Abstract
Prior research points to the importance of psychostimulants in improving self-control. However, the neural substrates underlying such improvement remain unclear. Here, in a pharmacological functional MRI study of the stop signal task, we show that methylphenidate (as compared with placebo) robustly decreased stop signal reaction time (SSRT), an index of improved control, in cocaine-dependent patients (a population in which inhibitory control is impaired). Methylphenidate-induced decreases in SSRT were positively correlated with inhibition-related activation of left middle frontal cortex (MFC) and negatively with activation of the ventromedial prefrontal cortex (vmPFC) in whole brain linear regressions. Inhibition-related MFC but not vmPFC activation distinguished individuals with short and long SSRT in 36 demographically matched healthy individuals, whereas vmPFC but not MFC activation, along with improvement in SSRT, was correlated with a previously implicated biomarker of methylphenidate response (systolic blood pressure). These results implicate a specific neural (i.e., vmPFC) mechanism whereby stimulants improve inhibitory control. Altered ventromedial prefrontal activation and increased blood pressure may represent useful CNS and peripheral biomarkers in individualized treatment with methylphenidate for patients with cocaine dependence.
Keywords: functional MRI, psychostimulant, catecholamine, cognitive control, ventromedial prefrontal cortex
Deficits in cognitive control have been implicated in the pathophysiology of several neuropsychiatric disorders, including attention deficit hyperactivity disorder (ADHD) and cocaine dependence (1–5), and a wide literature supports the utility of stimulants, including methylphenidate, in improving cognitive control (6–9, see ref. 10 for review). For example, studies have shown that stimulants alter cerebral activation and influence cognitive functions, including improving inhibitory control, in healthy individuals (11–21). Similarly, Overtoom et al. (22) reported that methylphenidate improved stopping performance and restored the electrophysiological potential of stopping in a stop signal task, whereas it did not affect go trial reaction time, in adult patients with ADHD. Although neuroimaging studies have examined the effects of stimulants on regional brain activation and connectivity, the neural substrates underlying stimulant-mediated improvements in inhibitory control remain unclear. A more complete understanding of the neural mechanisms whereby stimulants improve cognitive control will be critically important, not only for an improved understanding of its biological basis but also for understanding the pathophysiology and treatment of conditions associated with its impairment.
Using a stop signal task, we (and others) have demonstrated that patients with cocaine (and other stimulant) misuse display impaired inhibitory control and prefrontal activation during a stop signal task (5, 23–27). Whereas prior research has examined stimulants in clinical trials of therapeutic efficacy (28–33) and neuroimaging studies of regional brain activation and connectivity in cocaine users (34–37), none to our knowledge have specifically evaluated their association with the behavioral and neural aspects of inhibitory control. In the current study, we sought to address this gap in a pharmacological functional MRI (fMRI) study in which cocaine-dependent individuals received an i.v. injection of methylphenidate or saline placebo before they performed the stop signal task during fMRI. We used a race process to model stop signal performance and to compute the stop signal reaction time (SSRT) as a measure of inhibitory control, independent of other general task measures. In brief, methylphenidate improved SSRT as compared with placebo. We then used the change in SSRT to examine neural and physiological bases of individual variations in the improvement of inhibitory control.
Results
Psychological and Cardiovascular Assessments.
Fig. S1 shows the rating of euphoria, anxiety, and craving, as well as heart rate and systolic and diastolic pressure, before and after i.v. administration of saline (placebo or P session) or methylphenidate (M session). In the M as compared with the P session, cocaine-dependent (CD) volunteers showed an increase in heart rate (Z = 2.803, P = 0.005 during the first 30 min after injection and Z = 2.803, P = 0.005 during fMRI; two-tailed paired-sample Wilcoxon signed rank test), systolic blood pressure (Z = 2.803, P = 0.005 and Z = 2.703, P = 0.007), and diastolic blood pressure (Z = 2.701, P = 0.007 and Z = 2.293, P = 0.022). They also demonstrated an increase from the baseline in euphoria (Z = 2.803, P = 0.005 during the first 30 min after injection and Z = 1.836, P = 0.066 during fMRI; two-tailed paired-sample Wilcoxon signed rank test), cocaine craving (Z = 1.989, P = 0.047 and Z = 1.886, P = 0.059), and anxiety (Z = 2.803, P = 0.005 and Z = 2.668, P = 0.008).
Stop Signal Task Performance.
Table 1 shows behavioral results in the stop signal task. Compared with P session, CD subjects showed decreased SSRT during M session (P < 0.0024, two-tailed paired sample t test). CD subjects did not show differences in any other performance measures between M and P sessions.
Table 1.
General performance in the stop signal task
| Session | Median go RT, ms | % go | % stop | SSRT, ms | FP effect (effect size) | PES (effect size) |
| M | 605 ± 92 | 96.6 ± 1.5 | 52.6 ± 2.1 | 182 ± 38 | 1.2 ± 1.5 | 1.4 ± 1.7 |
| P | 631 ± 122 | 96.0 ± 1.7 | 52.4 ± 3.8 | 238 ± 41 | 1.3 ± 1.6 | 2.0 ± 1.2 |
| P value | 0.4108 | 0.3146 | 0.8387 | 0.0024 | 0.8493 | 0.3338 |
Note: %go and %stop: percentage of successful go and stop trials; SSRT: stop signal reaction time; FP: foreperiod; PES: posterror slowing; M: methylphenidate; P: saline placebo. All numbers are mean ± SD. P value based on two-tailed paired sample t test.
Regional Brain Activations.
We compared M and P sessions with the session order as a covariate in a flexible factorial design of statistical parametric mapping. The results showed that, compared with placebo, methylphenidate increased activation in the striatum and thalamus during stop success (SS), as compared with stop error (SE), largely as a result of decreased activation in these regions during SE as contrasted with SS during the M, as compared with P, session (Fig. S2 and Tables S1 and S2). We derived the effect size of the difference in activation during stop signal inhibition (SS − SE) and observed that these changes in striatothalamic activation did not correlate with changes in the SSRT, psychological assessments, or cardiovascular measures between the two sessions (Table S3).
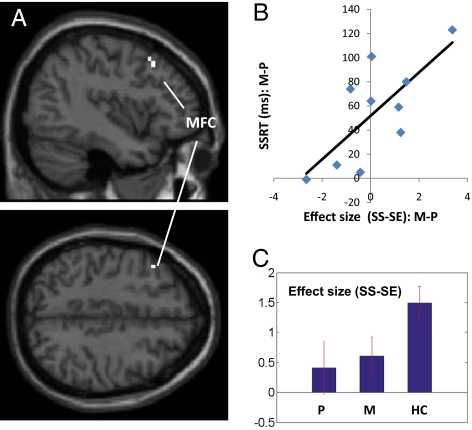
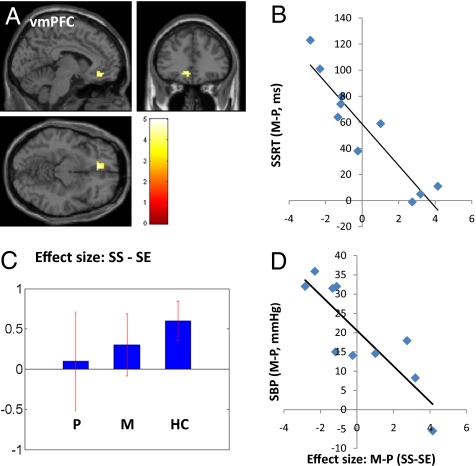
We regressed the whole brain (SS − SE, methylphenidate > placebo) against the improvement in SSRT (i.e., SSRTp − SSRTm) in a linear regression and observed a positive correlation in a small region of the left middle frontal cortex (MFC, x = −42, y = 20, z = 43, voxel Z = 2.05, 189 mm3) (Fig. 1 A and B) and a negative correlation with a region in the ventromedial prefrontal cortex (vmPFC, x = −6, y = 35, z = −11, voxel Z = 3.23, 702 mm3) (Fig. 2 A and B), in the area of the subgenual anterior cingulate cortex or Brodmann area 25. In both brain regions, the inhibition-related activation during SS as contrasted with SE trials appears to increase slightly toward the level of healthy control subjects during methylphenidate administration compared with placebo [Figs. 1C and 2C and Fig. S3 and Tables S4 and S5 for a whole-brain comparison between healthy subjects (HC) and P session of CD subjects]. MFC, but not vmPFC, activation differentiated HC with short and long SSRT (12 HC with shortest vs. 12 with longest SSRT: 151 ± 20 ms vs. 239 ± 11 vs., t = −11.50, P < 0.0001, two-tailed two-sample t test): 2.79 ± 1.25 vs. 0.97 ± 1.30 (MFC, t = 3.20, P < 0.005); 0.78 ± 1.58 vs. 0.00 ± 1.36 (vmPFC, t = 1.18, P < 0.26), whereas vmPFC, but not MFC, activation was linearly correlated with increase in systolic blood pressure (SBP) as a result of methylphenidate (compared with placebo) administration (vmPFC, R = −0.8537, P < 0.0017, Fig. 2D; MFC, R = 0.5774, P < 0.0805). The increase in SBP as elicited by methylphenidate was also linearly correlated with the improvement in SSRT: R = 0.7624, P < 0.0104). Session differences (M > P) in MFC and vmPFC activation (SS > SE) during fMRI were not correlated with changes in behavioral (i.e., euphoria, craving, anxiety) or other cardiovascular measures from baseline (Table S6).
Fig. 1.
Effects of methylphenidate on middle frontal cortical activation, in association with change in the SSRT. (A) Methylphenidate (M), as compared with saline placebo (P), altered inhibition-related activation (SS > SE) in the left MFC; (B) This altered activation in the MFC is linearly correlated with the decrease (improvement) of SSRT during methylphenidate, as compared with placebo, administration: R = 0.7322, P < 0.0161; (C) The histograms show mean ± SEM of the effect size of SS − SE, for M, P, and a control group of HC (n = 36).
Fig. 2.
Effects of methylphenidate on ventromedial prefrontal activation and blood pressure, in association with change in the SSRT. (A and B) Methylphenidate (M), as compared with saline placebo (P), decreased (improved) SSRT, and this improvement is inversely correlated with the change in inhibition-related activation (SS > SE) in the vmPFC: R = −0.9294, P < 0.0001; Color bar represents voxel T value of whole brain linear regression; (C) Mean ± SEM of the effect size of SS − SE for P, M, and a cohort of 36 HC n = 36) subjects; (D) The altered vmPFC activation during SS > SE is also inversely correlated with the change in systolic blood pressure (SBP) during M compared with P session: R = −0.8537, P < 0.0017.
Discussion
The main finding of this work is that methylphenidate improved inhibitory control in cocaine-dependent patients, in association with altered regional brain activation in the ventromedial prefrontal cortex. More broadly, the ventromedial prefrontal activation suggests a neural process whereby self-control might be improved, and highlights a potential neural substrate whereby stimulants and/or other catecholaminergic agents might ameliorate clinical deficits in self-control.
We showed that compared with saline placebo, methylphenidate improved inhibitory control by decreasing SSRT in the stop signal task. No other aspects of the stop signal performance, including go trial reaction time and stop success rate, were significantly affected, suggesting that the effects of methylphenidate were independent of potential cognitive confounds such as motor response processing and attentional monitoring (38–40).
Concomitant with the improvement in inhibitory control, we observed a positive correlation with inhibition-related activation in the MFC, an area shown to be associated with motor inhibitory control in our previous studies of healthy individuals (38, 39, 41), as well as studies of other psychiatric populations (e.g., schizophrenia, depression) (42–44). In the current study, activation of the MFC was also significantly different among healthy individuals with short and long SSRT, corroborating its role in motor inhibitory control. Consistent with these results, many previous studies have implicated this same region of the frontal cortex in cognitive control involving other behavioral tasks (45, 46). For instance, in a recent fMRI study of dieters engaged in real life decisions about food consumption, Hare et al. (47) reported greater activation in the broad area of the left dorsolateral prefrontal cortex in individuals who exercised self-control, choosing healthy over tasty food. Interestingly, the activation of the left frontal regions showed a negative psychophysiological interaction with the vmPFC, in the same area of the subgenual cingulate cortex as observed in the current study (47).
Specifically, we also observed a negative correlation in inhibition-related activity in the vmPFC and improved inhibitory control during methylphenidate administration. This finding was intriguing because unlike the MFC, which belongs to the task-related circuit, the vmPFC is part of the default, “anticorrelated” network of brain regions—one showing greater activity during resting as compared with behaviorally engaging conditions (i.e., tasks) (48, 49). Decreased activation of the vmPFC may thus indicate greater task engagement, in association with improved control of performance. Studies in rodents have shown that inactivation of the vmPFC causes disinhibited responses to unrewarded cues, suggesting that it is involved in inhibitory control of behavioral responses to environmental stimuli (50). Conversely, infusion of dopamine in the vmPFC has been shown to enhance controlled response to outcome valuations, suggesting a role of dopamine and vmPFC in overriding instrumental habits in rodents (51). Furthermore, vmPFC has been implicated in effortful control of phobia (52), regulation of fatigue (53), inhibitory control during experience of negative affect (54), and emotional intelligence for self-management (55). Thus, the current finding adds to a growing literature suggesting a critical role for the vmPFC in behavioral control during emotionally difficult situations and the effects of methylphenidate in normalizing control specifically via altering the activation of the vmPFC.
It is worth noting that along with these neural signatures, improved inhibitory control was also associated with increases in systolic blood pressure caused by methylphenidate. Whereas such findings are broadly consistent with a role for the CNS in mediating the cardiovascular effects of stimulants (56, 57) and are consistent with prior neuroimaging studies demonstrating correlations between CNS (e.g., stimulant-induced dopamine release as measured by positron emission tomography) and cardiovascular measures (e.g., stimulant-induced increases in systolic blood pressure) (58), an important methodological issue needs to be considered. Specifically, stimulants can potentially influence fMRI blood oxygenation level-dependent (BOLD) signals, which depend on the hemodynamic coupling of neuronal activities and local changes in blood flow and oxygenation. An earlier fMRI study showed that cocaine decreased cortical cerebral blood flow but did not obscure BOLD signals evoked by visual stimulation in humans (59). Another study of healthy subjects performing finger tapping showed that methylphenidate did not alter the rate-related increase in BOLD signals in motor cortices (60). A more recent study investigated the effects of cocaine-induced baseline hemodynamic changes on sensory-related hemodynamic and electrophysiological responses in the barrel cortex of rats during mechanical whisker stimulation (61). Cocaine infusion caused transient baseline increase in blood flow and volume, which peaked at 6 min and approached normal level after 25 min. Notably, even during the peak baseline increase, the electrophysiological responses to sensory stimulation were similar to saline, and, after the peak increase, the neural responses were faithfully accompanied by enhanced hemodynamic responses. Furthermore, peripheral blood pressure changes induced by an adrenergic agonist did not influence fMRI BOLD signals in humans (62). Taken together, these observations suggest that the current findings, obtained at 45 min after methylphenidate administration, are unlikely to be the result of general effects of the stimulant on cerebral hemodynamics.
An important limitation of the current study is its small sample size. Thus, the current results need to be considered preliminary and replicated in the future. Furthermore, despite being highly significant statistically, the current results are largely correlational in nature. More studies are required to clarify the mechanisms and links of the central neural and physiological actions of methylphenidate, both in healthy and other clinically affected populations.
To summarize, in a pharmacological fMRI study, we demonstrated that methylphenidate improved inhibitory control in CD volunteers and evoked changes in prefrontal regional brain activation suggesting specific neural substrates underlying deficits in cognitive control and its amelioration.
Methods
Subjects and Informed Consent.
Ten nontreatment seeking volunteers (eight men; 39.9 ± 5.5 y) with CD participated in the study. CD volunteers met criteria for current cocaine dependence (63). The sample had an average of 18.3 ± 7.9 y of cocaine use, with nine predominantly smoking and one preferring intranasal use of cocaine. All subjects were cigarette smokers, and all except one used alcohol with the amount ranging from one to two drinks per week to two to three drinks per day (none meeting DSM-IV criteria for dependence). Recent cocaine use was confirmed both by history and by urine toxicology screens upon admission. Participants were drug-free for a minimum of 5 d and an average of 7.6 d while staying in an inpatient unit before the current fMRI study. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None of them reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on another psychoactive substance (except nicotine) or past history of any substance abuse/dependence (except nicotine) and current or past history of psychotic disorders. Individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. To assess whether the effects of methylphenidate altered regional brain activations toward or away from the cerebral processes observed in healthy individuals, we also included 36 healthy subjects with matching age and gender (30 men; 36.0 ± 9.1 y) for comparison. The Human Investigation committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent before study participation.
Procedures of Behavioral Assessments and fMRI.
Each patient participated in two sessions, in which either saline (P session) or methylphenidate (M session, 0.5 mg/kg of body weight) was injected intravenously, approximately 45 min before fMRI. The two sessions were scheduled 48 h apart, the order of which was counter balanced across subjects, with five subjects participating in the P session first. On the day of fMRI, a research nurse evaluated and confirmed study consent of the patient before setting up an i.v. line and electronic monitoring of heart rate and blood pressure. For 30 min before the injection, a research assistant assessed patient's craving, anxiety, and euphoria (as experienced during cocaine use) each on a visual analog scale (from 0 to 10) every 5 min. Heart rate and blood pressure were recorded every 10 min. The psychological assessments and heart rate and blood pressure recordings continued every 5 min for 30 min after the injection and, after the patients were transported to the scanner, every 10 min until approximately 30 min after completion of imaging. Patients continued to be closely monitored for 6 h after they returned to the inpatient unit. No adverse events occurred throughout the entire study.
Behavioral Task.
We used a simple reaction time task in this stop signal paradigm (39, 40, 63–65). There were two trial types, “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval (foreperiod) ranging between 1 and 5 s, the dot turned into a circle (the go signal), which served as an imperative stimulus, prompting the subjects to quickly press a button. The circle vanished at a button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press before the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. The remaining one quarter were stop trials. In a stop trial, an additional “X,” the stop signal, appeared after and replaced the go signal. The subjects were told to withhold a button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop signal delay (SSD)—the time interval between the go and stop signal—started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 64 ms; conversely, if they failed, SSD decreased by 64 ms (66). There was an intertrial interval of 2 s. Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Before the fMRI study, each subject had a practice session outside the scanner. In the scanner each subject completed four 10-min runs of the task. Depending on the actual stimulus timing (trials varied in foreperiod duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure we anticipated that the subjects would succeed in withholding their response in approximately half of the stop trials.
One way to understand the stop signal task is in terms of a horse race model with a go process and a stop process racing toward a finishing line (67). The go process prepares and generates the movement, whereas the stop process inhibits movement initiation, whichever process finishes first determines whether a response will be initiated or not. Importantly, the go and stop processes race toward the activation threshold independently. Thus, the time required for the stop signal to be processed so a response is withheld (i.e., SSRT) can be computed on the basis of the go trial reaction time (RT) distribution and the odds of successful inhibits for different time delays between go and stop signals. This is done by estimating the critical SSD at which a response can be correctly stopped in approximately 50% of the stop trials. With the assumptions of this horse race model, the SSRT could then be computed for each individual subject by subtracting the critical SSD from the median go trial RT. Generally speaking, the SSRT is the time required for a subject to cancel the movement after seeing the stop signal. A long SSRT indicates poor response inhibition.
We also computed the foreperiod effect as an index of motor preparedness during the SST (39, 68). Briefly, longer foreperiod is associated with faster response time (69, 70). Thus, RT of go trials with a foreperiod between 3 and 5 s were compared with those with one between 1 and 3 s, and the effect size of RT difference was defined as foreperiod effect. It is also known that in an RT task, the RT of a correct response is prolonged following an error, compared with other correct responses, and this prolonged RT is thought to reflect cognitive processes involved in error monitoring (71). We thus computed the RT difference between the go trials that followed a stop error and those that followed another go trial and termed this RT difference “posterror slowing” (63). These measures, as well as the median go trial RT, go trial success rate, and stop trial success rate serve as indices of general task performance.
Imaging Protocol.
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the anterior commissure-posterior commissure (AC-PC) line with repetition time (TR) = 300 ms, echo time (TE) = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional, BOLD signals were then acquired with a single shot gradient echo echo-planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2,004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness of 4 mm and no gap. Three hundred images were acquired in each run for a total of four runs.
Data Analysis and Statistics.
Data were analyzed with Statistical Parametric Mapping (SPM8). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were coregistered with the high resolution structural image and then segmented for normalization to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (72, 73). Finally, images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
Four main types of trial outcome were distinguished: go success (G), go error (F), SS, and SE trial. A statistical analytical design was constructed for each individual subject, using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (74). Additional regressors with the go trial RT and stop trial SSD were included for parametric modulation. Realignment parameters in all six dimensions were also entered in the model. The data were high-pass filtered (1/128-Hz cutoff) to remove low frequency signal drifts. Serial autocorrelation was corrected by a first degree autoregressive (AR) (1) model. The GLM estimated the component of variance that could be explained by each of the regressors.
Following our previous studies, we constructed for each individual subject a contrast SS > SE to assess regional processes related to response inhibition (26, 39, 40). In group analyses, we used a whole brain linear regression to explore regions that showed activations during SS > SE that varied linearly with the difference in SSRT. Specifically, a contrast for SS > SE between methylphenidate and placebo conditions was made for each individual subject and the difference in SSRT between methylphenidate and placebo used as a vector for whole brain linear regressions.
In region of interest (ROI) analyses, we used MarsBaR (75) to compute for each individual subject the effect size of activity change for the ROIs derived from group analyses. The effect size rather than mean difference in brain activity was derived for correlation with behavioral measures to account for individual differences in the variance of the mean. The effect size measures of regional brain activation were correlated across subjects with the difference in the changes (from baseline) in euphoria, anxiety, and craving as well as the changes (from baseline) in heart rate, systolic, and diastolic blood pressure, and with changes in stop signal performance including the SSRT.
Supplementary Material
Acknowledgments
We thank Andreas Rodrigues and Nikkia Moss for their assistance in patient recruitment and staff at the Clinical Neuroscience Research Unit, Connecticut Mental Health Center, Connecticut Department of Mental Health and Addiction Services for their inpatient care. We are also grateful to Bill Hoffman and Anne O'Connor of the Yale Clinical Center of Investigation and staff at the Hospital Research Unit for their coordination and nursing support. This study was supported by National Institutes of Health Grants R03DA022395, K12DA000167, K02DA026990, and K24-DA017899 and Clinical Translational Science Award Grant UL1 RR024139 from the National Center for Research Resources and National Institutes of Health Roadmap for Medical Research. The study also receives partial support from the State of Connecticut.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002467107/-/DCSupplemental.
References
- 1.de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush G, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 7.Konrad K, Neufang S, Fink GR, Herpertz-Dahlmann B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: Results from a longitudinal functional MRI study. J Am Acad Child Adolesc Psychiatry. 2007;46:1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- 8.Rubia K, et al. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 10.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain SR, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Dodds CM, et al. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honey GD, et al. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson B, et al. Amphetamine modulates human incentive processing. Neuron. 2004;43:261–269. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Müller U, et al. Plasma level-dependent effects of methylphenidate on task-related functional magnetic resonance imaging signal changes. Psychopharmacology (Berl) 2005;180:624–633. doi: 10.1007/s00213-005-2264-9. [DOI] [PubMed] [Google Scholar]
- 16.Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology (Berl) 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- 17.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 18.Schlösser RG, et al. Dopaminergic modulation of brain systems subserving decision making under uncertainty: A study with fMRI and methylphenidate challenge. Synapse. 2009;63:429–442. doi: 10.1002/syn.20621. [DOI] [PubMed] [Google Scholar]
- 19.Udo de Haes JI, Maguire RP, Jager PL, Paans AM, den Boer JA. Methylphenidate-induced activation of the anterior cingulate but not the striatum: A [15O]H2O PET study in healthy volunteers. Hum Brain Mapp. 2007;28:625–635. doi: 10.1002/hbm.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaidya CJ, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND, et al. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overtoom CC, et al. Methylphenidate restores link between stop-signal sensory impact and successful stopping in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:614–619. doi: 10.1016/j.biopsych.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS ONE. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 25.Li C-SR, Milivojevic V, Kemp KA, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Li C-SR, et al. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Castells X, et al. Efficacy of central nervous system stimulant treatment for cocaine dependence: A systematic review and meta-analysis of randomized controlled clinical trials. Addiction. 2007;102:1871–1887. doi: 10.1111/j.1360-0443.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- 29.Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin Investig Drugs. 2002;11:491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- 30.Moeller FG, Schmitz JM, Herin D, Kjome KL. Use of stimulants to treat cocaine and methamphetamine abuse. Curr Psychiatry Rep. 2008;10:385–391. doi: 10.1007/s11920-008-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vansickel AR, Fillmorex MT, Hays LR, Rush CR. Effects of potential agonist-replacement therapies for stimulant dependence on inhibitory control in cocaine abusers. Am J Drug Alcohol Abuse. 2008;34:293–305. doi: 10.1080/00952990802013565. [DOI] [PubMed] [Google Scholar]
- 32.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gawin F, Kleber H. Pharmacologic treatments of cocaine abuse. Psychiatr Clin North Am. 1986;9:573–583. [PubMed] [Google Scholar]
- 34.Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 35.Kufahl PR, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Li SJ, et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: Relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duann J-R, Ide JS, Luo X, Li C-SR. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C-SR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C-SR, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao HH, Luo X, Chang JL, Li CS. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—an intra-subject analysis. BMC Neurosci. 2009;10:75. doi: 10.1186/1471-2202-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDonald AW, 3rd, Becker TM, Carter CS. Functional magnetic resonance imaging study of cognitive control in the healthy relatives of schizophrenia patients. Biol Psychiatry. 2006;60:1241–1249. doi: 10.1016/j.biopsych.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Smoski MJ, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner G, et al. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci. 2008;33:199–208. [PMC free article] [PubMed] [Google Scholar]
- 45.Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 46.Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 48.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- 52.Hermann A, et al. Emotion regulation in spider phobia: Role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–267. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pardini M, Krueger F, Raymont V, Grafman J. Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology. 2010;74:749–754. doi: 10.1212/WNL.0b013e3181d25b6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamm C, Lewis MD. Developmental change in the neurophysiological correlates of self-regulation in high- and low-emotion conditions. Dev Neuropsychol. 2010;35:156–176. doi: 10.1080/87565640903526512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krueger F, et al. The neural bases of key competencies of emotional intelligence. Proc Natl Acad Sci USA. 2009;106:22486–22491. doi: 10.1073/pnas.0912568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuncel M, et al. Mechanism of the blood pressure—raising effect of cocaine in humans. Circulation. 2002;105:1054–1059. doi: 10.1161/hc0902.104714. [DOI] [PubMed] [Google Scholar]
- 57.Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation. 1999;100:497–502. doi: 10.1161/01.cir.100.5.497. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, et al. Cardiovascular effects of methylphenidate in humans are associated with increases of dopamine in brain and of epinephrine in plasma. Psychopharmacology (Berl) 2003;166:264–270. doi: 10.1007/s00213-002-1340-7. [DOI] [PubMed] [Google Scholar]
- 59.Gollub RL, et al. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 1998;18:724–734. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Rao SM, et al. Effects of methylphenidate on functional MRI blood-oxygen-level-dependent contrast. Am J Psychiatry. 2000;157:1697–1699. doi: 10.1176/appi.ajp.157.10.1697. [DOI] [PubMed] [Google Scholar]
- 61.Devonshire IM, et al. Haemodynamic responses to sensory stimulation are enhanced following acute cocaine administration. Neuroimage. 2004;22:1744–1753. doi: 10.1016/j.neuroimage.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, et al. Peripheral blood pressure changes induced by dobutamine do not alter BOLD signals in the human brain. Neuroimage. 2006;30:745–752. doi: 10.1016/j.neuroimage.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 63.Li C-SR, et al. Neural correlates of post-error slowing in a stop signal task. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C-SR, et al. Error-specific medial cortical and subcortical activity during the stop signal task: A functional magnetic resonance imaging study. Neuroscience. 2008;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: A model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 66.Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- 67.Logan GD. In: Inhibitory Processes in Attention, Memory and Language. Dagenbach D, Carr TH, editors. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- 68.Li CS, Krystal JH, Mathalon DH. Fore-period effect and stop-signal reaction time. Exp Brain Res. 2005;167:305–309. doi: 10.1007/s00221-005-0110-2. [DOI] [PubMed] [Google Scholar]
- 69.Bertelson P, Tisseyre F. The time-course of preparation with regular and irregular foreperiods. Q J Exp Psychol. 1968;20:297–300. doi: 10.1080/14640746808400165. [DOI] [PubMed] [Google Scholar]
- 70.Woodrow H. The measurement of attention. Psychol Monogr. 1914;17:1–158. [Google Scholar]
- 71.Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- 72.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friston KJ, et al. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- 74.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 75.Brett M, Anton J-L, Valabregue R, Poline J-B. 2002. Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available on CD-ROM in NeuroImage 16.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.