Abstract
The histone variant H3.3 is implicated in the formation and maintenance of specialized chromatin structure in metazoan cells. H3.3-containing nucleosomes are assembled in a replication-independent manner by means of dedicated chaperone proteins. We previously identified the death domain associated protein (Daxx) and the α-thalassemia X-linked mental retardation protein (ATRX) as H3.3-associated proteins. Here, we report that the highly conserved N terminus of Daxx interacts directly with variant-specific residues in the H3.3 core. Recombinant Daxx assembles H3.3/H4 tetramers on DNA templates, and the ATRX–Daxx complex catalyzes the deposition and remodeling of H3.3-containing nucleosomes. We find that the ATRX–Daxx complex is bound to telomeric chromatin, and that both components of this complex are required for H3.3 deposition at telomeres in murine embryonic stem cells (ESCs). These data demonstrate that Daxx functions as an H3.3-specific chaperone and facilitates the deposition of H3.3 at heterochromatin loci in the context of the ATRX–Daxx complex.
Keywords: histone H3.3, epigenetics, ES cell, heterochromatin
The assembly of chromosomal DNA into nucleosomes represents the most fundamental step in the formation of eukaryotic chromatin structure. The deposition, remodeling, and eviction of nucleosomes have been shown to be important for a variety of DNA-templated processes such as replication, repair, and transcription. Histone deposition pathways are thought to play a critical role in the establishment and maintenance of epigenetic information encoded by histone modifications, nucleosome positioning, and higher-order chromatin structure (1–3). An additional layer of epigenetic regulation is achieved by the use of histone variants: paralogs of the core histones genes H3, H4, H2A, and H2B that have diverged from their canonical counterparts in primary structure and function.
In addition to a centromeric version, mammalian genomes encode three H3 variants. Histones H3.1 and H3.2 are primarily expressed in S-phase, whereas the H3.3 variant is expressed throughout the cell cycle. For its universal role in proliferating and nondividing cells, the function of H3.3 has been studied in a wide range of cell types and organisms (4). Differences in H3.3 and the canonical H3 species are confined to one residue in the histone tail and a cluster of three residues in the core histone fold. The three amino acid variations in the histone fold have been shown to be necessary for H3.3 replication-independent incorporation into chromatin (5, 6). Higher eukaryotes utilize separate chaperones and deposition pathways for the different histone H3 variants, and previous work identified two major pathways: replication-coupled deposition of H3.1/H3.2 by the CAF1 complex, and replication-independent deposition of H3.3 by the HIRA complex (6–8)
While originally associated with euchromatic sites of active transcription, H3.3 has recently been found associated with regulatory elements and constitutive heterochromatin at telomeres (9–12). We previously found that HIRA is required for localization of H3.3 to actively transcribed regions, while ATRX is essential for H3.3 incorporation at telomeres. Apart from ATRX, we also identified Daxx in H3.3 immunoprecipitations (IP) (9). Daxx and ATRX have been shown to form a complex (13) and colocalize at pericentric heterochromatin and promyelocytic leukemia bodies (PML bodies) (14, 15). Loss of ATRX leads to epigenetic alterations, including abnormal levels of DNA methylation at repetitive elements such as telomeres in murine cells (16). Moreover, ATRX and H3.3 have essential roles in maintaining telomere chromatin (9, 17).
To gain further insight into H3.3-specific deposition pathways, we sought to identify the direct binding partner of H3.3. Here we show that Daxx binds directly to H3.3, and importantly, this binding is specific for H3.3 and not H3.1. We find that Daxx alone, or when present in the ATRX–Daxx complex, can effectively assemble H3.3-containing nucleosomes. Additionally, we show that ATRX recruits Daxx to telomeres, and both complex subunits are required for H3.3 deposition at telomeric chromatin in murine ESCs.
Results
Daxx Associates Specifically with H3.3.
Having identified Daxx and ATRX as novel H3.3-associated factors, we wanted to determine if either of these polypeptides bound directly to H3.3 but not to H3.1. Using HeLa cells lines that stably express FLAG-HA-tagged H3.1 or H3.3 (hereafter e-H3.1 and e-H3.3) (6), we isolated minimal H3.1 and H3.3-containing complexes by tandem-affinity purification from soluble nuclear extract with a series of stringent salt washes of up to 1 M NaCl (Fig. 1A). After sequential anti-FLAG and anti-HA IP, we consistently recovered a single polypeptide band of ∼100 kD apparent molecular weight that copurified with H3.3 but not H3.1 (see arrowhead next to lane 4); mass spectrometric analysis of this band yielded a significant number of peptides matching Daxx. As reported previously (6), we also detected HIRA in the first H3.3 co-IP step (Fig. S1). While we recently found ATRX associated with H3.3 in whole-cell preparations (9), it was only weakly associated with H3.3 in soluble nuclear extracts (Fig. S1).
Fig. 1.
Identification of Daxx as an H3.3-specific binding protein. (A) Silver stain of H3.1 and H3.3 associated proteins. FLAG-M2 IP was performed on the nuclear extract (lanes 1, 2). The eluate from the FLAG IP was then subjected to HA IP (lanes 3, 4). HA IP was performed on untagged HeLa nuclear extract as a control (lane 5). Protein bands from the HA affinity column were identified by mass spectrometry. Arrowhead in A and B denotes Daxx. (B) Silver stain of FLAG eluate from H3.1 and H3.3 chromatin-associated fractions. (C) Immunoblot was performed on the FLAG eluate with anti-Daxx, anti-ATRX, anti-CAF150, and anti-ASF1a/b sera. Fractionation scheme for H3.3 chromatin-associated proteins. (D) Immunoblott analysis of Mono Q fractions with with anti-Daxx, anti-FLAG, and anti-ATRX. (E) Immunoblot of anti-HA and control rabbit IgG IP of e-H3.3 from pooled Mono Q complex I fractions (left). Silver stain of input and IP material (right).
As the tandem-affinity chromatography was performed on nuclear extracts that did not contain chromatin, we reasoned that the H3.3 and H3.1 complexes purified from these soluble nuclear extracts likely represent a pool of predeposition histones. We also sought to identify proteins that were enriched with H3.3 in the chromatin fraction. To this end, we solubilized oligonucleosomes from the tagged cell lines by brief digestion with micrococcal nuclease (MNase) and isolated H3.1- and H3.3-enriched chromatin by FLAG IP (Fig. 1B). By immunoblot analysis, we found that both Daxx and ATRX were associated with H3.3 but not H3.1 chromatin (Fig. 1C). The p150 subunit of the replication-coupled Chromatin Assembly Complex (CAF) was found primarily but not exclusively associated with the H3.1-associated fraction. The histone chaperone ASF1 was found in both e-H3.1 and e-H3.3 eluates.
We further fractionated proteins associated with H3.3 chromatin using Mono Q anion-exchange chromatography (Fig. 1D). Immunoblot analysis of the column fractions revealed two biochemically distinct populations of Daxx. The majority of Daxx eluted from the Mono Q column at ∼0.4 M KCl, (complex I). While this fraction contained H3.3 we did not detect any ATRX by immunoblot. The second population of H3.3-bound Daxx eluted from the Mono Q column at higher salt (0.75 M KCl). These fractions contained Daxx, ATRX, and H3.3 (complex II).
The isolation of two distinct populations of Daxx provided further evidence that Daxx is the direct binding partner of H3.3. In order to determine if Daxx was bound to H3.3 in complex I, we performed HA-IP with pooled fractions and immunoblotted for Daxx (Fig. 1E). We found that Daxx coimmunoprecipitated H3.3 in these fractions.
We failed to detect H2A, H2B, or endogenous untagged histone H3 in the Mono Q fractions that contained complex I. Immunoblot and silver stain analysis indicated that only epitope tagged-H3.3 and endogenous H4 are present in complex I (Fig. 1E). Because untagged H3 was not associated with Daxx, we concluded that the H3.3-Daxx predeposition complex includes a heterodimer of H3.3/H4. Consistent with this result, others had previously described purified predeposition complexes (e-H3.1 and e-H3.3) that contained H3/H4 heterodimers (6).
Daxx Directly Binds the Globular Core of H3.3.
To confirm the direct and variant-specific interaction with Daxx and H3.3, we next carried out in vitro pull-downs. Recombinant His-tagged human histone proteins from bacteria and recombinant human Daxx from insect cells were prepared. Interestingly, a small fraction of the purified Daxx isolated from insect cells was stably bound to endogenous histone H3/H4 (Fig. 2A). Recombinant Daxx protein bound our reconstituted H3.3/H4 tetramers over a wide range of salt concentrations, demonstrating that Daxx is a novel histone-binding protein. Notably, Daxx bound H3.1/H4 much less avidly at physiological, as well as at high salt concentrations (Fig. 2A).
Fig. 2.
Daxx directly interacts with histone H3.3. (A) Co-IP of recombinant H3.1/H4 or H3.3/H4 with Daxx on M2-FLAG agarose. Beads were washed with buffers of indicated KCl concentrations. (B) Domain structure of human Daxx. Shaded boxes indicate highly conserved regions (dark gray: highly conserved in all metazoan Daxx; light gray: highly conserved in vertebrates). GST-fusion Daxx constructs are shown in black. (C) GST pull-down with Daxx fragments shown in B with H3.3/H4 or H3.1/H4. (D) Schematic showing amino acid differences between H3.1 and H3.3. Relevant secondary structures are indicated. Solid lines represent histone deletion constructs use in E, biotinylated peptides used in G are shown below. (E) GST pull-down with the Daxx HBD (Δ3) and H3.3/H4 tetramers with histone tail deletions and the H3.3/H4 octamer with H2A/H2B. (F) Co-IP of single H3 point mutations as described in A. (G) Peptide pull-down with biotinylated peptides of residues 80–94 or 86–97 of H3.1 and H3.3.
The 740 amino acid human Daxx is comprised of an N-terminal, overall basic (pI 9.3), alpha-helical domain and an S/P/T-rich C-terminal fold connected by a likely unstructured highly acidic linker region (Fig. 2B). The N-terminal domain contains a highly conserved region found in all metazoan Daxx proteins (amino acids 183–417) with no homolog in yeast (Fig. S2B). The Glu/Asp-rich linker between N-and C-terminal domain is resembling similar acidic stretches found in many histone chaperones including NAP1, Asf1p, Rtt106, Vsp75, FACT, CAF-1p150. Additionally, two hydrophobic motifs at the far N and C termini are conserved in vertebrates (Fig. S2A).
Guided by conserved domains and secondary structure prediction, we designed a set of GST-fusion proteins that covered the N- and C-terminal domains. GST pull-downs with the Daxx fusion proteins and recombinant H3.3/H4 or H3.1/H4 were performed (Fig. 2C). We found that the fusion proteins containing the highly conserved region in the N terminus of Daxx all bound specifically to H3.3/H4. We termed this region the “histone-binding domain” (HBD) of Daxx. As a control, we found that a recombinant GST-Asf1a protein bound equally well to both H3.1/H4 and H3.3/H4 (Fig. 2C).
We further investigated the Daxx-H3.3 interaction by making a series of “tailless” recombinant H3.3/H4 (Fig. 2D). We found that the Daxx HBD fusion protein bound effectively to all H3.3/H4 tail deletions constructs (Fig. 2E), indicating that Daxx contacts H3.3/H4 via the histone globular domain. Furthermore, the Daxx HBD did not bind to the H2A/H2B dimer (Fig. 2E).
H3.1 and H3.3 differ in sequence at five residues; four residues found within the histone-fold domain and an A31S substitution in the unstructured N-terminal histone tail. Three substitutions are clustered at the base of helix 2 of the histone fold, and these residues were previously shown to confer binding specificity for particular histone deposition pathways (5, 6) (Fig. 2B). In order to identify which of the five residues are responsible for differential binding by Daxx, we constructed a series of single point mutations in the unique H3.1 or H3.3 residues. Each of these mutant H3/H4 complexes was subject to co-IP with recombinant full-length Daxx (Fig. 2F). We found that no single point mutation in H3.1 conferred markedly increased binding to Daxx. Similarly, we found that no single mutation in H3.3 abrogated binding to Daxx, demonstrating that single point mutations had little effect on the overall interaction. Mutants in position 31 had no effect on Daxx binding to H3.3, signifying that the primary mode of recognition occurs via the globular domain. Mutation of H3.3 Gly 90 to Met (the residue present in H3.1) had the largest single effect on Daxx binding.
To directly test if the interaction between Daxx and H3.3 occurs via the unique “AAIG” motif at the base of H3.3 helix 2, we performed pull-downs with peptides that corresponded to the residues 80–94 and from 86–97 of either H3.1 or H3.3 (Fig. 2D). Peptides corresponding to residues 80–94 of H3.3, but not H3.1, bound effectively, indicating that this region is necessary and sufficient for interaction with Daxx (Fig. 2G). H3 peptides spanning residues 86–97 failed to interact with Daxx in the pull-down assay.
Daxx Is a Histone H3.3-Specific Chaperone.
The purification of Daxx in a nucleoplasmic complex with H3.3/H4 indicated that Daxx might act as a histone chaperone for the deposition of newly synthesized H3.3. We saturated Daxx with excess H3.3/H4 and purified a stable stoichiometric complex over a Mono Q ion exchange column (Fig. 3A). Next, we added this complex to a relaxed plasmid template and found that it induced supercoiling in a concentration-dependent manner. The decreased mobility of the products in the presence of chloroquine indicated negative supercoiling characteristic for H3/H4 deposition onto the plasmid (Fig. 3B). Furthermore, a 148 bp DNA fragment formed a tetrasome upon addition of the Daxx-H3.3/H4 complex as indicated by a characteristic shift in electrophoretic mobility of the DNA and comigrating H3.3 (Fig. 3C). These results show that Daxx has intrinsic histone chaperone activity.
Fig. 3.
Daxx is a histone H3.3 chaperone. (A) In vitro reconstituted and purified Daxx-H3.3/H4 complex used for deposition assay (B) chromatin-induced supercoiling was analyzed by agarose gel electrophoresis in the absence (upper) and presence (lower) of chloroquine. (C) EMSA of tetrasome deposition on a 147 bp DNA fragment by the Daxx-H3/H4 complex. (D) Recombinant Daxx and (E) H3.1/H4 and H3.3/H4. (F) Daxx mediated H3.1/H4 or H3.3/H4 tetrosome deposition on topoisomerase I-relaxed plasmid.
Given the marked specificity of Daxx for H3.3 in our in vitro binding experiments, we wondered if its histone chaperone activity would be specific to H3.3, as well. We performed plasmid supercoiling assays as described above, but with purified Daxx (Fig. 3D) and H3.1 or H3.3 (Fig. 3E). While Daxx promoted some chromatin assembly in the presence of both H3.1/H4 and H3.3/H4, it proved more efficient in depositing H3.3/H4 tetramers (Fig. 3F). In contrast, NAP-1-mediated assembly was equally efficient with H3.1/H4 (Fig. S3). We therefore identified Daxx as a bona fide histone chaperone with intrinsic preference for H3.3.
The ATRX–Daxx Complex Has Chromatin-Remodeling and H3.3-Deposition Activity.
ATRX localizes to telomeric chromatin, and ATRX-/- ESCs fail to incorporate H3.3 into telomeres, suggesting that ATRX plays a direct role in incorporating H3.3 into chromatin (9). SNF2-family chromatin-remodeling proteins such as ATRX use ATP hydrolysis to translocate nucleosomes in cis along the DNA (18). Additionally, CHD1 and ISWI family remodelers function alone or in the context of protein complexes to facilitate the assembly of extended, periodic nucleosome arrays (19, 20). To investigate the histone deposition properties of the ATRX–Daxx complex, we performed chromatin assembly assays with a completely purified system containing recombinant ATRX–Daxx, recombinant Daxx and recombinant H3.1/H4, H3.3/H4, and H2A/H2B, and a DNA template that contained a series of tandem Sea Urchin 5S rDNA nucleosome positioning sequences. Micrococcal nuclease (MNase) digestion was used to analyze the reaction products for nuclease-protected mono- or oligonucleosomal fragments (Fig. 4 C and D). While we demonstrated above that Daxx has intrinsic histone deposition activity (Fig. 3), this analysis revealed that the ATRX–Daxx complex catalyzes the formation of extended nucleosome arrays (Fig. 4D). We compared the assembly activity of the ATRX–Daxx complex to recombinant human ACF complex and human NAP1. We found that the ATRX–Daxx complex assembled H3.3-containing nucleosomes to a greater extent than H3.1 nucleosomes, whereas ACF and NAP1 assembled both H3.1 and H3.3 nucleosomes equally well. ATRX assembly in the absence of Daxx could not be assessed because we were unable to purify recombinant full-length ATRX alone. In the absence of nucleosome positioning sequences, we failed to observe evenly spaced nucleosomes on a DNA template. Therefore, the ATRX–Daxx complex does not contain nucleosome spacing activity like ISWI or CHD1.
Fig. 4.
ATRX–Daxx is a histone deposition and remodeling complex. (A) Recombinant ACF complex (ACF1 and ISWI), hNAP1, Daxx, H3.1/H4, H3.3/H4, and H2A/H2B. (B) SMART Superdex 200 gel filtration fractionation of ATRX–Daxx complex. ATRX–Daxx complex used in C–E was purified from free Daxx and Daxx-H3.3/H4 complex. (C) MNase digestion of chromatin assembly reactions with H3.1/H4 or H3.3/H4 alone, or in combination with NAP1 and ACF complex. (D) MNase digestion of assembly reactions with H3.1/H4 or H3.3/H4 alone, or in combination with Daxx and ATRX–Daxx complex. (E) Analysis of remodeling activity of ACF and ATRX–Daxx complexes on H3.3 mononucleosomes by native TBE PAGE.
The enhanced deposition of nucleosome arrays suggested that the ATRX–Daxx complex contains remodeling activity in addition to its histone deposition activity. Previously, purified ATRX-containing complexes have been shown to have DNA-stimulated ATPase, DNA translocase, and chromatin-remodeling activities (13, 21, 22). The p185 isoform of Drosophila ATRX homolog (XNP) was found in complex with HP1, and exhibited in vitro chromatin-remodeling activity (22). We investigated whether the ATRX–Daxx complex could mobilize a positioned H3.3-containing nucleosome. Remodeling assays were performed with a 194-bp DNA template that contained a nucleosome positioning sequence. An H3.3-histone octamer was assembled onto the DNA and incubated with ATRX–Daxx and ATP. Analysis of the remodeled substrate by native gel electrophoresis indicated that the ATRX–Daxx complex could effectively mobilize the nucleosome along the DNA template (Fig. 4E).
Daxx Is Required for H3.3 Deposition at Telomeres.
Previously, we found that ATRX-/- ESCs exhibited a dramatic loss of H3.3 found at telomeres, indicating that ATRX may have a direct role in the incorporation or maintenance of H3.3-containing nucleosomes (9). We sought to determine if Daxx, like ATRX, is important for H3.3 deposition at telomeres.
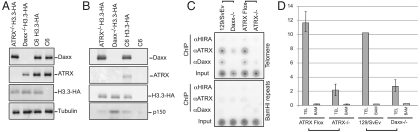
We have previously described the use of zinc finger nucleases to generate murine ESC lines that carry one H3.3B allele tagged with a C-terminal HA epitope (9). We targeted the H3.3B locus in 129/SvEv ESCs and Daxx-/- ESCs to generate cell lines that contained one HA-tagged allele of H3.3B. The Daxx-/- ESCs were generated from the 129/SvEv cell line (23). Immunoblot analysis of extract from the H3.3-HA-tagged ATRX-/-, Daxx-/-, and C6 ESCs indicated that levels of H3.3-HA were similar between the cell lines. Interestingly, we found that ATRX levels were reduced in Daxx-/- ESCs, suggesting that Daxx may be required for ATRX protein stability or expression (Fig. 5A).
Fig. 5.
ATRX–Daxx complex is required for H3.3-deposition at telomeres. (A) Immunoblots of nuclear extracts for Daxx, ATRX, and H3.3-HA. Tubulin immunoblot served as a loading control (B) HA-IP from the extracts in A. IP material was immunoblotted for Daxx, ATRX and H3.3-HA. (C) ChIP of HIRA, ATRX, and Daxx proteins was on 129/SvEv, Daxx-/-, ATRX–Flox, and ATRX-/- ESCs. Dot blot analysis of IP DNA using telomere and BamH1 repeat probes. (D) HA-H3.3 ChIP was performed on 129/SvEv, Daxx-/-, ATRX–Flox, and ATRX-/- ESCs. Bar graph showing the input-normalized signal from the dot blot on telomere and BamHI repeats with standard errors.
Immunoprecipitation of H3.3-HA was performed from nuclear extract from these cell lines (Fig. 5B). We found that H3.3-HA could efficiently co-IP Daxx in ATRX-/- cells, suggesting that ATRX is not required for Daxx interaction with H3.3. ATRX also failed to co-IP with H3.3-HA in Daxx-/- ESC. These data are consistent with our previous experiments that indicated a direct contact between Daxx and H3.3. Interestingly, the CAF1 p150 signal increased in Daxx-/- cells, suggesting that in the absence of Daxx, H3.3 may associate with other histone chaperone complexes.
We performed ChIP on H3.3-HA in the Daxx-/- ESCs to determine if Daxx, like ATRX, is required for H3.3 deposition at telomeres (Fig. 5C). The H3.3 signal was decreased to a similar degree in both ATRX-/- and Daxx-/- ESCs. We performed ChIP with Daxx antiserum and found that Daxx was bound to telomeric chromatin in wild-type murine ESCs (Fig. 5D). Daxx failed to ChIP to telomeric chromatin in ATRX-/- ESCs, suggesting that ATRX is required for targeting Daxx and H3.3 to telomeric chromatin. We therefore conclude that the ATRX–Daxx complex localizes to telomeres and directly mediates H3.3 deposition at telomeric chromatin.
Discussion
Daxx Is a H3.3 Chaperone.
We demonstrated that Daxx forms stable complexes with H3.3/H4 but not with H3.1/H4. We assessed Daxx histone chaperone activity alone and coupled to ATP-dependent remodeling by ATRX and have observed preference for H3.3 in both deposition assays. Similar H3.3-specificity has been observed in a minicircle assembly assay (24).
We identified a minimal 234 amino acid segment of Daxx that specifically interacts with H3.3/H4. The histone-binding domain also represents the most highly conserved segment of Daxx, suggesting that the H3.3-specific binding and chaperone activity may be conserved among Daxx homologs. Besides a poly-Glu/Asp acidic stretch, the Daxx histone-binding domain lacks sequence homology with any known histone-binding proteins, suggesting that the Daxx contains a novel histone chaperone domain (Fig. S2).
We have shown that Daxx distinguishes histone H3 variants through direct interaction with the variant-specific residues 87–90 in the core histone fold of H3.3 (Fig. 2 F and G). The three unique residues in the H3.3 “AAIG” motif cooperatively confer specificity for binding as single point mutants only have a modest effect. Our in vitro binding data closely resembles the composite specificity for replication-independent H3.3 deposition observed in Drosophila cells (5). While specific binding was achieved with a 15-residue peptide, it is conceivable that other surfaces of H3.3/H4 contribute to the overall binding affinity. These shared regions likely account for the residual binding to H3.1/H4 observed in all in vitro assays. We speculate that selectivity is enhanced in vivo by the existence of competing chaperone complexes and deposition pathways. In agreement with such a model, we found more H3.3 associated with CAF-1 in Daxx-/- ESCs (Fig. 5B).
Our previous ChIP-Seq studies indicated that H3.3-nucleosomes at telomeres and transcription factor binding sites (TFBS) were deposited independently of the HIRA chaperone (9). While the ATRX–Daxx complex may account for the HIRA-independent H3.3 deposition at telomeres, the factors involved in H3.3 deposition at TFBS remain unknown. Our purification of H3.3-bound proteins from cell extracts yielded two biochemically distinct Daxx populations. We speculate that Daxx may be recruited to TFBS and other genomic loci for assembly of H3.3-nucleosomes in an ATRX-independent manner.
Although Daxx has been studied extensively in many systems, its function in transcriptional regulation is not well understood. In light of our findings, the mechanism by which Daxx influences transcription may be directly linked to H3.3 deposition activity at TFBS or other regulatory elements.
ATRX Has Nucleosome Remodeling Activity.
We demonstrated that recombinant ATRX–Daxx complex has chromatin-remodeling activity and can assist Daxx in the assembly of H3.3-nucleosomes. Previously, immunoprecipitated human ATRX was shown to exhibit DNA translocase activity and a mononucleosome disruption activity that was focused near the DNA entry/exit site (21). The deposition of H3.3/H4 by Daxx, followed by the mobilization of H3.3-nucleosomes by the ATP-dependent remodeling activity of ATRX may serve important roles in the formation of higher-order chromatin structure. Human ATRX is a homolog of the repair and recombination protein, RAD54. Recent in vitro studies found that other human RAD54 homologs displayed similar remodeling activities to ISWI proteins that remodel near the nucleosome entry and exit sites (25, 26). These data are consistent with a model whereby ATRX and RAD54 family remodeling proteins mobilize the histone octamer, as opposed to catalyzing the formation of DNA loops on the nucleosome surface.
ATRX–Daxx Complex May Assemble Specialized Heterochromatin.
Our ChIP results indicate that the ATRX–Daxx complex is present at telomeric chromatin in murine ESCs (Fig. 5C). Moreover, we show that Daxx localization to telomeres is dependent on ATRX, suggesting that ATRX may be involved in recruitment of the complex to telomeres. While Daxx histone chaperone activity is sufficient to assemble chromatin in vitro, H3.3-deposition at telomeres is additionally dependent on ATRX (Fig. 5D), suggesting a dual role in both complex recruitment and ATP-dependent chromatin remodeling.
In differentiated cells, H3.3 has been found to localize to pericentric chromatin (10, 27). Recent work performed in murine embryonic fibroblasts (MEFs) found that H3.3 is deposited at pericentric DNA repeats in an ATRX–Daxx-dependent manner (24) ATRX–Daxx also localizes to PML bodies, and ATRX has been shown to colocalize with heterochromatin on both the inactive-X chromosome and Y-chromosome in mice (28, 29). These findings raise the intriguing possibility that the ATRX–Daxx complex may serve as a specialized chromatin assembly pathway for repetitive regions such as telomeres, centromeres, and other regions of constitutive heterochromatin. In agreement with this model, ATRX-depleted cells display centromere dysfunction exhibited by defective sister chromatid cohesion at the metaphase plate, as well as abnormal chromosome alignment (30, 31). Furthermore, cells from ATR-X syndrome patients display altered pericentric DNA methylation (15), and ESCs depleted for ATRX or H3.3 exhibit signs of telomere dysfunction (9, 17). Also, Drosophila xnp mutants display a position-effect phenotype (PEV) and have defects in pericentric heterochromatin (22, 32, 33).
Replication-independent H3.3-deposition is often associated with regions of high nucleosome turnover rate (1, 4). The replication-independent histone deposition complexes may therefore help to fill nucleosome-free regions created by RNA polymerase passage and ATP-dependent remodeling at transcribed genes and enhancer elements. Both telomeric and centromeric sequences are transcribed to produce long noncoding RNAs (34, 35). Telomere repeat-containing RNAs (TERRA) appear to affect telomere structure and replication (34), and centromeric RNA is important for pericentric heterochromatin formation (36). While heterochromatic regions are generally considered to have slow histone exchange rates, the intrinsic repetitive features of telomeric and centromeric DNA may promote nucleosome instability. Indeed, telomere repeats have a low propensity to form nucleosomes in vitro (37), and deposition of H3.3 outside of S-phase may help maintain a proper nucleosome density for heterochromatin formation.
Recruitment of the ATRX–Daxx Complex.
In addition to the chromatin-remodeling SNF2-like ATPase domain, ATRX contains an N-terminal ATRX–DMNT3L–DNMT3A (ADD) domain that consists of an N-terminal GATA-like zinc finger and a PHD finger. The ADD domain of DNMT3A and DNMT3L preferentially interact with the unmodified extreme N terminus of histone H3 (38, 39). Although the specific binding ligand is unknown, the ADD domain may serve to recruit or stabilize the ATRX–Daxx complex on a nucleosomal template for remodeling and histone deposition. Both telomeres and pericentric regions are enriched in DNA methylation and heterochromatin histone modifications such as H3K9me3 and H4K20me3 (34, 40), and these modifications may recruit the ATRX–Daxx complex to facilitate H3.3 deposition. In support, loss of the H3K9me3 methyltransferase, Suv39h1, had similar phenotypes as a reduction of ATRX, Daxx, or H3.3: abnormal telomeres, aberrant chromosome segregation and premature sister chromatid separation (41, 42). In addition to histone modifications, DNA methylation may be a means to recruit ATRX–Daxx to specific genomic loci. DNA methylation may also contribute to the recruitment of ATRX–Daxx through an interaction with the methyl-CpG binding protein 2 (MeCP2) (15).
Functional Significance of H3.3 Incorporation.
Viewed in a broader context, our results raise the question of whether newly incorporated H3.3 serves a specialized role beyond simply replacing the “old” histone H3. While it has been proposed that H3.3 nucleosomes are intrinsically less stable (43) and promote transcription (44), the exact role of transcription in heterochromatin still remains unclear and poorly defined.
Others and we have observed mitotic phosphorylation of H3.3 S31 at ESC telomeres as well as pericentric heterochromatin of differentiated cells (10, 27). Notably, S31 is unique to H3.3 as substituted by Ala in H3.1/2 making this heterochromatin-associated mark strictly dependent on H3.3 incorporation. Future studies will elucidate these unexpected heterochromatic functions of histone variant H3.3, initially discovered as a marker of “active” chromatin.
Materials and Methods
e-H3/H4 complexes were purified from nuclear extracts via FLAG/HA tandem-affinity purification and analyzed by SDS/PAGE. Deposition assay: H3/H4 tetramers and Daxx were mixed and incubated on ice for 30′. Prerelaxed pBluescript II KS+ was added in the presence of Topoisomerase and incubated 90 min at 37 °C. The assembly reaction was analyzed by agarose gel electrophoresis. Nucleosome assembly: Histone chaperones complexes were mixed with H3/H4 tetramers. pG5ML was added to the reaction. The reaction was incubated at 30 °C for 4 h with an ATP regenerating system. H2A/H2B were added to the reaction during incubation. Resulting chromatin was analyzed by MNase digestion. Detailed information regarding the reagent assembly and assay conditions can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Laura Banaszynski, Robert Kingston, Jaehoon Kim, David Picketts, Alex Ruthenburg, Agnel Sfeir, Christian Zierhut for reagents and experimental advice. P.W.L. was supported by a National Research Service Award fellowship. S.J.E. was supported by a Boehringer Ingelheim Fonds fellowship. K.M.N. was supported by a Rockefeller University Women & Science fellowship. S.C.S. was supported by a Deutsche Forschungsgemeinschaft fellowship. This work was funded by GM53122 and GM53512 (C.D.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008850107/-/DCSupplemental.
References
- 1.Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328(5982):1161–1164. doi: 10.1126/science.1186777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol. 2008;10(1):102–109. doi: 10.1038/ncb1674. [DOI] [PubMed] [Google Scholar]
- 3.Ohsawa R, Adkins M, Tyler J. Epigenetic inheritance of an inducibly nucleosome-depleted promoter and its associated transcriptional state in the apparent absence of transcriptional activators. Epigenetics & Chromatin. 2009;2:1–11. doi: 10.1186/1756-8935-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsaesser SJ, Goldberg AD, Allis CD. New functions for an old variant: No substitute for histone H3.3. Curr Opin Genet Dev. 2010;20(2):110–117. doi: 10.1016/j.gde.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002;9(6):1191–1200. doi: 10.1016/s1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 6.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116(1):51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 7.Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87(1):95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 8.Ray-Gallet D, et al. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol Cell. 2002;9(5):1091–1100. doi: 10.1016/s1097-2765(02)00526-9. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg AD, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140(5):678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong L, et al. Histone H3.3 incorporation provides a unique and functionally essential telomeric chromatin in embryonic stem cells. Genome Res. 2009;19:404–414. doi: 10.1101/gr.084947.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin C, et al. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet. 2009;41(8):941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mito Y, Henikoff JG, Henikoff S. Histone replacement marks the boundaries of cis-regulatory domains. Science. 2007;315(5817):1408–1411. doi: 10.1126/science.1134004. [DOI] [PubMed] [Google Scholar]
- 13.Tang J, et al. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J Biol Chem. 2004;279(19):20369–20377. doi: 10.1074/jbc.M401321200. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, et al. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci USA. 2003;100(19):10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nan X, et al. Interaction between chromatin proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation. Proc Natl Acad Sci USA. 2007;104(8):2709–2714. doi: 10.1073/pnas.0608056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons RJ, et al. Mutations in ATRX, encoding a SWI/SNF-like protein, cause diverse changes in the pattern of DNA methylation. Nat Genet. 2000;24(4):368–371. doi: 10.1038/74191. [DOI] [PubMed] [Google Scholar]
- 17.Wong LH, et al. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20(3):351–360. doi: 10.1101/gr.101477.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehouse I, et al. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature. 1999;400(6746):784–787. doi: 10.1038/23506. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Bulger M, Pazin MJ, Kobayashi R, Kadonaga JT. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell. 1997;90(1):145–155. doi: 10.1016/s0092-8674(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 20.Lusser A, Kadonaga JT. Chromatin remodeling by ATP-dependent molecular machines. Bioessays. 2003;25(12):1192–1200. doi: 10.1002/bies.10359. [DOI] [PubMed] [Google Scholar]
- 21.Xue Y, et al. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci USA. 2003;100(19):10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emelyanov AV, Konev AY, Vershilova E, Fyodorov DV. Protein complex of Drosophila ATRX/XNP and HP1a is required for the formation of pericentric beta-heterochromatin in vivo. J Biol Chem. 2010;285(20):15027–15037. doi: 10.1074/jbc.M109.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 1999;13(15):1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 2010 doi: 10.1101/gad.566910. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexeev A. Mazin A. Kowalczykowski SC. Rad54 protein possesses chromatin-remodeling activity stimulated by the Rad51-ssDNA nucleoprotein filament. Nat Struct Biol. 2003;10(3):182–186. doi: 10.1038/nsb901. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Fan HY, Goldman JA, Kingston RE. Homology-driven chromatin remodeling by human RAD54. Nat Struct Mol Biol. 2007;14(5):397–405. doi: 10.1038/nsmb1223. [DOI] [PubMed] [Google Scholar]
- 27.Hake SB, et al. Serine 31 phosphorylation of histone variant H3.3 is specific to regions bordering centromeres in metaphase chromosomes. Proc Natl Acad Sci USA. 2005;102(18):6344–6349. doi: 10.1073/pnas.0502413102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann C, Schmidtmann A, Muegge K, De La Fuente R. Association of ATRX with pericentric heterochromatin and the Y chromosome of neonatal mouse spermatogonia. BMC Mol Biol. 2008;9(29):1–18. doi: 10.1186/1471-2199-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann C, De La Fuente R. ATRX marks the inactive X chromosome (Xi) in somatic cells and during imprinted X chromosome inactivation in trophoblast stem cells. Chromosoma. 2009;118(2):209–222. doi: 10.1007/s00412-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De La Fuente R, Viveiros MM, Wigglesworth K, Eppig JJ. ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev Biol. 2004;272(1):1–14. doi: 10.1016/j.ydbio.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie K, et al. Loss of ATRX leads to chromosome cohesion and congression defects. J Cell Biol. 2008;180(2):315–324. doi: 10.1083/jcb.200706083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassett AR, Cooper SE, Ragab A, Travers AA. The chromatin remodelling factor dATRX is involved in heterochromatin formation. PLoS One. 2008;3(5):e2099. doi: 10.1371/journal.pone.0002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneiderman JI, Sakai A, Goldstein S, Ahmad K. The XNP remodeler targets dynamic chromatin in Drosophila. Proc Natl Acad Sci USA. 2009;106(34):14472–14477. doi: 10.1073/pnas.0905816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luke B, Lingner J. TERRA: Telomeric repeat-containing RNA. EMBO J. 2009;28(17):2503–2510. doi: 10.1038/emboj.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaratiegui M, Irvine DV, Martienssen RA. Noncoding RNAs and gene silencing. Cell. 2007;128(4):763–776. doi: 10.1016/j.cell.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Maison C, et al. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat Genet. 2002;30(3):329–334. doi: 10.1038/ng843. [DOI] [PubMed] [Google Scholar]
- 37.Cacchione S, Cerone MA, Savino M. In vitro low propensity to form nucleosomes of four telomeric sequences. FEBS Lett. 1997;400(1):37–41. doi: 10.1016/s0014-5793(96)01318-x. [DOI] [PubMed] [Google Scholar]
- 38.Ooi SKT, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448(7154):714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otani J, et al. Structural basis for recognition of H3K4 methylation status by the DNA methyltransferase 3A ATRX-DNMT3-DNMT3L domain. EMBO Rep. 2009;10(11):1235–1241. doi: 10.1038/embor.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoeftner S, Blasco MA. A ‘higher order’ of telomere regulation: Telomere heterochromatin and telomeric RNAs. EMBO J. 2009;28(16):2323–2336. doi: 10.1038/emboj.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehnertz B, et al. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol. 2003;13(14):1192–1200. doi: 10.1016/s0960-9822(03)00432-9. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Cao M, O’Sullivan R, Peters AH, Jenuwein T, Blasco MA. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet. 2004;36(1):94–99. doi: 10.1038/ng1278. [DOI] [PubMed] [Google Scholar]
- 43.Jin C, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21(12):1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura T, et al. Inducible deposition of the histone variant H3.3 in interferon-stimulated genes. J Biol Chem. 2009;284(18):12217–12225. doi: 10.1074/jbc.M805651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.