Abstract
Myotoxins play a major role in the pathogenesis of the envenomations caused by snake bites in large parts of the world where this is a very relevant public health problem. We show here that two myotoxins that are major constituents of the venom of Bothrops asper, a deadly snake present in Latin America, induce the release of large amounts of K+ and ATP from skeletal muscle. We also show that the released ATP amplifies the effect of the myotoxins, acting as a “danger signal,” which spreads and causes further damage by acting on purinergic receptors. In addition, the release of ATP and K+ well accounts for the pain reaction characteristic of these envenomations. As Bothrops asper myotoxins are representative of a large family of snake myotoxins with phospholipase A2 structure, these findings are expected to be of general significance for snake bite envenomation. Moreover, they suggest potential therapeutic approaches for limiting the extent of muscle tissue damage based on antipurinergic drugs.
Keywords: muscle damage, phospholipase A2, C2C12 cells
Snakes of the genus Bothrops are responsible for the majority of snakebite envenomations occurring in Latin America, from southern Mexico to northern Argentina (1–5). These envenomations are characterized by anatomical and pathophysiological alterations, which include prominent local tissue damage and major systemic disturbances that may lead to death (1, 2, 6). Common and abundant components of Bothrops venoms are myotoxins that adopt the fold of phospholipases A2 (PLA2) and play a major role in the pathogenesis of local tissue damage (6–9). These myotoxins are responsible for local myonecrosis, inflammation, and pain (6, 8). Venom PLA2s found in snakes of the family Viperidae are classified within the structural group IIA, with subunits of 121–122 amino acid residues characterized by a specific pattern of disulfide bonds (9, 10). Among them, two subgroups can be distinguished. One consists of enzymatically active PLA2s with a characteristic Asp49, a key residue for catalysis. The other subgroup includes proteins with a conserved PLA2 fold but devoid of PLA2 activity because the catalytically essential Asp49 has been replaced with Lys or other amino acids. Additional changes involve residues forming the Ca2+-binding loop, and members of this subgroup are therefore termed PLA2 homologs (6, 7, 10–12). Asp49 PLA2 myotoxins depend on their enzymatic activity to induce skeletal muscle fiber damage (6). In contrast, the catalytically inactive Lys49 PLA2 homologs use, as major determinant of toxicity, a C-terminal region (residues 115–129), which presents a variable combination of cationic and hydrophobic/aromatic residues and forms membrane pores (11–13). Both types of myotoxins cause a large influx of Ca2+ in muscle cells, which triggers a cascade of events such as loss of mitochondrial function, widespread proteolysis, myofibrillar hypercontraction, and additional degenerative events that still await a detailed description (6, 14). We recently reported that a Lys49 Bothrops myotoxin is more direct and rapid in its cytotoxic action than its Asp49 counterpart, although they both eventually cause cell death (15).
To gain further insight into the events involved in the pathogenesis of tissue injury caused by these myotoxins, we studied the effects of Bothrops asper myotoxins (Mt-I, an Asp49 myotoxin, and Mt-II, a Lys49 myotoxin) because they are representative of myotoxins present in many Bothrops spp., as well as in many other species belonging to different snake genera (11). The action of Lys49 myotoxins has been extensively studied in various animal models, in isolated muscle preparations, and in cells in culture. The key role of the Ca2+ entry from the extracellular medium into the cell, following a steep concentration gradient, is one of the best-characterized consequence of the rapid plasma membrane perturbation induced by these toxins (6, 15–17). However, no attention has been paid so far to the possible role of the efflux of cytosolic molecules that may act as alarm signals or amplifiers of the damage. Here we have focused on two major extracellular signaling molecules: ATP and K+ ions, which are well known to trigger a variety of pathophysiological reactions (18–21).
Using muscle cells in culture and isolated muscles, we have found that Bothrops myotoxins induce a very rapid efflux of K+ and ATP, which well accounts for the strong pain commonly reported after Bothrops bites (1). The strong structural similarities between B. asper myotoxins and related toxins produced by other snake species suggest that the present findings may have general relevance in the context of snakebite envenomation.
Results
Bothrops Lys49 Myotoxin Induces Rapid and Extensive Loss of K+ and ATP from C2C12 Muscle Cells.
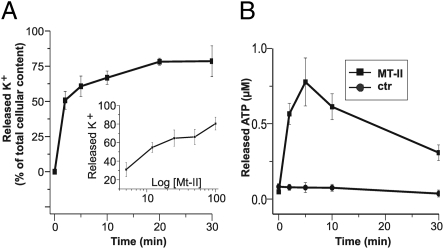
Figure 1A shows that the Bothrops asper Lys-49 Mt-II myotoxin induces C2C12 myotubes to release very rapidly their K+ content, and that this effect is dose dependent. Within 5 min of exposure to 50 μg/mL Mt-II, muscle cells have reduced their K+ content by 60% (Fig. 1A). It should be noted that this myotoxin concentration is probably lower than that reached in vivo close to the injection site if one considers that B. asper injects 50–75 mg of venom proteins in a bite and that myotoxins comprise ∼20% of venom weight. The effect of the myotoxin was dose dependent, and the half maximal effect was reached after 10 min with a Mt-II concentration of 12.5 μg/mL, which corresponds to ∼1 μM.
Fig. 1.
The Bothrops asper Lys49 Mt-II myotoxin induces C2C12 myotubes to rapidly release K+ and ATP in a dose-dependent mode. Murine C2C12 cells were plated on a 24-well plate and differentiated to myotubes for 5–7 d. After washing with modified Krebs-Ringer medium, Mt-II toxin was added to a final concentration of 50 μg/mL (medium was added to control samples) and incubated at 37 °C. At various time points, the supernatants were recovered and transferred to ice, and the cells were rapidly washed with the choline buffer (Materials and Methods) and lysed in choline buffer containing 0.5% Triton X-100. (A) Time course of toxin-induced release of K+, measured by atomic adsorption in cell lysates and expressed as percentage with respect to the total potassium content of untreated cells. (Inset) Dose dependence of K+ release after treatment with toxin for 10 min. (B) Time course of ATP released from intoxicated (■) or control (●) cells, expressed as ATP concentration in the medium, measured with a luciferase assay. Data are the average of four independent experiments; bars represent SD values.
The Mt-II myotoxin also induced a rapid ATP release from C2C12 muscle cells; the extracellular ATP level continued to increase for several minutes after toxin addition and then decreased, presumably owing to the action of ecto-ATP hydrolases (22, 23). The kinetics of K+ and ATP release from cells indicates that both Mt-II binding to the sarcolemma and alteration of its permeability to ions are very rapid events, and faster than those caused by bacterial pore-forming toxins (24–26).
ATP is an extracellular danger signal molecule (19, 27) that acts by binding to a variety of ATP purinergic receptors, which appeared early in evolution (28). The purinergic receptors of the P2X family is a cation-selective channel that is opened by ATP and allows the transmembrane passage of K+, Na+, and Ca2+ along their concentration gradients (19, 28). As purinergic receptors are present in murine muscle cells (29, 30), this led us to consider the possibility that the ATP released by muscle cells close to the site of toxin injection may amplify the toxin-induced damage by binding to neighboring cells with a consequent induction of Ca2+-overload toxicity and further release of K+ ions.
Released ATP Induces Spreading of Ca2+ Entry into Cells Close to Site of Lys-49 Myotoxin Cell Injection.
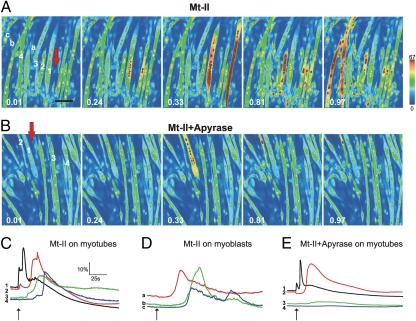
To test the possibility that the ATP released from myotoxin-damaged cells may spread around and induce Ca2+ entry into neighboring muscle fibers, we challenged C2C12 myotubes and myoblasts (loaded with the cytosolic Ca2+ indicator Oregon Green-AM) with a pulse of toxin through an adjacent patch-clamp micropipette (∼1-μm tip diameter) containing Mt-II. This experimental setting is different from that presented in Fig. 1, where the myotoxin was simply added to the medium, and is closer to the in vivo situation where the venom is released from the tip of the snake fangs into a limited region of the bitten muscle (31). The red arrows in Fig. 2 A and B indicate the position of the micropipette tip, wherefrom a pulse of Mt-II (100–500 pg) was released. This would correspond to an average concentration in the medium of 0.1–0.5 μg/mL, which is not by itself sufficient to trigger any release of ATP (Fig. 1). Figure 2 A and B are taken from two movies recorded by a confocal microscope that measures the emission fluorescence of the cytosolic Ca2+ indicator present within the C2C12 muscles cells (Movie S1). The pattern of cytosolic Ca2+ increase (given in a pseudocolor scale increasing from blue to red) within neighboring myoblasts and myotubes is particularly informative. The myotubes close to the micropipette tip increased their fluorescence immediately after toxin release (Fig. 2C, trace 1), indicating an intracellular Ca2+ rise induced directly by the toxin; additional myotubes then lighted up with time delays increasing with their distance from the toxin injection site (Fig. 2 A and C, traces 2– 4, and Movie S1). This latter increase could originate from the myotoxin diffusing out from the site of injection and from ATP released by the myotubes directly hit by the myotoxin, or from both effects. Myoblasts close to the injection site remained blue, in agreement with previous findings that they are Mt-II insensitive (15, 16); however, their cytosolic Ca2+ did increase at later times (Fig. 2 A and D, traces a–c). The most likely explanation is that this late rise is a consequence of the action of ATP, released by myotubes, on myoblast purinergic channels. If this is the case, micropipette injected ATP should induce a similar pattern of rise of cytosolic Ca2+ in the the C2C12 cell culture. Movie S2 shows that this is the case. The role of released ATP as mediator is also supported by the analysis of culture plates treated with Mt-II myotoxin in the presence of apyrase (Fig. 2 B and E and Movie S3). Under these conditions only myotubes that can be directly reached by the toxin did light up (Fig. 2 B and E, traces 1 and 2), whereas apyrase activity quenched the ATP signal spreading to more distant myotubes (Fig. 2 B and E, traces 3 and 4).
Fig. 2.
Imaging and time course analysis of the cytosolic Ca2+ concentration of myotubes and myoblasts induced by a pulse of Bothrops asper Lys49 Mt-II toxin released from a micropipette. C2C12 muscle cells were grown on 13-mm coverslips coated with poly-L-lysine and collagen. Cells were loaded with the Oregon Green 488 BAPTA-1 acetoxymethyl ester Ca2+ indicator. The 1-μm tip of a micropipette, filled with Mt-II (5 mg/mL in Hepes 10 mM, NaCl 150 mM, and 50% glycerol) was placed in the position indicated by the red arrow. The toxin was released from the micropipette tip by applying a positive pressure that caused the release of 20–100 pL. (A and B) Videoframes taken at indicated time points (minutes) from Movie S1 and Movie S3, respectively, of cultures treated with the Mt-II myotoxin and containing apyrase (B) or not containing apyrase (A). Corresponding time courses of cytosolic Ca2+ concentration of some myotubes (indicated by numbers in A and B) and myoblasts (indicated by lower-case letters in A) are given in C and D (Mt-II alone) and E (Mt-II plus apyrase). Scale for time courses of Ca2+ changes in C–E is given in C, with respect to basal fluorescence signal (ΔF/Fo). Toxin addition is indicated on traces by black arrows. (Scale bar, 100 μm.)
We showed previously that the snake myotoxins, including the Mt-II myotoxin, caused cell death of myotubes in cultured C2C12 cells (15), and this was evident also in the present study, as shown in Fig. S1 A and B. The large protective effect exerted by apyrase (Fig. S1 C and D) provides a further evidence for the role of ATP.
These results unravel a previously uncharacterized aspect of great significance in the action of B. asper Lys-49 Mt-II myotoxins, as the results show that this myotoxin may damage muscle cells at a distance from the injection site via an indirect action mediated by the ATP released from cells directly hit by the venom, i.e., those close to the tip of the snake fangs. This event can rapidly expand the anatomical dimensions of the direct myotoxin effect and, consequently, the ability of the snake to capture prey.
Bothrops Lys49 Myotoxin Induces ATP and K+ Release from Murine Muscles.
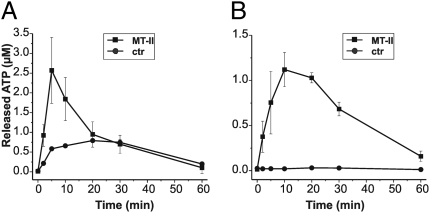
To further explore the hypothesis that ATP is indeed released from myotoxin-treated muscle and acts as a toxic mediator, we extended our studies to skeletal muscle. Different mouse hind leg muscles were isolated and injected with Mt-II or, alternatively, the toxin was added to the medium. Figure 3A shows that Mt-II injection caused ATP release from tibialis anterior muscle, whereas the untreated controls released smaller, although significant, amounts of ATP, most likely a consequence of tissue damage caused by the injection procedure. Indeed, when the toxin was added to the bathing medium, the toxin was markedly less effective in inducing ATP release, but no effect was seen in the mock-treated muscle (Fig. 3B). The lower toxin efficacy under the latter conditions is probably due to several factors, including diffusion through connective tissue to reach its target. The myotoxin action was not specific for the tibialis muscle, as similar findings were obtained with extensor digitorum longus and soleus mouse muscles.
Fig. 3.
Bothrops asper Lys49 Mt-II myotoxin induces release of ATP from the mouse tibialis anterior muscle. (A) Adult muscles were exposed by gently dissecting the skin and injected with 50 mg Mt-II toxin in 10 μL vehicle or with the same volume of vehicle alone (Hepes 10 mM and NaCl 150 mM with 50% glycerol) using a 26-G gauge syringe. Muscles were rapidly removed from the animal and suspended in 1 mL oxygenated physiological buffer (Materials and Methods) at 37 °C. Time course of release of ATP in medium was measured by taking, at different time points, small samples that were assayed with the luciferase assay. (B) Alternatively, muscles were dissected and suspended in the same oxygenated buffer for at least 15 min; 50 μg/mL Mt-II toxin was then added to one muscle, and the contralateral muscle was maintained in buffer and used as control. Medium ATP was similarly measured at indicated time points. Data are average of values obtained in three different experiments ± SD.
Five tibialis anterior muscles were assayed for their K+ content by atomic absorption of cryo-crushed and resuspended samples; muscles were found to contain 3.5 ± 0.3 μg K+/mg fresh tissue. Incubation of isolated muscle in choline-containing medium caused the release of 25 ± 3% of its K+ content after 15 min, whereas the presence of the Mt-II myotoxin in the medium caused release of ∼50 ± 4% of the initial content. These results strongly suggest that, following a snake bite, the K+ concentration may reach high values in the extracellular fluids before returning to the basal level as a result of lymphatic and blood circulation. Under such conditions of high, if transient, K+ concentrations, sensory nerves are well known to be stimulated and to relay a strong sensation of pain (21).
Purinergic P2X Channels Are Involved in Spreading of Lys49 Mt-II Myotoxin-Induced Damage.
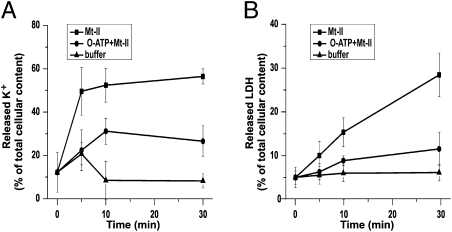
It is difficult to discern the individual contribution of the many purinergic channel isoforms by pharmacologic approaches (28, 32). Oxidized ATP (o-ATP) can be considered, in a pure culture of muscle cells, a specific inhibitor of P2X receptors with a preference for the P2X7 isoform. ATP binding to the P2X7 purinergic receptor opens a channel of high conductance for K+, Na+, and Ca2+ (18, 19, 28). Figure 4A shows that o-ATP inhibited the toxin effect, indicating that the toxin action is in part indirect and can be ascribed to an ATP-induced K+ release mediated by P2X channels. Figure 4 B shows the result of the assay of the amount of lactate dehydrogenase (LDH) released by the Mt-II–treated muscle cells, which suggests large, irreversible muscle damage, paralleling the cell death noticed in video imaging (Fig. S1). LDH release induced by myotoxins was used in previous studies as a marker of toxin myotoxicity (5). Considering the large size of the tetrameric LDH complex, it is not surprising that the time course of its release in the medium (Fig. 4B) was slower than that of K+ (Fig. 4A) and did not reach a plateau within 0.5 h. The finding that the P2X inhibitor o-ATP largely prevented the release of LDH (Fig. 4B) is an additional indication that cell damage is due in part to the indirect effect described above. Perhaps more importantly, this result indicates that some P2X inhibitors should be considered for their potential therapeutic value in human envenomings.
Fig. 4.
Oxidized ATP (o-ATP) inhibits K+ and LDH release from C2C12 cells. C2C12 myotubes were treated as described in Fig. 1 legend under three conditions: Mt-II at a final concentration of 12.5 μg/mL (■) in modified Krebs-Ringer medium, Mt-II 12.5 μg/mL with the additional presence of o-ATP 300 μM (●), and medium alone (▲). At given time points, K+ content of cells was measured as in Fig. 1. LDH activity was determined in both the supernatant and lysate; for each well, the sum of the two values was taken as 100% and the released LDH expressed as percentage of the total amount. Points are average of values obtained in four different experiments ± SD.
Bothrops Asp-49 Myotoxin Also Induces ATP and K+ Release from Muscle Cells.
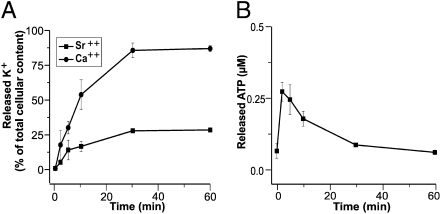
Another abundant pathogenic component of the venom of Bothrops snakes is the Asp-49 myotoxin Mt-I, which is an active PLA2 enzyme (33). Mt-I also was capable of inducing the release of K+ and ATP from C2C12 myotubes in culture (Fig. 5) and from mouse muscles (Fig. S2). The time course of K+ release was slower than that induced by the Lys-49 Mt-II myotoxin, in agreement with the different kinetics of Ca2+ entry reported previously (15). This is likely due to the fact that the Mt-I alteration of the sarcolemma is mediated by PLA2 phospholipid hydrolysis, which in turn requires some time to produce enough lysophospholipids and fatty acids to increase sarcolemmal permeability. Snake PLA2 toxins are highly dependent on Ca2+ for their enzymatic activity and are inhibited by Sr2+ (34,35). Indeed, the substitution of Sr2+ for Ca2+ in the medium largely prevented K+ release (Fig. 5A). On the other hand, the amount of ATP released by the PLA2 Mt-I myotoxin was less than that induced by the Lys49 Mt-II myotoxin and peaked at earlier time points. This rapid small ATP release has the features of a pool of vesicle-stored ATP that could be induced to exocytose by the Mt-I toxin. ATP is stored in vesicles in a variety of excitatory and nonexcitatory cells (36), and this mechanism would make PLA2 snake myotoxins very similar to the snake presynaptic PLA2 neurotoxins that induce exocytosis of synaptic vesicles from neurons via the action of lysophospholipids and fatty acids (37,38).
Fig. 5.
Bothrops asper Asp49 Mt-I myotoxin induces release of K+ and ATP from murine C2C12 muscle cells, dependent on its PLA2 activity. Cells were treated in Krebs-Ringer medium with 50 μg/mL (final concentration) of Mt-I myotoxin. (A) Time course of the loss of K+ from cells in normal medium (●) or in a medium where Ca2+ was replaced by an equal concentration of Sr2+ (squares), which is an inhibitor of the PLA2 activity of Mt-I, expressed as percentage of untreated controls taken as 100%. (B) Time course of the rise of the ATP concentration in medium. Points are the average of values obtained in three different experiments ± SD.
Discussion
Snakebite envenomation is a major, although neglected, health problem in many parts of the world, particularly in Africa, Asia, and Latin America (1–5, 39). In addition to lethality, one of the most serious consequences of these envenomations, particularly in the case of viperid and some elapid snakes, is associated with prominent tissue damage leading to permanent sequelae such as tissue loss and dysfunction, with important physical, psychological, and social consequences (2, 5). Many snakes produce venoms that include myotoxins as major determinants of their pathogenic action; these protein toxins damage the plasma membrane of muscle cells, causing myonecrosis (6, 14).
The present report provides a major advancement in the understanding of the pathogenesis of the muscle necrosis caused by these myotoxins and in the pain reaction characteristic of snakebite envenomation. Indeed we have shown that these myotoxins, in addition to inducing Ca2+ entry with the consequent Ca2+-mediated cell toxicity previously characterized (15–17), also promote an even faster efflux of K+ ions. At the same time, the toxin-damaged muscle fibers release ATP into the extracellular environment. These ATP molecules spread around the primarily damaged fibers, bind to the muscle P2X purinergic receptors (28), and induce Ca2+ and Na+entry and K+ efflux in cells that have not been directly hit by the myotoxins themselves. It appears that an important role is played by the P2X7 channel, but further studies are needed to address this point.
The present data also highlight the different mechanisms of sarcolemmal damage by the Lys49 and Asp49 myotoxins (15). Indeed, the fast time course and lower extent of ATP release induced by the Asp49 Mt-I myotoxin endowed with PLA2 activity suggests that ATP derives from a vesicular pool that is rapidly induced to exocytose by the local changes of lipid composition of the membrane, as previously defined for the snake PLA2 neurotoxins (37, 39). In the same samples, the release of K+, which derives from the cytosol, follows the slower time course of the increased membrane permeability to extracellular Ca2+ (15, 40). On the other hand, the Lys49 Mt-II myotoxin is believed to form a dimeric membrane pore that is large enough to mediate the passage of K+, Ca2+, and ATP, as it allows the passage of calcein and other rather large molecules (7, 10–12).
The present work represents a paradigm shift for the mechanism of action of these snake myotoxins. Besides direct plasma membrane damage, our findings reveal an indirect, ATP-mediated mechanism through which muscle cells are affected. Indeed, inhibition by o-ATP and by apyrase indicates that the indirect, ATP-based mechanism of damage plays a major role in the overall myotoxicity induced by the Bothrops Lys-49 myotoxin. Accordingly, the anatomical region reached by the activity of these myotoxins may extend well beyond the limited physical volume where they are injected by the snake fangs, resulting in a muscle damage that is much larger than that directly caused by the myotoxins. This contributes to explain systemic effects mediated by interleukins (8) because K+ efflux and extracellular ATP are activators of the inflammasome (41) and muscles have very recently been shown to possess inflammasome (42). It appears that these myotoxins induce a disease with features of a systemic inflammatory response syndrome (43). This mechanism of amplification sheds light on the evolutionary significance of the presence of these myotoxins in the venoms.
Immediate pain after venom injection is characteristic of viperid snake bites, and myotoxic PLA2s play a significant role in pain induced by B. asper venom (31). Previous studies have implicated several mediators in the onset of pain induced by these myotoxins (8, 44, 45). The present findings of K+ and ATP release provide a novel basis for the strong pain sensation reported by bitten individuals. Extracellular ATP and K+ are strong stimulants of peripheral sensory neurons (21, 46), and this reaction is predicted not to be limited to humans but to extend to a range of potential prey of viperid snakes, such as reptiles, amphibians, and birds, in addition to mammals. In fact, purinergic receptors are present in these animal classes (28), and K+-induced membrane depolarization is common to neurons of different origins (21). If one considers that pain has a strong immobilizing effect, it can be concluded that the snake myotoxins induced release of ATP and K+ (and possibly other mediators as well) contributes to prey immobilization and ingestion, providing an evident evolutionary advantage for the presence of myotoxins in snake venoms.
The present data also provide an additional explanation to the finding that the antitrypanosomal drug suramin protects from the toxic effects of the Lys49 myotoxins of the Bothrops jararacussu and Bothrops asper venoms (47). This was explained previously as a direct neutralization of the myotoxin by formation of a suramin-myotoxin complex (48). However, suramin also binds to P2X channels (32), and this property could, at least in part, account for suramin inhibitory activity because it would prevent the ATP-dependent spreading effect of the injected myotoxin. Furthermore, the suramin and the o-ATP effects indicate that the ATP-mediated indirect action of the myotoxin plays an important role in the overall extent of muscle tissue damage.
We conclude with two considerations of far-reaching consequences and of rather general interest. B. asper myotoxins Mt-I and Mt-II are prototypes of a large family of myotoxins present in many other viperid venoms. Therefore, the present findings could be extended to all snake myotoxins, although the potency and kinetics of action of these myotoxins may be different. It would be relevant to assess to what an extent this amplifying effect on tissue damage occurs in the muscles of potential prey other than rodents, to further reveal the adaptive biological impact of these observations. Furthermore, the present findings have significant potential implications for the therapy of these envenomations. Abrogation of acute muscle damage induced by viperid venoms and their purified myotoxins by the administration of antivenoms is difficult to achieve, mostly owing to the very rapid development of these effects (49, 50). The previously uncharacterized mechanism of amplification of cell damage presented in this study may be also responsible for this poor neutralization, as once ATP has been released from muscle cells, an amplification cascade of cellular damage is unleashed. Accordingly, this amplification effect may be reduced by the timely administration of antipurinergic drugs, thus opening possibilities for complementary therapies, a hypothesis that is currently being tested in animal models.
Materials and Methods
Myotoxins.
Myotoxins I and II were isolated from the crude venom of Bothrops asper, a pool obtained from at least 30 specimens kept at the serpentarium of Instituto Clodomiro Picado, University of Costa Rica, as described before (30, 51).
Cell Cultures.
Murine skeletal muscle C2C12 cells were obtained from the American Type Culture Collection (CRL-1772; ATCC), and were maintained at subconfluent levels in DMEM (Gibco) supplemented with 10% FBS (EuroClone). To induce differentiation (5–6 d), cells were grown to 80% confluence and then the medium was replaced with DMEM supplemented with 2% equine serum (Gibco) and changed every 24–48 h. For imaging analysis, cells were plated on coverslips, coated overnight with poly-L-lysine (Sigma) and for 2 h with collagen (BD Biosciences).
Muscle Isolation.
All experimental procedures were carried out in accordance to the European Communities Council Directive n° 86/609/EEC. Muscles (tibialis anterior, soleus, extensor digitorum longus) were isolated from CD-1 mice weighing 25–35 g and immediately transferred to vials containing 1 mL oxygenated (95% O2, 5% CO2) physiological buffer (139 mM NaCl, 12 mM NaHCO3, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM KH2PO4, and 11 mM glucose, pH 7.4) at 37 °C. Toxins were added to the physiological solution or injected in the muscles just before dissection, as indicated.
Potassium Measurement.
After treatments, cells were rapidly washed twice with a choline buffer (129 mM CholineCl, 1.5 mM CaCl2, 0.8 mM MgCl2, 5 mM H3PO4, 5 mM citric acid, and 5.6 mM glucose, pH 7.4) and then dissolved in 120 μL of the same buffer containing 0.5% wt/vol of Triton X100. Samples were diluted in bidistilled water and the K+ content was measured by flame photometry with a Perkin-Elmer AAnalyst atomic absorption photometer. The K+ content of control cells of the mock treated culture was taken as 100%.
ATP Measurement.
ATP was determined with the ATPlite luciferase assay (Perkin-Elmer). Briefly, the supernatant of control and intoxicated samples were collected in a white 96-well plate, and mammalian lysis solution was added. The plate was shaken for 5 min in an orbital shaker at 700 rpm at RT. ATP substrate solution was added and a 5-min shake was performed, protected from light. After 10 min, the luminescence was measured by Fluoroskan Ascent FL (Thermo Electron Corporation). The ATP concentration was calculated from a calibration curve obtained using ATP standard solutions.
LDH Measurement.
The release of LDH was measured as an index of cellular necrosis using the commercial kit TOX7 (Sigma), which is based on the LDH-catalyzed reduction of NAD+, which then converts a tetrazolium dye to a soluble colored formazan derivative.
Calcium Imaging.
Cells were plated on coverslips (13-mm diameter) and loaded with Oregon Green 488 BAPTA-1 acetoxymethyl ester (OGB-1 AM, 3 μM; Invitrogen.) by incubation at 37 °C for ∼30 min in modified Krebs–Ringer Buffer (described below) containing 0.04% pluronic (Molecular Probes). To prevent OGB-1 leakage and sequestration, 250 μM sulfinpyrazone was present throughout the loading procedure and [Ca2+] measurements. The coverslips were washed with a modified Krebs–Ringer buffer (140 mM NaCl, 2.8 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 11 mM glucose, pH 7.4), and emitted cell fluorescence at 530 nm was acquired with a TCS-SP5-RS confocal microscope (Leica) equipped with a 20× objective (NA, 1.0). Laser emission at 488 nm was used for stimulation of OGB-1. Time frame acquisition of 491 ms (with seven-line averaging) was used. Where indicated, apyrase (Sigma) was introduced (final concentration, 2 U/mL). The tip of a glass micropipette, filled with Mt-II myotoxin (5 mg/mL in Hepes 10 mM, NaCl 150 mM, and 50% glycerol), was placed at a 30-μm distance from the cell layer and a micropuff (0.3 bar of pressure and 2-s duration) was performed using a pressure ejection unit (PDES, NPI Electronics).
Supplementary Material
Acknowledgments
We thank Dr. P. Di Muro and Prof. M. Beltramini for the use of the atomic absorption spectrometer, Drs. D. Sandonà, O. Rossetto, and F. Tonello and Prof. F. Di Virgilio for discussions and suggestions, and Prof. P. Bernardi for critical reading the manuscript. M.C.F. was supported by a Ph.D. fellowship of the Fondazione CARIPARO. We gratefully acknowledge the support of Fondazione Cariparo Progetto “Physiopathology of the Synapse: Neurotransmitters, Neurotoxins and Novel Therapies” and of the Progetto Strategico of the University of Padova “An in Vivo Approach to the Physiopathology of Signal Transduction.” The work of Y.A., B.L., and J.M.G. is supported by Vicerrectoría de Investigación, Universidad de Costa Rica, and the ICGEB-CRP Program (Grant COS-08-03).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009128107/-/DCSupplemental.
References
- 1.Warrel D. Epidemiology, clinical features and management of snake bites in Central and South America. In: Campbell JA, Lamar WW, editors. The Venomous Reptiles of the Western Hemisfere. Ithaca: Cornell University Press; 2004. pp. 709–761. [Google Scholar]
- 2.Gutiérrez JM, Theakston RDG, Warrell DA. Confronting the neglected problem of snake bite envenoming: The need for a global partnership. PLoS Med. 2006;3:e150. doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan HW, Cardoso JL. Clinical toxicology of snakebites in South America. In: Meier J, White J, editors. Handbook of Clinical Toxicology of Animal Venoms and Poisons. Boca Raton: CRC Press; 1995. pp. 667–688. [Google Scholar]
- 4.World Health Organization . Rabies and envenomings: A neglected public health issue. Geneva: WHO; 2007. [Google Scholar]
- 5.Williams D, et al. Global Snake Bite Initiative Working Group; International Society on Toxinology The Global Snake Bite Initiative: An antidote for snake bite. Lancet. 2010;375:89–91. doi: 10.1016/S0140-6736(09)61159-4. [DOI] [PubMed] [Google Scholar]
- 6.Gutiérrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42:915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Lomonte B, Angulo Y, Calderón L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42:885–901. doi: 10.1016/j.toxicon.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42:947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Chioato L, Ward RJ. Mapping structural determinants of biological activities in snake venom phospholipases A2 by sequence analysis and site directed mutagenesis. Toxicon. 2003;42:869–883. doi: 10.1016/j.toxicon.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Lomonte B, Angulo Y, Sasa M, Gutiérrez JM. The phospholipase A2 homologues of snake venoms: Biological activities and their possible adaptive roles. Protein Pept Lett. 2009;16:860–876. doi: 10.2174/092986609788923356. [DOI] [PubMed] [Google Scholar]
- 12.dos Santos JI, Fernandes CAH, Magro AJ, Fontes MRM. The intriguing phospholipases A2 homologues: Relevant structural features on myotoxicity and catalytic inactivity. Protein Pept Lett. 2009;16:887–893. doi: 10.2174/092986609788923310. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosio ALB, et al. A molecular mechanism for Lys49-phospholipase A2 activity based on ligand-induced conformational change. J Biol Chem. 2005;280:7326–7335. doi: 10.1074/jbc.M410588200. [DOI] [PubMed] [Google Scholar]
- 14.Montecucco C, Gutiérrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell Mol Life Sci. 2008;65:2897–2912. doi: 10.1007/s00018-008-8113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cintra-Francischinelli M, et al. Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of acceptors. Cell Mol Life Sci. 2009;66:1718–1728. doi: 10.1007/s00018-009-9053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villalobos JC, Mora R, Lomonte B, Gutiérrez JM, Angulo Y. Cytotoxicity induced in myotubes by a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops asper: Evidence of rapid plasma membrane damage and a dual role for extracellular calcium. Toxicol In Vitro. 2007;21:1382–1389. doi: 10.1016/j.tiv.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Cintra-Francischinelli M, et al. The C-terminal region of a Lys49 myotoxin mediates Ca2+ influx in C2C12 myotubes. Toxicon. 2010;55:590–596. doi: 10.1016/j.toxicon.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- 21.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th Ed. New York: McGraw-Hill; 2000. [Google Scholar]
- 22.Plesner L. Ecto-ATPases: Identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 23.Gordon JL. Extracellular ATP: Effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacovache I, van der Goot FG, Pernot L. Pore formation: An ancient yet complex form of attack. Biochim Biophys Acta. 2008;1778:1611–1623. doi: 10.1016/j.bbamem.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte G, et al. Pore formation by Staphylococcus aureus alpha-toxin in lipid bilayers. Dependence upon temperature and toxin concentration. Eur Biophys J. 1987;14:349–358. doi: 10.1007/BF00262320. [DOI] [PubMed] [Google Scholar]
- 26.Skals M, Jorgensen NR, Leipziger J, Praetorius HA. α-Hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci USA. 2009;106:4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 29.Banachewicz W, Supłat D, Krzemiński P, Pomorski P, Barańska J. P2 nucleotide receptors on C2C12 satellite cells. Purinergic Signal. 2005;1:249–257. doi: 10.1007/s11302-005-6311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deli T, et al. Contribution from P2X and P2Y purinoreceptors to ATP-evoked changes in intracellular calcium concentration on cultured myotubes. Pflugers Arch. 2007;453:519–529. doi: 10.1007/s00424-006-0146-6. [DOI] [PubMed] [Google Scholar]
- 31.Campbell JA, Lamar WW. The Venomous Reptiles of the Western Hemisphere. Ithaca: Cornell University Press; 2004. p. 425. [Google Scholar]
- 32.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez JM, Ownby CL, Odell GV. Isolation of a myotoxin from Bothrops asper venom: Partial characterization and action on skeletal muscle. Toxicon. 1984;22:115–128. doi: 10.1016/0041-0101(84)90144-2. [DOI] [PubMed] [Google Scholar]
- 34.Strong PN, Goerke J, Oberg SG, Kelly RB. β-Bungarotoxin, a pre-synaptic toxin with enzymatic activity. Proc Natl Acad Sci USA. 1976;73:178–182. doi: 10.1073/pnas.73.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CC, Su MJ, Lee JD, Eaker D. Effects of Sr2+ and Mg2+ on the phospholipase A and the presynaptic neuromuscular blocking actions of beta-bungarotoxin, crotoxin and taipoxin. Naunyn Schmiedebergs Arch Pharmacol. 1977;299:155–161. doi: 10.1007/BF00498557. [DOI] [PubMed] [Google Scholar]
- 36.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 37.Rigoni M, et al. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- 38.Paoli M, et al. Mass spectrometry analysis of the phospholipase A(2) activity of snake pre-synaptic neurotoxins in cultured neurons. J Neurochem. 2009;111:737–744. doi: 10.1111/j.1471-4159.2009.06365.x. [DOI] [PubMed] [Google Scholar]
- 39.Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: A review. PLoS Negl Trop Dis. 2010 doi: 10.1371/journal.pntd.0000603. 10.1371/journal.pntd.0000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigoni M, et al. Calcium influx and mitochondrial alterations at synapses exposed to snake neurotoxins or their phospholipid hydrolysis products. J Biol Chem. 2007;282:11238–11245. doi: 10.1074/jbc.M610176200. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 42.Rawat R, et al. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol. 2010;176:2891–2900. doi: 10.2353/ajpath.2010.090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992;268:3452–3455. [PubMed] [Google Scholar]
- 44.Chacur M, et al. Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: Pharmacological mediation and molecular determinants. Toxicon. 2003;41:667–678. doi: 10.1016/s0041-0101(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 45.Chacur M, et al. Snake venom components enhance pain upon subcutaneous injection: An initial examination of spinal cord mediators. Pain. 2004;111:65–76. doi: 10.1016/j.pain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15:1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- 47.de Oliveira M, et al. Antagonism of myotoxic and paralyzing activities of bothropstoxin-I by suramin. Toxicon. 2003;42:373–379. doi: 10.1016/s0041-0101(03)00166-1. [DOI] [PubMed] [Google Scholar]
- 48.Murakami MT, et al. Inhibition of myotoxic activity of Bothrops asper myotoxin II by the anti-trypanosomal drug suramin. J Mol Biol. 2005;350:416–426. doi: 10.1016/j.jmb.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 49.Gutiérrez JM, et al. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon. 1998;36:1529–1538. doi: 10.1016/s0041-0101(98)00145-7. [DOI] [PubMed] [Google Scholar]
- 50.Gutiérrez JM, et al. Trends in snakebite envenomation therapy: Scientific, technological and public health considerations. Curr Pharm Des. 2007;13:2935–2950. doi: 10.2174/138161207782023784. [DOI] [PubMed] [Google Scholar]
- 51.Lomonte B, Gutiérrez JM. A new muscle damaging toxin, myotoxin II, from the venom of the snake Bothrops asper (terciopelo) Toxicon. 1989;27:725–733. doi: 10.1016/0041-0101(89)90039-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.