Abstract
Erythropoietin (EPO), originally identified for its critical hormonal role in regulating production and survival of erythrocytes, is a member of the type 1 cytokine superfamily. Recent studies have shown that EPO has cytoprotective effects in a wide variety of tissues, including the heart, by preventing apoptosis. However, EPO also has undesirable effects, such as thrombogenesis. In the present study, we investigated whether a helix B-surface peptide (HBSP), a nonerythropoietic, tissue-protective peptide mimicking the 3D structure of erythropoietin, protects cardiomyocytes from apoptosis in vitro and in vivo. In cultured neonatal rat cardiomyocytes, HBSP clearly inhibited apoptosis (≈80%) induced by TNF-α, which was comparable with the effect of EPO, and activated critical signaling pathways of cell survival, including Akt, ERK1/2, and STAT3. Among these pathways, Akt was shown to play an essential role in HBSP-induced prevention of apoptosis, as assessed by using a small interfering RNA approach. In the dilated cardiomyopathic hamster (J2N-k), whose cardiac tissues diffusely expressed TNF-α, HBSP also inhibited apoptosis (≈70%) and activated Akt in cardiomyocytes. Furthermore, the levels of serum creatine kinase activity and of cardiac expression of atrial natriuretic peptide, a marker of chronic heart failure, were down-regulated in animals treated with HBSP. These data demonstrate that HBSP protects cardiomyocytes from apoptosis and leads to a favorable outcome in failing hearts through an Akt-dependent pathway. Because HBSP does not have the undesirable effects of EPO, it could be a promising alternative for EPO to treat cardiovascular diseases, such as myocardial infarction and heart failure.
Keywords: apoptosis, cardiomyocyte, cytoprotection, signal transduction
Erythropoietin (EPO) was originally identified for its critical hormonal role in regulating survival, proliferation, and differentiation of erythroid progenitors through the interaction with a preformed receptor homodimer, and is widely used in the clinical setting for the treatment of anemia caused by chronic kidney disease, cancer, or nonmyeloid hematologic malignancies [reviewed by Fisher (1)]. Since the early 1990s, it has emerged that EPO has cytoprotective effects in a wide variety of tissues, including the brain, kidney, and heart, from ischemic or nonischemic injury [reviewed by Brines and Cerami (2)]. In its nonerythropoietic functions, EPO is produced locally by many tissues in response to physical or metabolic stress and acts in a paracrine-autocrine manner. EPO's tissue-protective actions have been shown to be mediated by a tissue-protective receptor complex consisting of the EPO receptor and the β common-receptor (CD131) subunit that is also used by GM-CSF, IL-3, and IL-5 (3). However, administration of EPO can cause serious adverse effects mediated by the homodimer receptor, such as hypertension (4) and thrombosis (5). For avoiding these undesirable effects associated with EPO therapy, a number of nonerythropoietic, tissue-protective compounds (TPCs) have been developed that interact specifically with the tissue-protective receptor, including carbamylated EPO (CEPO) (6), as well as peptides mimicking the 3D structure of EPO (7): for example, a helix B-surface peptide (HBSP). This peptide is composed of 11 amino acids (QEQLERALNSS) derived from the aqueous face of helix B of EPO and exhibits tissue-protective activities comparable with EPO, as demonstrated in models of ischemic stroke and renal ischemia/reperfusion (I/R) (7).
Chronic heart failure (CHF) caused by ischemic heart disease or dilated cardiomyopathy (DCM) remains the leading cause of mortality in developed countries. Therefore, novel therapeutic approaches that prevent the development and progression of CHF are critical for improving the prognosis of patients with CHF. A number of studies have shown that circulating levels of TNF-α are elevated in patients with end-stage CHF and are correlated with the severity of the disease (8–10). Previous work has demonstrated that the failing human myocardium, but not nonfailing human hearts, expresses abundant amounts of TNF-α (11–13) and that TNF-α induces apoptosis in cardiomyocytes and results in the development of CHF (14, 15). It is notable that EPO limits the destructive potential of TNF-α and other proinflammatory cytokines in many tissues, including the heart (2). EPO and CEPO have been shown to protect the myocardium from I/R injury in an acute model that mimics the clinical situation of an acute myocardial infarction with early reperfusion (16–18). In this model, both EPO and CEPO prevent cardiomyocyte apoptosis and lead to a favorable outcome in left ventricular function. However, it remains unclear whether EPO and its nonerythropoietic derivatives, including HBSP, protect failing hearts from apoptosis in a chronic model that mimics a patient with CHF such as, for example, an animal model of DCM.
In the present study, we determined whether HBSP protects cardiomyocytes from apoptosis induced by TNF-α using cultured neonatal rat cardiomyocytes in vitro and a hamster strain of DCM, J2N-k (19), in vivo. Cardiomyocyte protection by HBSP was observed both in vitro and in vivo, and a critical role of the serine/threonine kinase Akt in HBSP-mediated inhibition of apoptosis was also demonstrated. Furthermore, chronic administration of HBSP beginning at 20 wk of age, long after the onset of myocardial damage as assessed by myocardial necrosis (19), resulted in the down-regulation of serum creatine kinase (CK) activity and of cardiac expression of atrial natriuretic peptide (ANP), critical markers of CHF (20), in the DCM hamster. These data confirm the protective effects of HBSP on cardiomyocytes as a promising alternative to EPO for treatment of cardiovascular diseases.
Results
Apoptosis of Cardiomyocytes in Vitro.
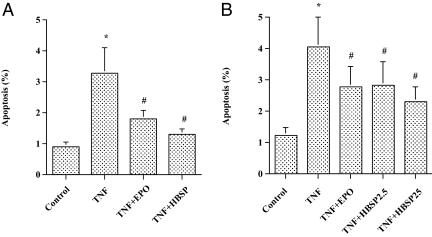
In cultured cardiomyocytes, incubation with TNF-α (50 pg/mL) resulted in a significant increase in the number of apoptotic cells (Fig. 1A). Pretreatment with HBSP (2.5 ng/mL, the molar equivalent of 10 IU/mL of EPO) clearly decreased the number of apoptotic cells (≈80%) induced by TNF-α, which was comparable to the effect of EPO (10 IU/mL) (Fig. 1A). To test the importance of pretreatment with HBSP in preventing apoptosis, cardiomyocytes were simultaneously treated with TNF-α and HBSP. Without pretreatment, HBSP effectively inhibited apoptosis (≈60%) in cultured cardiomyocytes (Fig. 1B). The inhibitory effects of HBSP on cardiomyocyte apoptosis in pretreatment and simultaneous treatment showed no significant difference between the two treatment groups.
Fig. 1.
Effect of HBSP on TNF-α-induced cardiomyocyte apoptosis. (A) Pretreatment. Neonatal rat cardiomyocytes were pretreated with EPO (10 IU/mL), HBSP (2.5 ng/mL), or vehicle for 12 h, and then TNF-α (50 pg /mL) was added to the culture. After an additional 12-h incubation, the cells were harvested by trypsinization, and flow cytometric analysis was performed to evaluate apoptosis. Mean ± SEM, n = 9 each group; *P < 0.05 vs. Control; #P < 0.05 vs. TNF. (B) Simultaneous treatment. Cardiomyocytes were simultaneously treated with TNF-α (50 pg /mL) and EPO (10 IU/mL), or TNF-α and HBSP (2.5 or 25 ng/mL) for 12 h. Next, the cells were harvested for flow cytometric analysis to evaluate apoptosis. Mean ± SEM, n = 12 each group; *P < 0.01 vs. Control; #P < 0.05 vs. TNF.
Activation of Critical Signaling Pathways of Cell Survival.
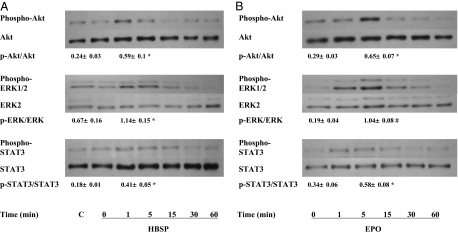
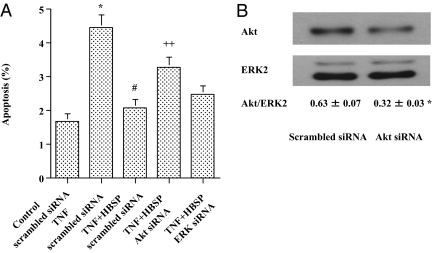
To investigate the mechanisms by which HBSP inhibits cardiomyocyte apoptosis, we examined intracellular signaling pathways of cell survival. HBSP rapidly activated Akt, ERK1/2, and STAT3 in cultured cardiomyocytes with a peak at 1 min after incubation (Fig. 2A). EPO also activated these molecules, but time to peak activation was delayed compared with HBSP (Fig. 2B). In contrast, a scrambled version of HBSP did not activate any signaling pathways (Fig. 2A). To determine which pathway is critical for preventing apoptosis, we assessed the effect of small interfering RNA (siRNA) against Akt1 (Akt siRNA) and ERK1 (ERK siRNA) on HBSP-induced inhibition of apoptosis. Akt siRNA specifically attenuated apoptosis inhibition induced by HBSP in cultured cardiomyocytes (Fig. 3A). On the other hand, ERK siRNA and scrambled siRNA did not show significant changes in the inhibitory effect of HBSP on cardiomyocyte apoptosis (Fig. 3A). Densitometric analysis of the Western blot showed a significant down-regulation of Akt by Akt siRNA at the protein level (Fig. 3B).
Fig. 2.
Critical signaling pathways of cell survival. (A) Activation by HBSP. Cardiomyocytes were incubated with HBSP (2.5 ng/mL) for the indicated times or a control peptide (C; a scrambled version of HBSP, 2.5 ng/mL, 5 min) and cell lysates were prepared. Then Western blot was performed as described in Materials and Methods. (B) Activation by EPO. The cells were incubated with EPO (10 IU/mL) for the indicated times and treated in the same manner as in A. Immunoreactive bands were analyzed by densitometry using NIH Image 1.62 and ratios of the phospho/total antibody immunolabeling were calculated. The ratios were compared between C and 1 min in A, and between 0 and 5 min in B. Mean ± SEM n = 3 each group; *P < 0.05; #P < 0.01.
Fig. 3.
Critical role of Akt in cardiomyocyte protection and Akt down-regulation by Akt siRNA at the protein level. (A) Critical role of Akt in cardiomyocyte protection. Small interfering RNA targeting Akt1 or ERK1, or negative control (scrambled) siRNA was transfected to cardiomyocytes at a concentration of 20 nM with a Silencer siRNA Transfection II Kit. TNF-α (50 pg/mL), TNF-α+HBSP (25 ng/mL), or vehicle was added in the culture 24 h after transfection and the cells were incubated for an additional 12 h. Next, the cells were harvested by trypsinization and flow cytometric analysis was performed to detect apoptotic cells. Mean ± SEM, n = 12 each group; *P < 0.01 vs. Control scrambled siRNA; #P < 0.01 vs. TNF scrambled siRNA; ++P < 0.05 vs. TNF+HBSP scrambled siRNA and TNF+HBSP ERK siRNA. (B) Akt down-regulation by Akt siRNA at the protein level. Scrambled siRNA or Akt siRNA was transfected to cardiomyocytes in the same manner, as in A, without treatment of TNF-α or HBSP, and cell lysates were prepared. Next, Western blot was performed as described in Materials and Methods using an antibody against Akt or ERK2 (a loading control). Immunoreactive bands were analyzed by NIH Image 1.62 and ratios of densitometric analysis (Akt/ERK2) were calculated. Mean ± SEM, n = 3 each group; *P < 0.05.
Cardiomyocyte Protection in the DCM Hamster.
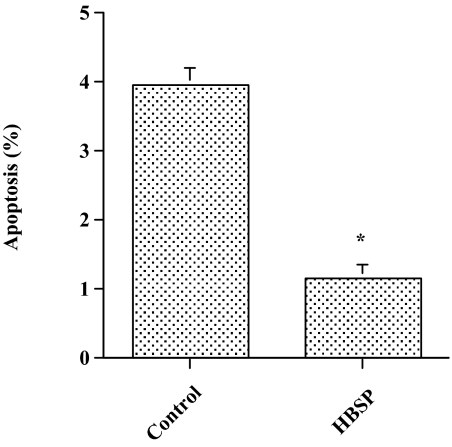
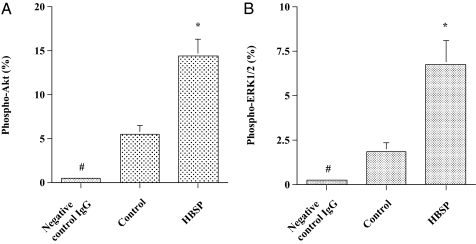
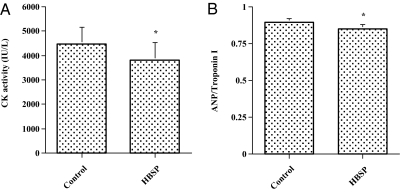
To confirm an antiapoptotic effect of HBSP on cardiomyocytes in vivo, 30 μg/kg body weight HBSP or a control scrambled peptide was administered s.c., three times a week, to DCM hamsters (J2N-k) for 6 mo, although by week 5 there is already significant myocardial injury in this genetic mutant (19). Administering HBSP resulted in a significant decrease in the number of apoptotic cardiomyocytes in the cardiac sections compared with the control scrambled peptide, as assessed by in situ TdT assay (Fig. 4). Considering the data obtained from the in vitro experiments for signaling pathways of cell survival, we evaluated activation of Akt and ERK1/2 in cardiac tissue sections. Activation of both Akt and ERK1/2 was more prominent in the cardiac sections from animals treated with HBSP compared with those treated with the control scrambled peptide (Fig. 5). To investigate the effect of HBSP on myocardial injury, we measured serum CK activity, which is markedly elevated in J2N-k hamsters (19). There was a significant decrease in CK activity in animals treated with HBSP (Fig. 6A). Next, we assessed whether HBSP has an effect on the expression of ANP, one of prognostic markers of CHF (20). Densitometric analysis of Western blots showed a significant decrease of ANP expression in the cardiac tissues from animals treated with HBSP (Fig. 6B).
Fig. 4.
Effect of HBSP on cardiomyocyte apoptosis in the DCM hamster. After drawing blood, the heart was transversely cut and the basal half was fixed with 10% buffered formalin and embedded in paraffin. Next, the heart was sectioned and in situ TdT assay was performed as described in Materials and Methods. Mean ± SEM, n = 12 each group; *P < 0.01.
Fig. 5.
Survival signaling pathways in cardiomyocytes of the DCM hamster. (A) Activation of Akt. The cardiac sections were incubated with a primary antibody against phospho-Akt or negative-control IgG and immunostaining of the deparaffinized sections was performed. The number of cardiomyocytes positive for phospho-Akt was determined as described in Materials and Methods. Mean ± SEM negative control IgG, n = 6; control and HBSP, n = 12; *P < 0.01 vs. control; #P < 0.01 vs. control and HBSP. (B) Activation of ERK1/2. The cardiac sections were incubated with a primary antibody against phospho-ERK1/2 or negative-control IgG. Immunostaining and determination of the number of cardiomyocytes positive for phospho-ERK1/2 was performed in the same manner as in A. Mean ± SEM negative-control IgG, n = 6; control and HBSP, n = 12; *P < 0.05 vs. control; #P < 0.05 vs. control and HBSP. The SEM bars for negative control IgG are not drawn because of small SEM.
Fig. 6.
Decrease in serum CK activity and ANP down-regulation in cardiomyocytes of the DCM hamster. (A) Decrease in serum CK activity in the DCM hamster. Blood was drawn from the heart and serum CK activities were measured with a CPKII Test-Wako kit. Mean ± SEM, n = 10 each group; *P < 0.01. (B) ANP down-regulation in cardiomyocytes of the DCM hamster. Protein (200 μg) was extracted from the frozen cardiac tissues and Western blot was performed as described in Materials and Methods using an antibody against ANP or troponin I (a loading control). Immunoreactive bands were analyzed by NIH Image 1.62 and ratios of densitometric analysis (ANP/troponin I) were calculated. Mean ± SEM, n = 7 each group; *P < 0.05.
With respect to involvement of TNF-α in developing CHF in the DCM hamster, we assessed the levels of TNF-α expression in cardiac tissues by Western blot. Densitometric analysis, as shown by the ratio of TNF-α/troponin I, showed no significant difference between the two groups (control, 0.94 ± 0.05 vs. HBSP, 0.91 ± 0.04; n = 7 each group). In these animals, in which therapy began after the onset of myocardial damage, there were no significant differences in body weight at death (control, 124.6 ± 5.6 g vs. HBSP, 127.2 ± 3.3 g) and body weight change during administration of peptides (control, 28.6 ± 6.0 g vs. HBSP, 31.5 ± 4.7 g; n = 12 each group). Survival rate at 6 mo did not differ significantly between the two groups (control, 17% vs. HBSP, 25%; n = 12 each group).
Discussion
The results obtained from the in vitro experiments directly show that HBSP, a nonerythropoietic, tissue-protective peptide mimicking the 3D structure of erythropoietin, inhibits cardiomyocyte apoptosis induced by TNF-α. This effect is comparable with that observed for EPO on a molar basis, and similarly obtained regardless of timing of treatment. Recent studies have demonstrated that EPO and TPCs, nonerythropoietic derivatives of EPO, protect a wide variety of tissues (21–24), including the heart, from I/R injury (16–18). These protective effects of EPO and TPCs have been shown in part to be mediated by preventing apoptosis (16–18, 24). Our data are consistent with previous findings observed for EPO and TPCs and confirm the antiapoptotic effect of HBSP on cardiomyocytes.
The molecular mechanisms by which HBSP provides the antiapoptotic effect on cardiomyocytes remain to be elucidated, although the signaling pathways of EPO have been studied extensively (17, 25, 26). We have demonstrated that both EPO and HBSP activate critical signaling pathways of cell survival in cardiomyocytes, and Akt has been shown to play a pivotal role in HBSP-mediated prevention of apoptosis induced by TNF-α. Recent work has shown that pharmacological inhibition of Akt abolishes the protective effects of EPO (17) and insulin (27) on I/R injury of the heart in animal models. Interestingly, selective overexpression of TNF-α in the mouse heart has been shown to reduce basal and insulin-stimulated Akt activation, which results in the development of a cardiomyopathy that closely mimics that observed in failing human hearts (28). Given these observations and the data obtained from our signaling experiments, it is likely that Akt is a key molecule for prevention of apoptosis in the heart and that its activators, including HBSP and EPO, provide the cytoprotective effects through an Akt-dependent pathway, in part by antagonizing the effects of TNF-α. With respect to timing of signaling activation, HBSP phosphorylates Akt, as well as ERK1/2, more rapidly than EPO. This rapid effect of HBSP compared with that of EPO could be explained by the difference in time-dependent molecular diffusion as HBSP (11 amino acids) is much smaller than EPO (165 amino acids).
Based on the data obtained from the in vitro experiments, we have assessed the cardioprotective effects of HBSP in vivo using a hamster strain of DCM, J2N-k (19). This DCM hamster shows clear evidence of cardiomyocyte necrosis and dropout by 5 to 6 wk of age. A compensatory increase in myocardial work and associated remodeling ultimately leads to cardiac dilatation and dysfunction, and a markedly elevated serum CK activity at about 20 wk of age. Death from CHF occurs at ≈43 wk of age. In this model, cardiac tissues expressed abundant TNF-α, as assessed by immunohistochemistry. In clinical settings, medical therapy is started after the onset of CHF symptoms. Therefore, we started administration of HBSP at 20 wk of age and terminated at 46 wk of age (6-mo administration). As expected, the results from the in situ TdT assay show a significant decrease of cardiomyocyte apoptosis in HBSP-treated animals. With respect to Akt and ERK1/2 signaling pathways, recent work has reported that baseline phosphorylation levels of Akt, as well as ERK1/2, in cardiac tissues are similar in the Golden hamster, a healthy control, and the UMX7.1 hamster, a subline of the BIO 14.6 cadiomyopathic hamster, the same as the J2N-k hamster used in the present study (29). Activation of Akt and ERK1/2 by HBSP in the heart was confirmed, and phosphorylation of Akt was more prominent compared with that of ERK1/2, suggesting the role of Akt in preventing apoptosis in vivo. In addition, serum CK activity (a marker of myocardial injury) in HBSP-treated animals exhibits a significant decrease compared with that in control animals. A major pathophysiologic component of myocardial injury is related to an increased work load of individual cardiomyocytes, which HBSP could attenuate because of ongoing cardiomyocyte salvage by preventing apoptosis. A direct beneficial effect of HBSP on myocardial function is also possible, as observed for CEPO (30), but was not assessed in the current experiments.
Considering the pleiotropic effects of EPO and TPCs (31–34), including anti-inflammatory effects (35, 36) other than antiapoptosis, HBSP may reduce myocardial injury by counteracting proinflammatory cytokines, such as TNF-α in chronic failing hearts. In view of antagonizing the effect of TNF-α, recent large-scale, multicenter trials of TNF-α antagonist in patients with moderate-to-severe CHF did not demonstrate any clinical benefits associated with the use of infliximab, a chimeric monoclonal antibody to TNF-α, or the soluble TNF-α antagonist etanercept (37, 38). However, these results do not rule out the possibility of beneficial effect of TNF-α antagonism in a select group of patients with CHF. Furthermore, chronic administration of HBSP results in a significant decrease in cardiac ANP expression in the DCM hamster, indicating a favorable prognosis in animals treated with HBSP (20), although survival rate did not show a significant difference between the two groups. In addition to the possibility that a different dosing paradigm may positively affect survival, beginning treatment much earlier before significant myocardial injury has occurred could improve survival rate. Further studies are needed to assess the outcome of different treatment protocols, consisting of different dosing paradigms of chronic HBSP administration and beginning earlier when myocardial injury is minimal.
In conclusion, using cardiomyocyte cultures and the DCM hamster, an animal model of CHF, we have shown that HBSP protects cardiomyocytes from apoptosis induced by TNF-α in vitro and in vivo. This cardioprotective action is mediated by activation of Akt. Chronic administration of HBSP shows the down-regulation of serum CK activity and of cardiac ANP expression in the DCM hamster, confirming the chronic protective effects of HBSP on the heart in addition to the acute cardioprotective effects previously observed for EPO and CEPO in I/R injury. Because HBSP does not have the adverse effects of EPO (e.g., hypertension and thrombosis), administration of HBSP may provide novel insights into therapeutic approaches that prevent the development and progression of CHF.
Materials and Methods
All experiments were approved by the Animal Use and Care Committees of Jichi Medical University and Takara Bio in accordance with the directives of the Guidelines for Proper Conduct of Animal Experiments of the Science Council of Japan.
Materials.
HBSP and a control peptide (a scrambled version of HBSP) were obtained from commercial manufacturers. Recombinant human EPO was purchased from Kyowa Hakko Kirin. Recombinant mouse TNF-α was obtained from R&D Systems. All other materials were from Sigma-Aldrich, except where indicated.
Cardiomyocyte Culture.
Neonatal rat cardiomyocytes were cultured, as previously described (39, 40), with some modifications. Briefly, heart ventricles were isolated from 1-d-old Sprague-Dawley rats and digested five times with 0.1% collagenase type II (Worthington) in Ads buffer (116 mM NaCl, 20 mM Hepes, 1 mM NaH2PO4, 5.5 mM glucose, 5.4 mM KCl, 0.8 mM MgSO4; pH 7.35) at 37 °C for 20 min. The dispersed cells from each digestion were combined and purified by centrifugation through a discontinuous Percoll gradient of 1.050, 1.060, and 1.082 g/mL, respectively. The cells at the 1.060/1.082 g/mL interface were collected and used for cardiomyocyte cultures. The purified cell cultures were plated on 60-mm dishes (2 × 106 cells per dish) or 6-well plates (8 × 105 cells per well) in minimum essential medium supplemented with 5% calf serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The percentage of cardiomyocytes in the cultures was >95%, as assessed by immunohistochemistry with an anti-troponin I antibody (Santa Cruz Biotechnology). After 12-h incubation at 37 °C in humidified air with 5% CO2, serum was reduced to 0.5% and the cultures were subjected to various analyses.
Determination of Apoptosis by Flow Cytometric Analysis.
TNF-α (50 pg/mL) rather than oxygen deprivation was used to induce apoptosis for mimicking the clinical situation observed in patients with CHF (11–15), whose circulating TNF-α levels have been reported to be elevated to ≈30 to 70 pg/mL (8–10). Apoptosis of cardiomyocytes was evaluated by using the TACS Annexin V-FITC Apoptosis Detection Kit (R&D Systems) according to the manufacturer's instructions. Briefly, 5 × 105 cells were harvested by trypsinization, washed in cold PBS, and stained with FITC-conjugated annexin V and propidium iodide for 15 min at room temperature in the dark. The percentage of positive cells determined over 10,000 events acquired was analyzed by a FACSCalibur system equipped with a 488-nm argon laser and CellQuest software (BD Biosciences).
Small Interfering RNA Transfection.
Small interfering RNAs targeting Akt1 and ERK1 were purchased from Vicgene. Negative control (scrambled) siRNA was obtained from Ambion. Cardiomyocytes were transfected with the siRNA targeting Akt1 or ERK1, or scrambled siRNA at a concentration of 20 nM with a Silencer siRNA Transfection II Kit (Ambion). TNF-α, TNF-α and HBSP, or vehicle was added in the cultures 24 h after transfection and the cells were incubated for an additional 12 h. Next, the cells were harvested and apoptosis was evaluated by flow cytometric analysis. The percentage of target-gene knockdown at the protein level was ≈70%, as assessed by the KDalert GAPDH Assay Kit (Ambion) according to the manufacturer's instructions.
In Vivo Experimental Protocol.
Male J2N-k hamsters (20 wk of age, 95.8 ± 1.4 g), were purchased from Japan SLC and bred at Takara Bio. J2N-k is a novel hamster strain of DCM and one of the best animal models for X-linked DCM and many DCMs in humans (19). HBSP or a control scrambled peptide was administered to J2N-k hamsters (n = 12 each group) by s.c. injection at a dosage of 30 μg/kg body weight, three times a week for 6 mo. Before being killed, animals were given an overdose of sodium pentobarbital and blood was drawn from the heart. Next, the heart was removed and transversely cut at the center between the atrioventricular groove and the apex. The basal half was fixed with 10% buffered formalin for immunohistochemistry and the rest was frozen by dry ice for Western blot analysis. Serum CK activities from blood samples were measured with a CPKII Test-Wako kit (Wako) according to the manufacturer's instructions.
Immunohistochemistry and in Situ Apoptosis Detection.
Cardiac sections (3-μm) from paraffin-embedded tissue were incubated with a primary antibody against phospho-Akt, or phospho-ERK1/2 (Cell Signaling Technology). A LSAB+ Kit (DAKO) was used for the immunostaining of the deparaffinized sections with diaminobenzidine as the chromogen and nuclei were counterstained with hematoxylin. In situ TdT assay was performed to detect apoptotic cells using an ApopTag Plus In Situ Apoptosis Detection Kit (Chemicon) according to the manufacturer's instructions. Positive-control sections were from rat mammary gland. The number of cardiomyocytes positive for phospho-Akt, phospho-ERK1/2 or TdT labeling was determined in a blinded fashion by counting an average of >400 cells in each section on a digital microscopic system (BX51/DP72, Olympus) at 400× magnification.
Western Blot Analysis.
Antibodies used were as follows: anti-phospho-Akt, anti-phospho-ERK1/2, anti-phospho-STAT3 and anti-Akt (Cell Signaling Technology); anti-ERK2, anti-ANP, anti-TNF-α and anti-troponin I (Santa Cruz Biotechnology); anti-STAT3 (Upstate Biotechnology). Cardiomyocytes were treated with HBSP, EPO, or vehicle, and then cell lysates were prepared as previously described (41). Cell lysates were subjected to SDS/PAGE, and transferred to nitrocellulose membranes (Amersham Biosciences). The membrane was blocked for 1 h at room temperature with PBS containing 2% BSA and 0.05% Tween 20. The blots were incubated overnight at 4 °C or for 4 h at room temperature with a primary antibody against phospho-Akt, phospho-ERK1/2 or phospho-STAT3, followed by incubation for 1 h with a secondary, horseradish peroxidase-conjugated antibody. Then the blots were reprobed with an antibody against Akt, ERK2, or STAT3, respectively, to show equal protein loading. Immunoreactive bands were visualized using ECL (Amersham Biosciences). For the frozen cardiac tissues from the in vivo study, 200 μg of protein was extracted from tissues and Western blot was performed as mentioned above using a primary antibody against ANP, TNF-α, or troponin I. Densitometric analysis of immunoreactive bands was performed using NIH Image 1.62 to assess the levels of protein expression.
Statistical Analysis.
Data are shown as mean ± SEM. Differences were analyzed with paired-t tests between two groups, and one-way analysis of variance (ANOVA) among more than three groups. Values of P < 0.05 were considered statistically significant.
Acknowledgments
We thank Kazuko Futaka and Kimiko Aoki for expert technical assistance. This work was funded in part by Grants-in-Aid for Scientific Research 20590887 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to H.U. and M.K.).
Footnotes
Conflict of interest statement: A.C., M.B., and M.Y. are or have been employees of Warren Pharmaceuticals and Araim Pharmaceuticals, which are developing tissue-protective compounds. These authors also own stock or stock options in these companies.
*This Direct Submission article had a prearranged editor.
References
- 1.Fisher JW. Erythropoietin: Physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 2.Brines M, Cerami A. Erythropoietin-mediated tissue protection: Reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 3.Brines M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common β-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varet B, Casadevall N, Lacombe C, Nayeaux P. Erythropoietin: Physiology and clinical experience. Semin Hematol. 1990;27(3) Suppl 3:25–31. [PubMed] [Google Scholar]
- 5.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–2829. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 6.Leist M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 7.Brines M, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci USA. 2008;105:10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurray J, Abdullah I, Dargie HJ, Shapiro D. Increased concentrations of tumour necrosis factor in “cachectic” patients with severe chronic heart failure. Br Heart J. 1991;66:356–358. doi: 10.1136/hrt.66.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz SD, et al. Pathophysiological correlates of increased serum tumor necrosis factor in patients with congestive heart failure. Relation to nitric oxide-dependent vasodilation in the forearm circulation. Circulation. 1994;90:12–16. doi: 10.1161/01.cir.90.1.12. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari R, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–1486. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- 11.Torre-Amione G, et al. Tumor necrosis factor-α and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 12.Habib FM, et al. Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–1155. doi: 10.1016/s0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M, et al. Inducible nitric oxide synthase and tumor necrosis factor-alpha in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol. 1997;29:716–724. doi: 10.1016/s0735-1097(96)00567-0. [DOI] [PubMed] [Google Scholar]
- 14.Krown KA, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota T, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 16.Calvillo L, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsa CJ, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiordaliso F, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuhashi S, et al. Defect of delta-sarcoglycan gene is responsible for development of dilated cardiomyopathy of a novel hamster strain, J2N-k: Calcineurin/PP2B activity in the heart of J2N-k hamster. J Biochem. 2003;134:269–276. doi: 10.1093/jb/mvg140. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:1534–1539. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 21.Brines ML, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erbayraktar S, et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci USA. 2003;100:6741–6746. doi: 10.1073/pnas.1031753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong H, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004;66:683–695. doi: 10.1111/j.1523-1755.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 24.Salahudeen AK, et al. Antiapoptotic properties of erythropoiesis-stimulating proteins in models of cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F1354–F1365. doi: 10.1152/ajprenal.00131.2008. [DOI] [PubMed] [Google Scholar]
- 25.Sirén AL, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittorf T, Büchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: Structural requirements and biological significance. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 27.Jonassen AK, Sack MN, Mjøs OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi Y, et al. Cardioprotection afforded by NF-kappaB ablation is associated with activation of Akt in mice overexpressing TNF-α. Am J Physiol Heart Circ Physiol. 2006;290:H590–H598. doi: 10.1152/ajpheart.00379.2005. [DOI] [PubMed] [Google Scholar]
- 29.Miyata S, et al. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon C, et al. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 32.Ransome MI, Turnley AM. Systemically delivered Erythropoietin transiently enhances adult hippocampal neurogenesis. J Neurochem. 2007;102:1953–1965. doi: 10.1111/j.1471-4159.2007.04684.x. [DOI] [PubMed] [Google Scholar]
- 33.Harder Y, et al. Erythropoietin reduces necrosis in critically ischemic myocutaneous tissue by protecting nutritive perfusion in a dose-dependent manner. Surgery. 2009;145:372–383. doi: 10.1016/j.surg.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Erbayraktar Z, et al. Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing. Mol Med. 2009;15:235–241. doi: 10.2119/molmed.2009.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villa P, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman TR, et al. Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities. Proc Natl Acad Sci USA. 2006;103:5965–5970. doi: 10.1073/pnas.0601377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti-TNF Therapy Against Congestive Heart Failure Investigators Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 38.Mann DL, et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 39.Iwaki K, Sukhatme VP, Shubeita HE, Chien KR. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990;265:13809–13817. [PubMed] [Google Scholar]
- 40.Tamamori-Adachi M, et al. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res. 2003;92:e12–e19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 41.Ueba H, et al. Glimepiride induces nitric oxide production in human coronary artery endothelial cells via a PI3-kinase-Akt dependent pathway. Atherosclerosis. 2005;183:35–39. doi: 10.1016/j.atherosclerosis.2005.01.055. [DOI] [PubMed] [Google Scholar]