Abstract
Mutations of the KCNJ10 (Kir4.1) K+ channel underlie autosomal recessive epilepsy, ataxia, sensorineural deafness, and (a salt-wasting) renal tubulopathy (EAST) syndrome. We investigated the localization of KCNJ10 and the homologous KCNJ16 in kidney and the functional consequences of KCNJ10 mutations found in our patients with EAST syndrome. Kcnj10 and Kcnj16 were found in the basolateral membrane of mouse distal convoluted tubules, connecting tubules, and cortical collecting ducts. In the human kidney, KCNJ10 staining was additionally observed in the basolateral membrane of the cortical thick ascending limb of Henle's loop. EM of distal tubular cells of a patient with EAST syndrome showed reduced basal infoldings in this nephron segment, which likely reflects the morphological consequences of the impaired salt reabsorption capacity. When expressed in CHO and HEK293 cells, the KCNJ10 mutations R65P, G77R, and R175Q caused a marked impairment of channel function. R199X showed complete loss of function. Single-channel analysis revealed a strongly reduced mean open time. Qualitatively similar results were obtained with coexpression of KCNJ10/KCNJ16, suggesting a dominance of KCNJ10 function in native renal KCNJ10/KCNJ16 heteromers. The decrease in the current of R65P and R175Q was mainly caused by a remarkable shift of pH sensitivity to the alkaline range. In summary, EAST mutations of KCNJ10 lead to impaired channel function and structural changes in distal convoluted tubules. Intriguingly, the metabolic alkalosis present in patients carrying the R65P mutation possibly improves residual function of KCNJ10, which shows higher activity at alkaline pH.
Keywords: Bartter, Gitelman, kidney, Kir4.1, SeSAME
The kidneys play a key role in electrolyte and water homeostasis of the body. In renal salt-wasting disorders, specific transport functions of tubular epithelial cells are impaired. Defects of salt transport in the thick ascending loop of Henle and the distal convoluted tubule underlie the salt-wasting states observed in Bartter's syndrome(s) and Gitelman's syndrome, respectively (1). We and others described a unique autosomal recessive form of Gitelman-like renal salt wasting caused by mutations in the potassium channel KCNJ10 (2, 3). KNCJ10 (Kir4.1) is expressed in various tissues, including brain, inner ear, eye, and kidney (4, 5). Patients suffering from KCNJ10 mutations display a complex combination of features we called EAST syndrome: epilepsy, ataxia, sensorineural deafness, and (a salt-wasting) renal tubulopathy. The renal features resemble those of Gitelman's syndrome and comprise urinary Na+ loss, activation of the renin-angiotensin-aldosterone system, hypokalemic metabolic alkalosis, hypomagnesemia, and hypocalciuria (2).
In C57BL6 mouse kidney, Kcnj10 is expressed in distal convoluted tubules starting from the macula densa down to the early cortical collecting duct (2, 6). In CD1 mice, Kcnj10 and related Kcnj16 (Kir5.1) are also found in the cortical thick ascending limb (7). Kcnj10 and Kcnj16 are localized in the basolateral membrane, where they establish the hyperpolarized membrane voltage needed for electrogenic ion transport (e.g., Cl− exit and Na+-coupled Ca2+ and Mg2+ export) (8). Additionally, KCNJ10/KCNJ16 activity is required for Na+/K+-ATPase pump activity. Basolateral Na+/K+-ATPases take up K+ from the narrow space of the basolateral invaginations of the plasma membrane. During Na+/K+-ATPase activity, basolateral K+ becomes a rate-limiting factor limiting further pump activity. K+ efflux through KCNJ10/KCNJ16 allows K+ to recycle, and thereby permits continuous Na+/K+-ATPase activity [so-called “pump-leak coupling” (9–12)]. Although KCNJ10 and KCNJ16 are not the only K+ channels expressed in these nephron segments, they appear to be critical for the pump-leak coupling, because human patients and Kcnj10−/− mice display deficits of the reabsorptive function in the nephron segments mentioned above (2).
In this study, we have investigated the localization of KCNJ10 in mouse and human kidney and the functional consequences of mutations of KCNJ10 that we have found in our patients with EAST syndrome. Notably, the renal biopsy of a patient with EAST syndrome disclosed loss of basolateral infoldings of distal convoluted tubular cells as a morphological correlate of the impaired transport function resulting in salt wasting. The KCNJ10 mutations G77R and R199X showed almost complete loss of function. The R65P mutation and the newly described R175Q mutation resulted in mutated proteins with small residual function and substantially changed pH sensitivity with IC50 values in the alkaline range. The changed pH sensitivity in these mutations may therefore have implications for the treatment of patients carrying these mutations.
Results
Localization of KCNJ10 and KCNJ16 in Mouse and Human Kidney.
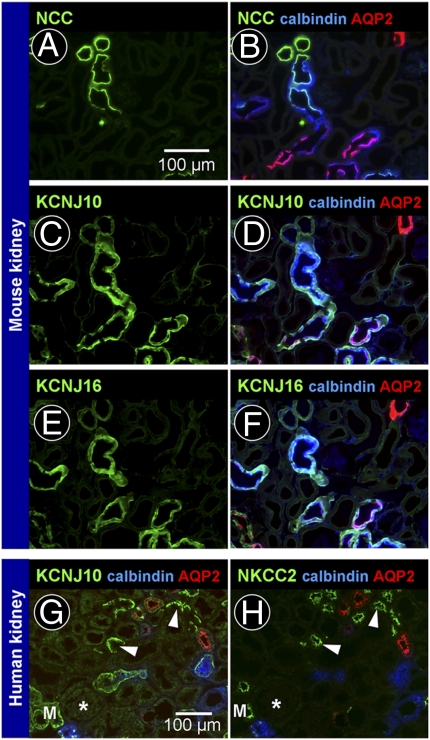
KCNJ10 and KCNJ16 are inwardly rectifying K+ channels expressed in renal tubules. It has been proposed that both channels form heterotetramers to build functional channels in native tissues. We therefore examined the renal localization of both channel proteins by immunofluorescence. As described previously, in C57BL6 mouse kidney, Kcnj10 labeling was found in early and late distal convoluted tubules, starting sharply at the macula densa, and also in connecting tubules and early cortical collecting ducts. Aquaporin-2-negative intercalated cells were not labeled by the KCNJ10 antibody. Using consecutive sections, Kcnj16 showed a distribution similar to Kcnj10, suggesting that both channels could form heterotetramers in native tubular cells (Fig. 1 A–F). In human kidney, localization distal to the macula densa was similar to that observed in C57BL6 mouse kidney. However, in addition to this, KCNJ10 was found in cortical thick ascending limbs, as evidenced by staining of Na+2Cl−K+ cotransporter (NKCC2) in consecutive sections (Fig. 1 G and H).
Fig. 1.
KCNJ10 and KCNJ16 in the nephron. (A–F) Localization of Kcnj10 and Kcnj16 in mouse kidney. A and B, C and D, and E and F are consecutive sections. (A and B) Markers of distal convoluted tubules [NaCl cotransporter (NCC), green], connecting tubules and collecting ducts (calbindin, blue), and principal cells of connecting tubules and collecting ducts [aquaporin-2 (AQP2), red]. (C and D) Kcnj10 (green) was localized in the basolateral membrane of early and late distal convoluted tubules, connecting tubules, and early cortical collecting ducts. (E and F) Distribution of Kcnj16 (green) was similar to that of Kcnj10. (G and H) Localization of KCNJ10 in consecutive sections of human kidney. KCNJ10 labeling (green) was observed in the same segments as in mouse kidney. In addition, KCNJ10 was found in cortical thick ascending limbs (arrowheads, NKCC2-positive tubules; asterisk, glomerulus; M, macula densa).
Changes in the Distal Convoluted Tubule in EAST Syndrome.
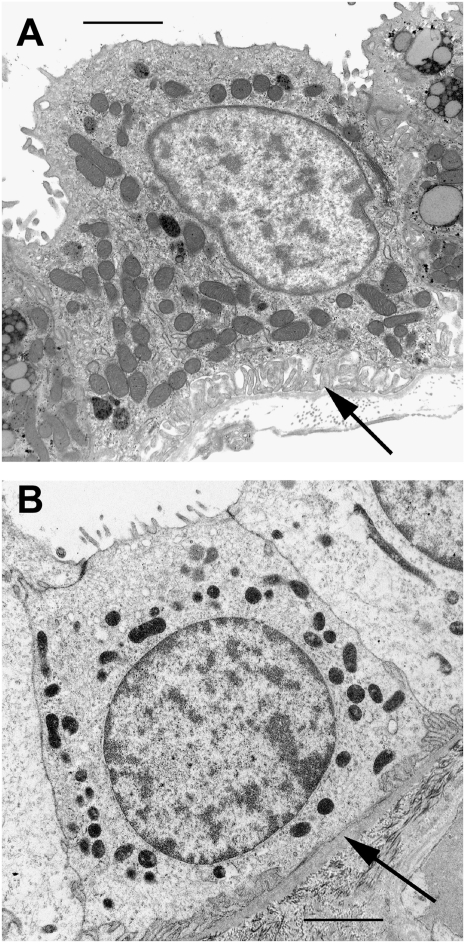
Patients with EAST syndrome display renal salt wasting and electrolyte disturbances that resemble the clinical features of impaired distal tubular salt transport in Gitelman's syndrome. We investigated the distal tubular morphology of a patient who had EAST syndrome using renal biopsy material. EM of the distal convoluted tubule revealed a decreased number of mitochondria and reduction of basolateral infoldings (Fig. 2). These morphological data confirm the concept of reduced reabsorptive capacity of the distal convoluted tubule when basolateral K+ efflux is impaired.
Fig. 2.
Morphological changes of distal convoluted tubule cells in EAST syndrome. Electron micrographs of distal convoluted tubule cells of a control (A) and an EAST patient (B). The patient with EAST syndrome shows a decreased number of mitochondria and reduction of basolateral infoldings (arrows). The apparent difference in the density of mitochondria between A and B is attributable to slight differences in fixation. (Scale bars: 2 μm.)
Functional Consequences of KCNJ10 Mutations Found in EAST Syndrome.
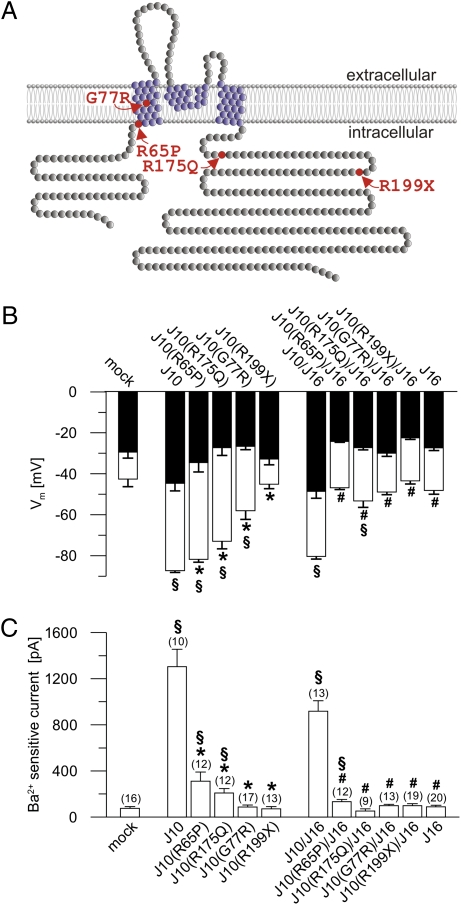
We have identified mutations of the potassium channel KCNJ10 that are causative for a renal salt-wasting disease, EAST syndrome. A first set of experiments using Xenopus oocytes suggested a functional deficit of two disease-related KCNJ10 mutations (2). In this study, we performed a more detailed electrophysiological analysis of four KCNJ10 mutations found in our patients with EAST syndrome: c.194G > C resulting in p.R65P, c.229G > C resulting in p.G77R, c.524G > A resulting in p.R175Q, and c.595C > T resulting in p.R199X. The KCNJ10 mutation p.R175Q has not been described previously. Fig. 3A shows a scheme of human KCNJ10 and the localization of the above-mentioned mutations. The mutated KCNJ10 channels were heterologously expressed in CHO cells.
Fig. 3.
Mutated KCNJ10 channels display reduced function in whole-cell experiments. (A) Predicted model of the human KCNJ10 membrane topology according to Uniprot (ID P78508; available at http://www.uniprot.org/). Four of these subunits are thought to build a functional channel. KCNJ10 may also form heteromers with KCNJ16, which has similar topology. The KCNJ10 mutations of the patients with EAST syndrome investigated in this study are colored in red. (B) Effects of KCNJ10 mutations found in patients with EAST syndrome on the membrane voltage (Vm) of transfected CHO cells. The group on the right displays the effect of cotransfection with KCNJ16. White columns show Vm under control conditions, and black columns show Vm after inhibition with Ba2+ (5 mM). Numbers of experiments are shown in parentheses in C. All mutant channels led to a more depolarized Vm compared with the WT KCNJ10 channel (J10). KCNJ16 (J16) alone or in cotransfection with KCNJ10 mutants did not hyperpolarize the cell membrane. (C) Ba2+-sensitive K+ outward current of the cells shown in B [clamped voltage (Vc) = −30 mV]. Transfection with mutated KCNJ10 channels led to a strong reduction in the current (R65P > R175Q) or to a total loss (G77R, R199X). Cotransfection of KCNJ16 with the mutant KCNJ10 channels also showed a strongly diminished current compared with cells cotransfected with KCNJ10/KCNJ16. *, different from WT KCNJ10; #, different from WT KCNJ10/KCNJ16; §, different from mock-transfected cells.
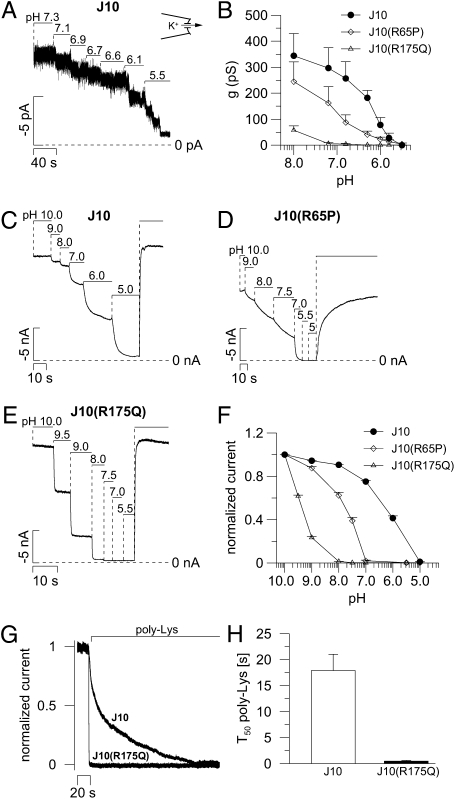
The membrane voltage of cells expressing human WT KCNJ10 was hyperpolarized close to the equilibrium potential of K+ (Fig. 3B). Cells expressing R65P > R175Q > G77R were hyperpolarized compared with mock-transfected cells but less so than WT KCNJ10-expressing cells. R199X-expressing cells were not different from mock-transfected cells, indicating a complete loss of function by this mutation. Application of Ba2+ (5 mM) led to a strong depolarization attributable to inhibition of Ba2+-sensitive KCNJ10 channels. As expected, the Ba2+-induced depolarization in R199X cells was not different from that observed in mock-transfected cells (Fig. 3B). As a measure of the KCNJ10-specific K+ current, the Ba2+-sensitive outward current was determined (Fig. 3C). To minimize endogenous Cl− currents, a clamp voltage of −30 mV was chosen. Expression of WT KCNJ10 induced large Ba2+-sensitive outward currents. Currents of mutated channels were reduced (R65P > R175Q) or not different from those of mock-transfected cells (G77R and R199X).
In native tissues, KCNJ10 is thought to form heterotetramers with KCNJ16. Therefore, we tested the effect of KCNJ16 cotransfection. To avoid contamination with homomeric KCNJ10 channels, KCNJ16 was cotransfected with KCNJ10 in a 10:1 stoichiometry ratio (13, 14). As reported previously, KCNJ16 alone did not hyperpolarize the membrane and did not induce measurable currents (15). Cells coexpressing WT KCNJ10 with KCNJ16 were strongly hyperpolarized and exhibited a large Ba2+-sensitive outward current (5) (Fig. 3 B and C). By contrast, the currents of cells coexpressing mutated KCNJ10 with KCNJ16 were much smaller than those of cells transfected with WT KCNJ10/KCNJ16, and the membrane voltage was similar to that of mock-transfected cells. All KCNJ10 mutations found in our patients therefore led to complete or partial loss of function when expressed alone or together with KCNJ16.
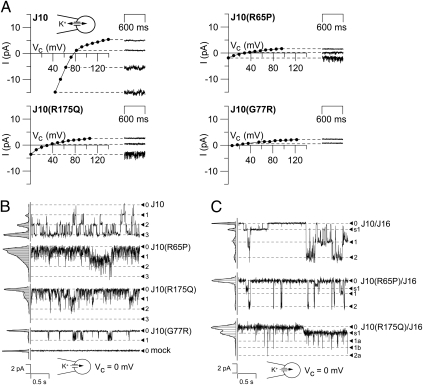
Effects of KCNJ10 Mutations at the Single-Channel Level.
The mutant channels R65P, R175Q, and, to a lesser extent, G77R showed residual function in whole-cell experiments. Here, we investigated the single-channel properties of mutated channels using transfected HEK cells. Using the cell-attached configuration with a cytosol-like pipette solution, WT KCNJ10-expressing cells showed large inwardly rectifying currents across the patch membrane. In contrast to this, R65P-, R175Q-, and G77R-expressing cells showed strongly reduced current amplitudes and slight or no inward rectification (Fig. 4A). WT KCNJ10 channels showed clear single-channel levels (25–30 pS) and a high open probability (70–80%, n = 10) (Fig. 4B). R65P and R175Q showed channel flickering with no clear single-channel levels. Because of channel flickering, it was very difficult to determine single-channel conductances of mutant channels. Apparently, they were in the same range as the WT channels. The channel open probability was strongly reduced to 20–30% for R65P (n = 11) and to 10–15% for R175Q (n = 3). In G77R-expressing cells, channel activity was almost absent and only rare channel events were observed corresponding to an open probability of about 0.5% (n = 10).
Fig. 4.
Single-channel properties. (A) Patch current in cell-attached configuration with a cytosol-like pipette solution. HEK293 cells transfected with WT KCNJ10 channels (J10) showed large inwardly rectifying currents across the patch membrane. In contrast to WT cells, expression of the R65P, R175Q, and G77R mutants led to a strongly reduced current amplitude (patches showed predominantly nonspecific baseline currents) with diminished inward rectification. (B and C) Single-channel experiments on transfected HEK293 cells in cell-attached configuration at clamped voltage (Vc) = 0 mV. (B) Cells transfected with WT KCNJ10 channels showed clear single-channel levels and a high open probability (70–80%). Transfection with R65P and R175Q led to a strongly reduced open probability with channel flickering, and levels were hardly detectable. In G77R-expressing cells, channel events were rare. (C) Coexpression of KCNJ10 with KCNJ16 resulted in single-channel events with variable current amplitude. Large amplitudes of about 50–70 pS and sublevels with smaller sizes were observed. Heteromers of KCNJ16 with R65P and R175Q showed similar single-channel amplitude but reduced mean open probability.
Coexpression with KCNJ16 resulted in heteromeric KCNJ10/KCNJ16 channels with biophysical properties different from those of homomeric KCNJ10 channels (Fig. 4C). KCNJ10/KCNJ16-containing patches showed single-channel events of variable current amplitude. The most frequently observed amplitude was 50–70 pS [double the amplitude of homomeric KCNJ10 channels (15)], but sublevels of smaller size were regularly observed, as reported previously (13). Heteromers of KCNJ16 with R65P and R175Q (the mutations with relatively large residual function) exhibited reduced open probability caused by shortening of the mean open time. Single-channel amplitude of these channels was similar to that of KCNJ10/KCNJ16 heteromers, and channel substates were also observed (Fig. 4C).
pH Sensitivity of Mutated KCNJ10 Channels.
KCNJ10 channels are known to be strongly regulated by variations of cytosolic pH with activation by alkaline pH and channel inhibition by acidic pH values. Here, we explored the effects of R65P and R175Q mutations on pH sensitivity of KCNJ10 channels in excised inside-out patches (Fig. 5). Similar to published data (16), half-maximal activity (IC50) of WT KCNJ10 channels expressed in HEK293 cells was observed at pH 6.3 (Fig. 5 A and B). Channel activity of R65P at physiological pH was strongly reduced, and the IC50 value was shifted to a more alkaline pH. R175Q channels were almost inactive in the range of physiological pH, and only small currents could be measured at pH 8. To investigate pH sensitivity further at more alkaline pH values, excised membrane patches from Xenopus laevis oocytes were used. Similar to the results in HEK293 cells, R65P showed a shift of the IC50 from pH 6.37 (WT KCNJ10; Fig. 5 C and F) to pH 7.86 (Fig. 5 D and F). The mutant R175Q exhibited large currents at alkaline pH and a dramatic shift of the IC50 to pH 9.35 (Fig. 5 E and F).
Fig. 5.
KCNJ10 mutations leading to changed pH sensitivity. (A) Typical trace of the pH effect in excised inside-out patch of a KCNJ10-transfected HEK293 cell (125 mM K+ in pipette and bath). For simplicity, the negative inward current at clamped voltage (Vc) = −20 mV is upwardly reflected. (B) Summary of experiments as shown in A. The IC50 of WT KCNJ10 channels (n = 5) was pH 6.3, and the IC50 of the R65P mutant was pH 7.1 (n = 5). R175Q channels (n = 7) were almost inactive at physiological pH, and only small currents were detected at pH 8. (C–E) Typical traces for pH regulation in excised inside-out macropatches of X. laevis oocytes. For simplicity, the negative inward current at Vc = −80 mV is upwardly reflected. (F) Summary of experiments as shown in C–E. Currents were normalized to the maximal currents of the patches. (G) Estimation of channel PIP2 affinities by competitive PIP2 binding of poly-Lys (250 mg/L, saturated solution) obtained at pH 8.5. (H) Summary of experiments as shown in G. The time of half-maximal current inhibition (T50) is depicted (n = 4 each).
Phosphatidylinositol 4,5-Bisphosphate Sensitivity of R175Q.
The KCNJ1 residue R188 (corresponding to R175 of KCNJ10) was shown to bind phosphatidylinositol 4,5-bisphosphate (PIP2), which regulates channel activity (17). By competitive binding of PIP2, polylysine (poly-Lys; 250 mg/L, saturated solution) led to channel inhibition. The time constant of the poly-Lys inhibition was much faster in R175Q (0.47 ± 0.1 s, n = 4) compared with WT KCNJ10 (17.89 ± 3.1 s, n = 4), indicating reduced PIP2 affinity of the R175Q mutant. The velocity for inhibition of R175Q was in the range of our solutions exchange rate (~0.5 s); thus, the real rate of inhibition might be even faster (Fig. 5 G and H).
Discussion
Mutations of human KCNJ10 cause the EAST [or seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME)] syndrome, an autosomal recessive disorder (2, 3). The cause of the tubulopathy is the loss of KCNJ10 K+ channel activity in the basolateral membrane of the distal nephron, resulting in impaired tubular transport capacity. Here, we investigated the localization of KCNJ10 in mouse and human kidney and elucidated the functional consequences of KCNJ10 mutations found in our patients who had EAST syndrome.
We found Kcnj10-specific immunofluorescence in the basolateral membrane of distal convoluted tubules, connecting tubules, and early collecting ducts of C57BL6 mouse kidney similar to that described in rat kidney (4). Kcnj10 expression started at the macula densa and was absent in the cortical thick ascending limb of Henle's loop. In contrast to our findings, Lachheb et al. (7) also observed Kcnj10 expression in the cortical thick ascending limb of CD1 mice, suggesting strain-dependent variability of Kcnj10 localization. In the present study, we also performed experiments on human kidney. In human cortex, strong KCNJ10 staining was found in cortical thick ascending limbs, distal convoluted tubules, and, to a lesser extent, aquaporin-2-positive principal cells of connecting tubules and cortical collecting ducts. The relatively broad expression of KCNJ10 in human distal tubules possibly accounts for the severity of renal salt wasting and electrolyte imbalance in patients with EAST syndrome and might have an impact on our understanding of ion transport in the cortical thick ascending limb.
In distal convoluted tubules, salt transport occurs mostly transcellularly, requiring high Na+/K+-ATPase pump activity. As a functional adaptation, these tubular cells are mitochondria-rich and have deep basolateral infoldings, leading to expansion of the basolateral membrane surface. The infoldings are decorated with Na+/K+-ATPases and with KCNJ10 and KCNJ16 channels for the recycling of K+. Under conditions with lowered transport activity [e.g., in mice lacking the luminal NaCl cotransporter (18)], the number of mitochondria and the depth of basolateral infoldings are strongly reduced. Our previous study suggested that transport in distal tubules is similarly decreased in patients who have EAST syndrome (2). Here, we present EM data indicating that distal convoluted tubular cells of a patient with EAST syndrome exhibit morphological changes consistent with reduced transport capacity of this segment (i.e., reduced number of mitochondria and basolateral infoldings). These morphological changes support the concept of impaired function of distal convoluted tubules as a cause for salt wasting and electrolyte disturbance in patients who have EAST syndrome.
In patients with EAST/SeSAME syndrome, seven different disease-related mutations have been described so far. In this study, we explored the functional properties of our patients’ four KCNJ10 mutations expressed in mammalian cell systems and X. laevis oocytes. One of them (R175Q) has not been described previously. All the mutations examined showed severely impaired channel activity. In whole-cell experiments, the residual channel function was dependent on the mutation R65P > R175Q > G77R > R199X (R199X displayed complete loss of function). In cell-attached experiments, R65P, R175Q, and G77R channels showed flickering, with a strongly decreased mean open time. In native tissues, KCNJ10 is thought to form heterotetramers with KCNJ16 (7, 13, 13, 15, 19). Coexpression of KCNJ10 mutants with KCNJ16 produced heteromeric channels with impaired activity similar to those observed for homomeric KCNJ10 channels. Heteromers of KCNJ16 with R65P or R175Q showed flickering and shortening of the mean open time. These data are in agreement with the substantial loss of channel function in affected tissues of patients with EAST syndrome.
A characteristic of inwardly rectifying K+ channels (KCNJ or Kir family) is the regulation by intracellular pH (15, 19–23). The mutation R65P is in close proximity to a lysine residue at position 67 that has been shown to determine pH sensitivity of KCNJ10 (24, 25) and KCNJ1 channels (23). Interestingly, R65P showed a significant change of pH sensitivity (i.e., the IC50 values were shifted toward alkaline pH values). In patches from mammalian cells expressing R175Q, measurable currents could only be elicited at pH 8. More alkaline pH values were not applicable in that system. Experiments with more robust macropatches from X. laevis oocytes disclosed a dramatic shift of pH sensitivity in the R175Q mutant, with an IC50 of pH 9.35. The arginine residue R175 is conserved in all 15 human KCNJ channels. In ROMK [KCNJ1 (26)], the corresponding mutation R188Q also led to a substantial shift in pH sensitivity (17, 27) and it was reported to reduce the PIP2 sensitivity of ROMK and Kir6.2 channels (17, 27, 28). Using the PIP2-binding substance poly-Lys, we disclosed a strongly reduced PIP2 affinity of R175Q. This result suggests that this amino acid residue, which is conserved in all human inward rectifiers, is involved in the PIP2 regulation of this channel family. In contrast to the KCNJ10 mutation R175Q of our patient with EAST syndrome, the R188Q mutation of ROMK has not yet been found in patients.
In the KCNJ10 mutant R65P, the alkaline shift of pH sensitivity was smaller than in R175Q and was still in the pathophysiologically relevant pH range. Hypokalemic metabolic alkalosis is characteristic of EAST syndrome. Given the shifted pH sensitivity of R65P, patients with this mutation probably have improved residual channel function in the alkalotic state. Conversely, conditions that lead to acidification, such as exercise or physical stress, could reduce the function of mutated KCNJ10 in patients. This will lead to diminished renal transport and impaired K+ buffering of glial cells, conditions that worsen ataxia and provoke epilepsy. Thus, the acid-base balance of patients with EAST syndrome may have a substantial impact on the severity of the symptoms in EAST syndrome.
Methods
Patients.
Our patients were diagnosed with EAST syndrome as a result of typical clinical findings (epilepsy, ataxia, sensorineural deafness, and renal tubulopathy). Genetic and histological studies were approved by the Institute of Child Health–Great Ormond Street Hospital Research Ethics Committee and were performed after having obtained parental informed consent. Mutations in KCNJ10 were confirmed in all our patients, as reported previously (2). One patient was homozygous for G77R (2); one patient was compound heterozygous for R65P and R199X (3); and another patient, a 14-y-old boy of consanguineous offspring, was homozygous for R175Q. This patient presented with epilepsy from infancy onward and showed ataxia and sensorineural deafness. Blood chemistry tests documented hypomagnesemia and hypokalemia.
Animal Experiments.
C57BL6 mice (3 mo of age) had free access to standard chow and tap water. The experimental protocols were approved by the local councils for animal care and were conducted according to the German law for animal care and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunofluorescence.
Anesthetized mice (isoflurane) were killed by replacement of blood by 0.9% NaCl solution containing 10 IU/mL heparin via a catheter placed into the abdominal aorta. For tissue fixation, mice were then perfused with paraformaldehyde (30 g/l) dissolved in a solution containing 100 mM sucrose, 90 mM NaCl, 15 mM K2HPO4, 1 mM EGTA, and 2 mM MgCl2 (pH 7.4). The kidneys were removed, incubated in a sucrose solution (170 g/l) overnight, and frozen in isomethylbutane. Cryosections (5 μm) were mounted on poly-Lys slides (Kindler). Before incubation with the primary antibodies, sections were incubated in 0.1% SDS (5 min), rinsed, and blocked with BSA (50 g/L, 15 min). Primary and secondary antibodies were diluted in PBS, pH 7.4, containing 0.04% Triton X-100 (Sigma) and 0.5% BSA. Primary antibodies were applied overnight at 4 °C. Polyclonal rabbit antibodies for NaCl cotransporter and NKCC2 were kind gifts from Mark Knepper (National Heart Lung and Blood Institute, Bethesda, MD) (29, 30). Other antibodies were KCNJ10 (Alomone Labs; Fig. S2) and KCNJ16 (custom-made by Davids, Regensburg, Germany; Fig. S1), aquaporin-2 (Santa Cruz), and calbindin (Sigma). Appropriate Alexa dye-coupled secondary antibodies (Invitrogen) were used. Slides were washed in PBS (2 × 5 min) and mounted with fluorescence-free glycergel mounting medium (DakoCytomation).
EM.
A renal biopsy was taken at the age of 7 y from a boy with EAST syndrome and the R65P/R199X mutation. A piece fixed in glutaraldehyde was processed and sectioned for EM. A control specimen that showed no abnormality from a boy of the same age who was biopsied for intermittent hematuria was handled similarly.
Patch Clamp of Mammalian Cells.
For patch-clamp experiments, transient transfected HEK293 cells (single-channel and pH analysis) and CHO cells (whole-cell experiments) were used. Patch-clamp recordings were performed using a custom-made EPC-7–like amplifier (obtained from U. Fröbe, Institute of Physiology, Freiburg, Germany) and an EPC-10 amplifier (HEKA). The patch pipette solution was composed of 95 mM K-gluconate, 30 mM KCl, 4.8 mM Na2HPO4, 1.2 mM NaH2PO4, 5 mM glucose, 2.38 mM MgCl2, 0.726 mM CaCl2, 1 mM EGTA, and 3 mM ATP (pH 7.2). The standard bath solution for whole-cell experiments contained 145 mM NaCl, 1.6 mM K2HPO4, 0.4 mM KH2PO4, 1.3 mM Ca-gluconate, 1 mM MgCl2, 5 mM D-glucose, and 5 mM Hepes. For excised patches, the bath solution was replaced by the pipette solution.
Macropatches of Oocytes.
cRNA from all KCNJ10 constructs, cloned by inserting PCR amplificates (using primers carrying restriction sites) from patient DNA into the pTLB expression vector (a kind gift from T. J. Jentsch, Leibniz-Institut für Molekulare Pharmakologie, Berlin, Germany) using primers carrying restriction sites, was injected into X. laevis oocytes and measured 1–2 d after injection. Patch-clamp recordings were done in the inside-out configuration at a constant membrane voltage of −80 mV under symmetrical K+ conditions (120 mM K+ intra/extra). Different pH solutions were applied to the intracellular side of the membrane via a multibarrel perfusion system that allowed solution exchange within 1 s. All traces shown in Fig. 5 are upward reflections of inward currents at −80 mV. To test the PIP2 sensitivity of KCNJ10, negatively charged PIP2 was clustered by the polycation poly-Lys (P4158; Sigma). The time course of inhibition was used to estimate the strength of KCNJ10/PIP2 interactions (17).
Statistics.
Data are shown as mean values ± SEM from n observations. Paired as well as unpaired Student's t test was used as appropriate. Differences were considered significant if P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Roger D. G. Malcomson (Birmingham Children's Hospital) for EM material and Prof. Dr. Thomas Jentsch (Leibniz-Institut für Molekulare Pharmakologie, Berlin, Germany) for instruments and materials. The expert assistance by Helga Schmidt is acknowledged. This study was supported by Deutsche Forschungsgemeinschaft Grants SFB699 (to M.R., R. Warth, and R. Witzgall) and BA1793/4-2 (to T.B).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003072107/-/DCSupplemental.
References
- 1.Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol. 2006;104:p73–p80. doi: 10.1159/000094001. [DOI] [PubMed] [Google Scholar]
- 2.Bockenhauer D, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholl UI, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito M, et al. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- 5.Takumi T, et al. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- 6.Lourdel S, et al. An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: Similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachheb S, et al. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294:F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- 8.Xi Q, Hoenderop JG, Bindels RJ. Regulation of magnesium reabsorption in DCT. Pflugers Arch. 2009;458:89–98. doi: 10.1007/s00424-008-0601-7. [DOI] [PubMed] [Google Scholar]
- 9.Koefoed-Johnsen V, Ussing HH. The nature of the frog skin potential. Acta Physiol Scand. 1958;42:298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 10.Schultz SG. Pump-leak parallelism in sodium-absorbing epithelia: The role of ATP-regulated potassium channels. J Exp Zool. 1997;279:476–483. doi: 10.1002/(sici)1097-010x(19971201)279:5<476::aid-jez10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Beck JS, Laprade R, Lapointe JY. Coupling between transepithelial Na transport and basolateral K conductance in renal proximal tubule. Am J Physiol. 1994;266:F517–F527. doi: 10.1152/ajprenal.1994.266.4.F517. [DOI] [PubMed] [Google Scholar]
- 12.Giebisch GH. A trail of research on potassium. Kidney Int. 2002;62:1498–1512. doi: 10.1046/j.1523-1755.2002.t01-2-00644.x. [DOI] [PubMed] [Google Scholar]
- 13.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- 14.Konstas AA, Korbmacher C, Tucker SJ. Identification of domains that control the heteromeric assembly of Kir5.1/Kir4.0 potassium channels. Am J Physiol Cell Physiol. 2003;284:C910–C917. doi: 10.1152/ajpcell.00479.2002. [DOI] [PubMed] [Google Scholar]
- 15.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol. 2000;525:587–592. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 18.Loffing J, et al. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman's syndrome. J Am Soc Nephrol. 2004;15:2276–2288. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 19.Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- 20.Bleich M, Schlatter E, Greger R. The luminal K+ channel of the thick ascending limb of Henle's loop. Pflugers Arch. 1990;415:449–460. doi: 10.1007/BF00373623. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Jiang C. Opposite effects of pH on open-state probability and single channel conductance of kir4.1 channels. J Physiol. 1999;520:921–927. doi: 10.1111/j.1469-7793.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNicholas CM, et al. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am J Physiol. 1998;275:F972–F981. doi: 10.1152/ajprenal.1998.275.6.F972. [DOI] [PubMed] [Google Scholar]
- 24.Rapedius M, et al. Control of pH and PIP2 gating in heteromeric Kir4.1/Kir5.1 channels by H-Bonding at the helix-bundle crossing. Channels (Austin) 2007;1:327–330. doi: 10.4161/chan.5176. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, et al. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4.1-Kir5.1 channels by CO2 and pH. J Gen Physiol. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho K, et al. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 27.Leung YM, Zeng WZ, Liou HH, Solaro CR, Huang CL. Phosphatidylinositol 4,5-bisphosphate and intracellular pH regulate the ROMK1 potassium channel via separate but interrelated mechanisms. J Biol Chem. 2000;275:10182–10189. doi: 10.1074/jbc.275.14.10182. [DOI] [PubMed] [Google Scholar]
- 28.Schulze D, Krauter T, Fritzenschaft H, Soom M, Baukrowitz T. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J Biol Chem. 2003;278:10500–10505. doi: 10.1074/jbc.M208413200. [DOI] [PubMed] [Google Scholar]
- 29.Kim GH, et al. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol. 1999;276:F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 30.Kim GH, et al. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.