloride (20 μm; Tocris) and tetrodotoxin (0.5 μm; Latoxan) were added to block GABAA receptor-mediated signals and action potentials, respectively. Whole-cell voltage-clamp recordings were made with an Axopatch 200B amplifier (Axon Instruments) using borosilicate glass electrodes (impedance 4–6 MΩ, 1.5-mm outer diameter; Hilgenberg) pulled with a micropipette puller (Brown/Flaming P-87; Sutter Instruments). For mEPSC recordings, the intracellular pipette solution contained 120 mM Cs methane sulphonate, 17.5 mM CsCl, 10 mM Hepes, 5 mM BAPTA, 2 mM MgATP, and 0.1 mM Na GTP (295 mOsm, pH 7.4 adjusted with CsOH). BAPTA was obtained from Molecular Probes; all other chemicals were purchased from Sigma.
Neurons in the BLA were selected for recording if they displayed a pyramidal-shaped cell body. All mEPSCs were recorded with a holding potential of −70 mV. If the neuron under study displayed stable mEPSC properties during baseline recording (at least 10 min), corticosteroids were applied for ≈20 min via the perfusion medium; drug testing comprised (i) 100 nM corticosterone (Sigma), (ii) 10 nM aldosterone (Fluka) in the presence of 500 nM RU 38486 (Sigma), (iii) 100 nM RU 28362 (generous gift from Benno Roozendaal, University Medical Center Groningen, Groningen, The Netherlands), (iv) 100 nM corticosterone in the presence of 100 μM cycloheximide (Fluka), and (v) 100 nM corticosterone-BSA (Sigma). In one set of cells, we compared cells 1–4 h after a 20-min application of corticosterone or vehicle, as described in ref. 14. Corticosterone, aldosterone, and RU28362 were prepared weekly in a stock solution (1 mM in 95% ethanol) and diluted to their final concentration in aCSF just before application. Cycloheximide and corticosterone-BSA were dissolved directly in ACSF and freshly prepared on the day of the experiment. RU38486 was dissolved in distilled water (1 mM) with a few drops of 5 M HCl and diluted to the final concentration in ACSF.
Series resistance compensation was >70% in all recordings. Series resistance and capacitance were monitored during the whole recording. Responses were filtered at 5 kHz and digitized at 10 kHz (Digidata 1322A; Axon Instruments). All data were acquired, stored, and analyzed on a PC using pClamp 9.0 and Clampfit 9.2 (Axon Instruments). Minimal cutoff for mEPSC analysis was 6 pA.
Mutant Mice.
To generate mutant mice with forebrain ablation of either MR or GR we crossed transgenic mice expressing the CreERT2 fusion protein under the control of the regulatory elements of the CaMKIIa gene (16) to mice harboring either a conditional MR allele (23) or a conditional GR allele (24). Eight- to 9-wk-old MRCaMKCreERT2 (MRflox/floxCaMKCreERT2) and their littermate controls (MRflox/flox), as well as GRCaMKCreERT2 (GRflox/floxCaMKCreERT2) and their littermate controls (GRflox/flox), were treated with 1 mg of tamoxifen twice a day for 5 d to induce in mutants the loss of MR or GR in forebrain neurons that express CreERT2, including principal neurons in the BLA (Fig. S1).
Statistical Analyses.
In most cases, we applied paired t test comparisons between mEPSC properties determined during the final 5 min of baseline recording and the final 5 min of recording in the presence of corticosterone [or other (combinations of) drugs] in the same cells. If mEPSC properties determined 1–4 h after corticosterone treatment were compared with those after vehicle treatment, we used unpaired statistics. Cumulative frequency distributions were subjected to a Kolmogorov–Smirnov analysis. We removed one observation (a cell tested with corticosterone in the presence of a CB-R1 antagonist) from the dataset, because this value was >2 SD removed from the mean and considered to be an outlier.
Supplementary Material
Acknowledgments
This work was supported by Dutch Organization for Scientific Research (NWO-ALW) Grant 817.02.017 (to H.K.), as well as by the Deutsche Forschungsgemeinschaft through SFB 636 and European Union Grant LSHM-CT-2005-018562 (CRESCENDO).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914381107/-/DCSupplemental.
References
- 1.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Neurosci Rev. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress repsonses. Nat Neurosci Rev. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu NZ, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- 4.Joëls M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: Implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Karst H, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olijslagers JE, et al. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- 7.Joëls M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Neurosci Rev. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Neurosci Rev. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 11.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 13.Duvarci S, Paré D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebmann L, et al. Differential effects of corticosterone on the slow afterhyperpolarization in the basolateral amygdala and CA1 region: Possible role of calcium channel subunits. J Neurophysiol. 2008;99:958–968. doi: 10.1152/jn.01137.2007. [DOI] [PubMed] [Google Scholar]
- 15.Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Erdmann G, Schütz G, Berger S. Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci. 2007 doi: 10.1186/1471-2202-8-63. 10.1186/1471-2202-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 22.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann NY Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 23.Berger S, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci USA. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.