Abstract
Advances in systems biology have allowed for global analyses of mRNA and protein expression, but large-scale studies of protein dynamics and turnover have not been conducted in vivo. Protein turnover is an important metabolic and regulatory mechanism in establishing proteome homeostasis, impacting many physiological and pathological processes. Here, we have used organism-wide isotopic labeling to measure the turnover rates of ~2,500 proteins in multiple mouse tissues, spanning four orders of magnitude. Through comparison of the brain with the liver and blood, we show that within the respective tissues, proteins performing similar functions often have similar turnover rates. Proteins in the brain have significantly slower turnover (average lifetime of 9.0 d) compared with those of the liver (3.0 d) and blood (3.5 d). Within some organelles (such as mitochondria), proteins have a narrow range of lifetimes, suggesting a synchronized turnover mechanism. Protein subunits within complexes of variable composition have a wide range of lifetimes, whereas those within well-defined complexes turn over in a coordinated manner. Together, the data represent the most comprehensive in vivo analysis of mammalian proteome turnover to date. The developed methodology can be adapted to assess in vivo proteome homeostasis in any model organism that will tolerate a labeled diet and may be particularly useful in the analysis of neurodegenerative diseases in vivo.
Keywords: isotopic labeling, protein, turnover, degradation, in vivo
Protein molecules are in dynamic equilibrium in vivo: they are continuously synthesized and degraded during the lifetime of the organism (1, 2). The turnover rate of proteins can vary from minutes to years, often conforming to their biological functions (3, 4). The constant renewal of the protein population is an energy-intensive process, yet it allows the cell to rapidly modulate protein levels in response to changes in the environment (5, 6). Proper proteome dynamics are critical to normal development and maintenance of health (7, 8). For example, the dysregulation of protein turnover has been implicated in the aging process (9), increased degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is a primary cause of cystic fibrosis (10), and the inability to clear protein aggregates leads to pathogenic accumulations in Alzheimer's, Parkinson's, Creutzfeldt-Jakob, and other age-related diseases (11).
The turnover rate of a protein is established by its relative rates of synthesis and catabolism. Thus, the lifetime of a protein is influenced by a number of regulated processes at the level of the cell (transcription, translation, proteolysis, and autophagy) and tissue (development and regeneration) as well as its biochemical properties (structural stability, hydrophobicity, and sequence motifs) (1, 12–15). The ability to measure turnover rates on a proteome-wide scale can help elucidate the interplay between these regulated processes and identify novel variables that play a role in proteome homeostasis. It can also identify proteins whose dysregulation influences or results from pathological processes.
Traditionally, protein turnover has been studied by measuring the incorporation of radioactive, tracer amino acids into proteins or bulk tissues (16–20). The advent of modern proteomics has enabled scientists to use mass spectrometry to detect the incorporation of stable isotopes into proteins (21, 22). In measuring turnover rates, the latter approach offers three potential advantages. First, a large number of proteins can be simultaneously analyzed within a biological sample. Second, labeling can be conducted with fully (100%) labeled amino acids. Therefore, turnover rates can be assessed by the single-step kinetics of amino acid incorporation without conducting complex pulse-chase analyses. Third, whereas tracer methods can only measure the total incorporation of label, mass spectrometry can analyze the population distribution of partially labeled species for a given protein. Thus, turnover rates can be measured in instances in which upstream processes, such as label uptake into tissue, are rate limiting (7, 8).
Recent studies have shown that rats can be isotopically labeled using a diet source supplemented with 15N-enriched, blue-green algae (Spirulina platensis) (23). Here, we have used this approach to measure the in vivo turnover kinetics of proteins in the brains of wild-type, inbred mice (FVB) and provide a comparison of these dynamics to the blood and liver proteomes.
Results
Large-Scale Production of Ubiquitously 15N-Labeled Algae.
To obtain the algae necessary for long-term labeling studies, we constructed a closed-loop bioreactor based on a bubble-lift circulator (Fig. S1A and SI Text). Using 15N-enriched NaNO3 as the sole nitrogen source, we produced near-uniform 15N-labeled Spirulina at a yield of 3 g of algae per liter of broth. Mass spectral analysis of the Spirulina indicated that the final isotopic enrichment was >99.5% 15N (Dataset S1). The labeled feed required for the entirety of our studies was supplied by ~60 L of Spirulina culture.
Maintaining Mice on Algae Diet.
Mice fed a diet based on Spirulina had no subchronic toxicities after 13 wk of continuous feeding (24). Our diet formulation was similar to published protocols (21, 23). Mice were examined daily for general health and at least twice a week for body mass. Body mass fluctuated daily (Fig. S1B), but no mice lost or gained a significant fraction (>20%) of their body mass subsequent to the introduction of the algae diet. At nine time points, mice were sacrificed and the labeling kinetics of proteins in the brain, liver, and blood were analyzed (Fig. 1, Methods).
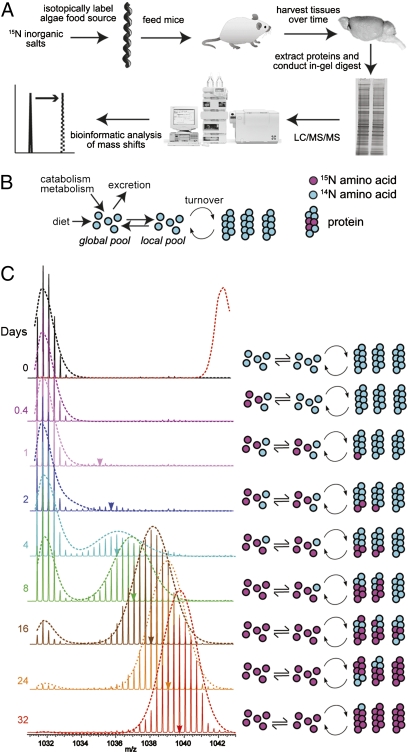
Fig. 1.
Protocol, model, and analysis of global turnover rates. (A) Experimental protocol. 15N inorganic salts are used to make broth for cultures of Spirulina. Dried 15N-labeled algae is used to supply protein in mouse diet. At designated time points, samples were collected. Tissues were homogenized and fractionated according to molecular weight in a 1D SDS/PAGE gel. In-gel digests liberate peptides from the gel, which are then analyzed by LC/MS/MS. The change in molecular weight and the relative populations of labeled peptides are compared in proteome-wide bioinformatic analyses. (B) Three-pool kinetic model for the incorporation of 15N-labeled amino acids into proteins. Amino acids in the global pool circulate between local tissue pools. (C) Sample spectral data showing the incorporation of 15N over time in a Cofilin-1 tryptic peptide. A shift in centroid mass (colored arrowheads) as well as the change in the relative ratios of unlabeled to labeled peptide populations (integrated peak areas) increase with time. The observed data fit with predictions in mass spectral changes as a function of labeling time as calculated by a three-pool model (dotted line overlaying each spectrum.) The dotted red line overlaying the 0-d time point represents the predicted fully labeled spectrum. The corresponding schematics on the Right represent the theorized labeling of the protein, local amino acid, and global amino acid pools in accordance with the three-pool model.
Peptide Identification.
The computational analysis of the data was conducted using a novel suite of computer scripts (SI Text). All processed data, including the peptides, proteins, and their measured kinetic parameters, are included in Dataset S2. All raw data, including LC elution profiles, survey scans, and MS/MS spectra, are available upon request. Only peptides assigned with a greater than 95% confidence in a Protein Prospector reverse database search (25) were used in subsequent analysis. For samples collected after the initiation of labeling, 15N-labeled peptides were identified on the basis of (i) molecular weight region of the gel; (ii) the expected LC retention time, as observed in the 0-d sample; and (iii) expected mass distribution based on the elemental composition of the peptide, natural isotopic distribution of C, O, S, and H atoms, and 14N/15N ratio ranging from 0.0037 to 0.995 (the natural and algal abundance of 15N, respectively). For 4,619 brain peptides, 7,226 liver peptides and 1,968 blood peptides, we were able to identify and quantify the mass distribution at all nine time points with high statistical confidence (Table 1). Data obtained from duplicate animals at any given time point showed minimal variation (Fig. S2).
Table 1.
Numbers of peptides and proteins analyzed in this study
| Brain | Liver | Blood | |
| Detected peptides (0 d) | 14,971 | 14,653 | 4,670 |
| Peptides used in protein analysis* | 4,619 | 7,226 | 1,968 |
| Total proteins analyzed | 1,010 | 1,122 | 334 |
| 1 peptide† | 379 | 313 | 107 |
| 2–4 peptides† | 353 | 343 | 111 |
| 5–10 peptides† | 165 | 244 | 71 |
| >10 peptides† | 113 | 222 | 45 |
| Unique proteins (total)‡ | 1,716 |
*Only peptides detected in all nine time points were used for protein analysis.
†Number of proteins for which the stated number of peptides were analyzed and averaged.
‡Number of unique proteins in the combined analysis of the three tissues.
As an example of a typical kinetic labeling pattern, the time-dependent mass increase of the brain-derived Cofilin-1 peptide (NIILEEGKEILVGDVGQTVDDPYTTFVK) is shown in Fig. 1C. Two independent parameters of 15N incorporation are evident. First, there is a time-dependent increase in the fraction of the area of the peptide peaks that fall outside the expected unlabeled mass distribution (0 d). Second, the centroid mass of the labeled population (Fig.1C, arrowheads) increases over time. We refer to these two measurements, normalized with respect to the mass difference between unlabeled and fully labeled samples, as “labeled population” and “mass shift,” respectively. For the Cofilin-1 peptide, a 15N-labeled population is clearly visible in samples obtained after 1 d of labeling (Fig. 1C). The labeled protein population is well resolved from the natural isotopic distribution even at the earliest time points, negating the need for the deconvolution of the natural and isotopic mass distributions.
Plots of the labeled population and mass shift for Cofilin-1 and several other peptides are shown in Fig. 2. Several kinetic trends are evident in the data. First, the increase in mass shift is biphasic, with a rapid initial burst followed by an extended (slow) phase. Second, mass shift kinetics are similar among peptides extracted from the same tissue but different between the three analyzed tissues. Specifically, in the brain, the initial fast phase has a lower amplitude and the second phase has a slower rate in comparison with liver and blood. Third, labeled population has an initial lag phase of ~1 d, followed by a single exponential increase. And lastly, the kinetics of labeled population is highly variable among the analyzed peptides.
Fig. 2.
The kinetics of peptide-labeled populations (A) and mass shifts (B). Measurements (symbols) were made for individual peptides from the designated proteins extracted from brain, liver, and blood. UniProtKB/Swiss-Prot accession codes are indicated.
Calculating Protein Turnover.
Historically, various simplified models have been used to interpret the kinetics of protein turnover in tracer labeling experiments (26–28). These models attempt to reconcile the observed kinetic trends of label uptake with reaction mechanisms consisting of kinetic influx and efflux of theoretical pools of amino acids and proteins (17, 26). Here, we use a three-pool model (Fig. 1B) to explain the observed kinetic trends (exemplified by the Cofilin-1 peptide in Fig. 1C and the peptides plotted in Fig. 2). The global (organism-wide) pool of amino acids can be supplied by two sources: external diet and internal metabolism/catabolism. The global pool provides amino acids for local (tissue-wide) pools used in protein synthesis. Amino acids can exit the system (excreted) from the global pool. According to this model, the labeled population in our studies represents the fraction of the protein pool that has turned over at a given time and mass shift of the labeled population reflects the enrichment of the labile nitrogen in the local amino acid pool. The extent of mass shift is a characteristic of the tissue of origin and not a feature of any individual protein. Conversely, the kinetics of labeled population represent the turnover rates of peptides and as such are expected to be highly variable. After a lag period, the labeled population increases exponentially. Fitting this phase to a single exponential equation allows the measurement of the rate of peptide turnover (kturnover, Dataset S2) (29).
Most proteins in our dataset were represented by more than one peptide (Table 1). The variability in turnover kinetics among peptides encompassing a single protein was quite low, with the typical coefficient of variation of ~0.25 (Fig. S3). Peptides belonging to a single protein and having similar kinetic profiles of labeled population were averaged to obtain labeled population curves for each protein (Dataset S2). Outliers, defined as peptides with Pearson correlations less than 0.9 with respect to the protein average, were excluded. The averaged protein curves were fit to a single exponential equation and the turnover rate for each protein was measured, as shown for blood-extracted serum albumin (Fig. S3).
The measured rates of turnover spanned four orders of magnitude, from 0.002 d−1 to 10 d−1 (Dataset S2). In the brain, proteins had longer turnover times whereas the distributions of the blood and liver proteins were skewed toward faster turnover rates (Fig. 3). The median turnover rate for the brain peptides was 0.075 d−1 compared with 0.23 and 0.20 d−1, respectively, for the blood and liver proteins. Thus, the average lifetimes of proteins in the brain, liver, and blood are 9.0, 3.0, and 3.5 d, respectively.
Fig. 3.
Distribution and comparison of protein turnover rates in the brain, liver, and blood. (A) Distribution of turnover rates. In the brain, proteins had longer turnover times whereas the distributions of the blood and liver proteins were skewed toward faster turnover rates. The median turnover rate for the brain peptides was 0.075 d−1 compared with 0.23 and 0.20 d−1, respectively, for the blood and liver proteins. Thus, the average lifetimes of proteins in the brain, liver, and blood are 9.0, 3.0, and 3.5 d, respectively. Each bar represents the fraction of the total protein population within the rate bin. The x axis represents the low limit of the bin at 0.25 log d−1 intervals. (B) Comparison of turnover rates of proteins shared between tissues. Gray dots represent mitochondrial proteins.
We found that many of the proteins uniquely expressed in the brain had slow rates of turnover. For example, myelin basic protein, an abundant constituent of the myelin sheath, had a half-life of up to a year. Furthermore, proteins present in all three tissues showed longer turnover times in the brain (Figs. 3–5). In particular, mitochondrial proteins (Fig. 3B) tend to have a much slower turnover rate in the brain in comparison with the liver and blood.
Fig. 5.
Turnover rates of analyzed subunit proteins that comprise multiprotein complexes. Boxes show the interquartile range (IQR) of turnover rates of protein complex subunits. The error bar represents the entire range of rates and the dots represent outliers (1.5 IQR). Numbers in parentheses indicate the number of protein subunits analyzed and represented in the distribution. Complexes observed in multiple tissues share the same box color; white boxes indicate that complex was detected in that tissue only.
Correlation of Turnover Rates to Function.
We next sought to uncover statistically significant correlations between turnover rates and other biological properties of the proteins kinetically analyzed in our studies. We created a list of Gene Ontology (GO) terms (30) associated with the proteins in blood, liver, and brain, and then separated the proteins into bins according to turnover rates, ranging from −3 to 2 log d−1 and overlapping by 0.5 log d−1 at intervals of 0.25 log d−1. The relative prevalence of GO terms in each rate bin was calculated as the ratio between the number of observed proteins belonging to the GO term in that bin to the number expected by random chance. The statistical significance of the enrichment was determined by calculating the Fisher exact-test P value (31). We identified 330 GO terms that were enriched in one or more rate bins with a statistical significance of P < 0.001, which included 108 terms for brain, 124 for liver, and 98 for blood (Fig. 4). Multiple GO terms can be related to one another in a hierarchical fashion (30). Thus, the same group of genes can cause the enrichment of multiple, related GO terms. To negate this redundancy, GO terms that were represented by overlapping groups of proteins in the data (overlap of 30% or more) were grouped into empirically named clusters (Table S1). The bin enrichments for each cluster were determined by averaging the enrichments for each of the constituent GO terms.
Fig. 4.
Correlations between function and turnover rates. Functional categories based on the Gene Ontology (GO) Database were clustered into the categories listed along the y axis. The turnover rates for proteins belonging to the GO clusters were enriched (shown by grayscale shading) with high statistical significance (P < 0.001) for the indicated rate bin. The constituent GO terms, proteins, and turnover rates for each cluster are listed in Table S1.
Secreted proteins (apolipoprotein, chylomicron, and complement factors) and proteins involved in signaling and protein folding (e.g., chaperones) have the fastest rates of turnover. We measured half-lives of 2–10 h for these proteins. Proteins contained in the nucleosome (e.g., histones) and those involved in the maintenance of the myelin sheath showed the longest turnover rates, with measured half-lives of up to 1 y. Proteins associated with a particular organelle often turn over at similar rates. For example, mitochondrial proteins generally showed long half-lives, and many proteins residing in the ER had half-lives of 6–10 d (Fig. 4). It should be noted that Fig. 4 is not an exhaustive list of functional categories that are enriched for proteins with specific half-lives. The figure is limited to functional categories that were represented by a sufficient number of proteins in our dataset to enable the measurement of enrichments with a high degree of statistical confidence (P < 0.001).
Turnover of Protein Complex Subunits.
GO annotations were used to identify proteins in the dataset belonging to multiprotein complexes, excluding highly heterogeneous protein complexes (e.g., microtubule, nucleosome, etc.) We identified complexes that contained five or more protein subunits in our dataset and plotted the distribution of their turnover rates (Fig. 5). The proteins contained within each complex and their respective turnover rates are listed in Table S2. Without exception, all protein complexes identified in both brain and liver turned over more slowly in the brain than in the liver. For example, 12 subunits of the proteasome were identified in both the brain and the liver. The average half-life for the observed subunits in the brain was 8 d, whereas the average half-life for subunits in the liver was 4 d. The half-lives among subunits of the proteasome were similar, with a SD of only 1.3 d in the brain. The subunits of many large, abundant complexes such as ATP synthase and the ribosome have similarly narrow ranges of turnover rates (Table S2).
Discussion
The recent advent of global genomic and proteomic approaches has enabled scientists to gain valuable insights into biological processes at a system-wide level. These techniques have mainly focused on analyzing steady-state levels of mRNA or proteins under varying biological conditions. However, it is clear that additional insights can be gained by furthering these studies to include the dynamics of protein expression and degradation (7, 8). In the brain, for example, protein turnover is critical to synaptic plasticity (32, 33). Here, we report a large-scale analysis of in vivo protein turnover in mice, a commonly used model organism for the study of mammalian biology. We have shown that the rates of protein turnover span four orders of magnitude and correlate with a number of biological properties.
Typically, in vivo labeling is achieved by the introduction of food source in which 1 of 20 natural amino acids has been isotopically labeled (SILAC) (22, 34, 35). Here, we have chosen to instead use a ubiquitously labeled food source—an approach that was initially developed by Wu et al. (23). In measuring turnover rates, the utilization of a ubiquitously labeled food source offers a number of advantages. First, most peptides produced by trypsin digestion lack the full complement of amino acids. For example, ~40% of the tryptic peptides analyzed in this study lacked lysines (the most commonly used SILAC probe) and could not have been studied by the introduction of labeled lysine alone (Fig. S4). Second, in cases where only a single amino acid in a peptide can be labeled, it is not possible to distinguish between the effects of local amino acid pool labeling and protein turnover. For example, analysis of a peptide with a single amino acid probe cannot distinguish between a scenario where 50% of the proteins have turned over within a fully labeled amino acid pool and one where 100% of the proteins have turned over within a half-labeled amino acid pool (Fig. S4). Doherty and coworkers addressed this complexity computationally through the careful analysis of peptides containing multiple valine residues (36). In our approach, the presence of multiple exchange probes enables the analysis of partially labeled protein populations, allowing the independent measurement of these two parameters. This ability enables the measurement of turnover rates in lieu of the multiphasic and tissue-specific label uptake kinetics observed in vivo. Third, we have shown that ubiquitously labeled algae can be produced in a typical laboratory setting and is significantly more cost effective than the utilization of commercially purified, labeled amino acids.
Our data are consistent with a three-pool model of amino acid incorporation. If the cellular amino acid pools of a tissue are in fast equilibrium, the kinetics of mass shift are expected to be congruent among all of the peptides in a given tissue. This trend is clearly observed in our measurements (Fig. 2B). Our data show bursts in mass shifts, indicating rapid increases in 15N-labeled amino acids in the local pool from the dietary algae. The initial increase was lower in brain than in liver and blood, reflecting the trafficking of dietary amino acids. The 15N-labeled amino acids enter the blood and traverse multiple tissues, including the gut and liver, before they reach the brain. The sequential flux through multiple tissues enables the introduction of 14N-labeled amino acids (through local metabolic and catabolic processes) before flow into the local brain pool. Thus, in the brain the initial burst of 15N labeling of the local brain pool is reduced in comparison with upstream tissues. The second, slower phase of the mass shift corresponds to the “recycling” of amino acids through catabolic and metabolic processes. In other words, the breakdown of internal proteins constantly dilutes the dietary supply of 15N-labeled amino acids. Before complete labeling of the amino acid pool can be achieved, the internal pool of 14N-containing proteins needs to be completely depleted. In the brain, the prolonged recycling phase is slower in comparison with the liver and blood. This result is expected given that brain proteins are relatively long lived in comparison with liver and blood proteins, leading to an extended recycling phase. Future analysis of the free amino acid enrichment kinetics in these various tissues could be used to refine this model.
It is important to note that steady-state protein levels, in themselves, are not predictive of turnover rates. Whereas the static level of a protein is established by the relative ratio of synthesis and degradation rates, its lifetime is determined by the magnitude of these rates. This idea is supported by our data. In proteomic analyses of tryptic peptides, the relative steady-state level of a protein can be crudely estimated by the ratio of observable peptides to the theoretical number of peptides expected from that protein [Protein Abundance Index (PAI)] (37). Within our data, there is no significant correlation between turnover rates and PAI (Fig. S5A), suggesting that proteins with similar abundances can have a wide range of turnover rates.
Our data suggest that protein turnover is regulated at the level of the tissue, organelle, and protein complex. The rate of turnover is generally slower in the brain compared with the blood and the liver. The relatively slow rate of bulk protein turnover in the brain had been previously observed (18, 21). We show that this is not only due the presence of stable proteins that are uniquely expressed in the brain, but also because proteins that are shared between the three tissues have a longer half-life in the brain—by a factor of two to five. It is interesting to note that in rats, the organ-specific metabolic rate per gram of liver has been estimated to be five times greater than the equivalent mass of brain (38). The observation suggests that the difference in the metabolic rate of these two tissues may be largely due to differences in energy commitment to proteome turnover.
We observe statistically significant similarities within turnover rates of proteins localized to specific organelles. Mitochondrial proteins, whether encoded by mitochondrial or nuclear DNA, have similar turnover rates (Fig. 4 and Dataset S2). Mitochondrial and nuclear proteins tend to have longer half-lives than cytosolic proteins, which in turn, are more stable than proteins of the endoplasmic reticulum. For some organelles, this coordinated turnover may reflect autophagy as a primary route of degradation. The turnover of mitochondria as a unit through autophagy (mitophagy) is known to be a primary method for mitochondrial regulation in the cell (39, 40). Mitochondrial protein lifetimes vary between the liver and the brain, suggestive of different tissue-specific mitophagy rates.
For many protein complexes, turnover rates of constituent subunits fall within a small range. The 20S proteasome core complex in the brain and liver has a narrow range of turnover rates. Although it has been suggested that multiple 20S subtypes are present in cells and tissues (41), our data suggest that alternative proteasome compositions are either rare or have the same lifetime as the canonical core complex. Synthesis of abundant multiprotein complexes, such as the ribosome and the proteasome, represent a considerable energy investment for the cell. The coordinated turnover of these complexes may represent an energy-conservation strategy by the cell to avoid the presence of orphan subunits. For example, the regulation of turnover among protein and RNA ribosomal subunits had been previously established (20). For a few complexes, such as the Cop9 signalosome complex (CSN), we observed a broad range of turnover rates. CSN is a regulated component of the ubiquitine–proteasome degradation pathway associated with specific developmental processes (42). Distinct CSN populations with varying subunit compositions and activities have been identified (43). Consistent with these observations, our data suggest the presence of multiple CSN populations with distinct half-lives.
A recent analysis of cultured HeLa cells succeeded in measuring the turnover rate of ~600 proteins (22). Of these, we found ~150 homologous mouse proteins in our in vivo dataset for at least one tissue. A comparison of the data reveals little correlation between turnover rates in culture with turnover rates in mice (Fig. S5B). Indeed, the turnover rates measured in culture were significantly faster than the in vivo measurements. This variability may be due to the continuously proliferating nature of transformed cell lines. Unlike differentiated cells, a dividing cell line is in continuous need of protein production to supply newly generated daughter cells. The regeneration of liver mass that occurs through the proliferation of hepatic cells was shown to reduce apparent protein half-life (20). Additionally, the range of half-lives in HeLa cells appears to be much broader than the corresponding measurements in vivo, perhaps because some of the mechanisms that regulate protein turnover in vivo (e.g., autophagy and tissue regeneration) are absent in culture. These results highlight the limitations of cultured cell lines as models of in vivo proteome homeostasis.
Future studies that combine protein turnover measurements with quantitative proteomic strategies will allow the absolute protein degradation rates to be established on a proteomewide scale. This work provides the methodology and theoretical framework necessary to conduct proteome-wide analyses of in vivo protein turnover in any model organism and environmental condition where a labeled diet can be incorporated into the experimental design. The approach will be generally useful in analyzing relationships between proteome homeostasis and biological phenotypes of interest, particularly in the brain, where protein turnover is critical to normal function (32, 33) and accumulation of misfolded protein aggregates is a primary characteristic of neurodegenerative disease (44, 45).
Methods
Additional methods are provided in SI Text.
Isotopic Labeling and Sample Collection.
Labeling experiments were performed on 9-wk-old male mice. At this age, mice are fully developed and tissue growth and differentiation are expected to play minimal roles in protein turnover (20). Following a 7-d habituation period using unlabeled Spirulina, 15N-labeled Spirulina was introduced as the sole dietary protein source for 32 d (Fig. 1A). After the initiation of labeling, tissues were collected from two mice at 0, 0.4, 1, 2, 4, 8, 16, 24, and 32 d. Liver and brain were flash-frozen on solid CO2 immediately after collection. Blood (1 mL) was obtained by cardiac puncture and mixed thoroughly with anticoagulant buffer. The buffered blood was fractionated by centrifugation, and a cell population depleted of red blood cells was used for analysis of the blood proteome. Alkylated total protein was resolved according to molecular weight (Mr) with SDS/PAGE. Each gel lane (representing a single time point) was divided into 9-Mr sections spanning 10–250 kDa. Cut gel fragments were trypsinized, and the extracted peptides were analyzed by LC/MS/MS.
Gene Ontology Analysis.
We created a list of gene ontology (GO) terms (30) associated with the analyzed proteins. The proteins were binned according to turnover rates, ranging from −3 to 2 log d−1. The relative prevalence of GO terms in each rate bin was calculated as the ratio between the number of observed proteins belonging to the GO term in that bin to the number expected by chance. The statistical significance of the enrichment was determined by calculating the Fisher exact-test P value (31). To negate redundancy, GO terms that were represented by overlapping groups of proteins in the data were grouped into empirically named clusters (Table S1). For the analysis of multiprotein complexes, we identified all proteins within our dataset that mapped to “GO:0043234 protein complex.” In cases where overlapping, hierarchical protein-complex GO terms were identified, the higher-order GO term was culled. Highly heterogeneous protein-complex GO terms (e.g., microtubule, nucleosome, etc.) were not included for analysis because they do not represent single discrete complexes.
Supplementary Material
Acknowledgments
We thank the staff at the Hunters Point animal facility for assistance in animal maintenance and tissue collection. We also thank Jonathan Weissman for providing valuable comments and Hang Nguyen for editing the manuscript. This work was supported by grants from the National Institutes of Health (NIH) (AG10770, NCRR P41RR001614, and NCRR RR019934) and gifts from the G. Harold and Leila Y. Mathers Charitable Foundation and Sherman Fairchild Foundation. J.C.P. was supported by the Larry L. Hillblom Foundation. S. Ghaemmaghami was supported by the John Douglas French Alzheimer’s Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006551107/-/DCSupplemental.
References
- 1.Goldberg AL, St John AC. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- 2.Mortimore GE, Pösö AR, Lardeux BR. Mechanism and regulation of protein degradation in liver. Diabetes Metab Rev. 1989;5:49–70. doi: 10.1002/dmr.5610050105. [DOI] [PubMed] [Google Scholar]
- 3.Rousset S, et al. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007;581:479–482. doi: 10.1016/j.febslet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Verzijl N, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 5.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 7.Pratt JM, et al. Dynamics of protein turnover, a missing dimension in proteomics. Mol Cell Proteomics. 2002;1:579–591. doi: 10.1074/mcp.m200046-mcp200. [DOI] [PubMed] [Google Scholar]
- 8.Hellerstein MK. New stable isotope-mass spectrometric techniques for measuring fluxes through intact metabolic pathways in mammalian systems: Introduction of moving pictures into functional genomics and biochemical phenotyping. Metab Eng. 2004;6:85–100. doi: 10.1016/j.ymben.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.van Remmen H, Ward WF, Sabia RV, Richardson A. Gene expression and protein degradation [in aging] In: Masoro EJ, editor. Handbook of Physiology Section 11: Aging. New York: Oxford Univ Press; 1995. pp. 171–234. [Google Scholar]
- 10.Sun F, et al. Derlin-1 promotes the efficient degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR folding mutants. J Biol Chem. 2006;281:36856–36863. doi: 10.1074/jbc.M607085200. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 13.Dice JF, Goldberg AL. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch Biochem Biophys. 1975;170:213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- 14.Dice JF, Goldberg AL. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci USA. 1975;72:3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tompa P, Prilusky J, Silman I, Sussman JL. Structural disorder serves as a weak signal for intracellular protein degradation. Proteins. 2008;71:903–909. doi: 10.1002/prot.21773. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan DL. Total carbon turnover measured by feeding a uniformly labeled diet. Arch Biochem Biophys. 1961;94:500–511. [Google Scholar]
- 17.Garfinkel D, Lajtha A. A metabolic inhomogeneity of glycine in vivo. I. Experimental determination. J Biol Chem. 1963;238:2429–2434. [PubMed] [Google Scholar]
- 18.Lajtha A. Amino acid and protein metabolism of the brain. V. Turnover of leucine in mouse tissues. J Neurochem. 1959;3:358–365. doi: 10.1111/j.1471-4159.1959.tb12643.x. [DOI] [PubMed] [Google Scholar]
- 19.Lajtha A, Berl S, Waelsch H. Amino acid and protein metabolism of the brain. IV. The metabolism of glutamic acid. J Neurochem. 1959;3:322–332. doi: 10.1111/j.1471-4159.1959.tb12638.x. [DOI] [PubMed] [Google Scholar]
- 20.Nikolov EN, Dineva BB, Dabeva MD, Nikolov TK. Turnover of ribosomal proteins in regenerating rat liver after partial hepatectomy. Int J Biochem. 1987;19:159–163. doi: 10.1016/0020-711x(87)90326-0. [DOI] [PubMed] [Google Scholar]
- 21.McClatchy DB, Dong MQ, Wu CC, Venable JD, Yates JR., 3rd 15N metabolic labeling of mammalian tissue with slow protein turnover. J Proteome Res. 2007;6:2005–2010. doi: 10.1021/pr060599n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty MK, Hammond DE, Clague MJ, Gaskell SJ, Beynon RJ. Turnover of the human proteome: Determination of protein intracellular stability by dynamic SILAC. J Proteome Res. 2009;8:104–112. doi: 10.1021/pr800641v. [DOI] [PubMed] [Google Scholar]
- 23.Wu CC, MacCoss MJ, Howell KE, Matthews DE, Yates JR., 3rd Metabolic labeling of mammalian organisms with stable isotopes for quantitative proteomic analysis. Anal Chem. 2004;76:4951–4959. doi: 10.1021/ac049208j. [DOI] [PubMed] [Google Scholar]
- 24.Salazar M, Martínez E, Madrigal E, Ruiz LE, Chamorro GA. Subchronic toxicity study in mice fed Spirulina maxima. J Ethnopharmacol. 1998;62:235–241. doi: 10.1016/s0378-8741(98)00080-4. [DOI] [PubMed] [Google Scholar]
- 25.Chalkley RJ, et al. Comprehensive analysis of a multidimensional liquid chromatography mass spectrometry dataset acquired on a quadrupole selecting, quadrupole collision cell, time-of-flight mass spectrometer: II. New developments in Protein Prospector allow for reliable and comprehensive automatic analysis of large datasets. Mol Cell Proteomics. 2005;4:1194–1204. doi: 10.1074/mcp.D500002-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Garfinkel D. A metabolic inhomogenity of glycine in vivo. II. Computer simulation. J Biol Chem. 1963;238:2435–2439. [PubMed] [Google Scholar]
- 27.Schimke RT. Regulation of protein degradation in mammalian tissues. In: Munro HN, Allison JB, editors. Mammalian Protein Metabolism. Vol. 4. New York: Academic; 1970. pp. 177–228. [Google Scholar]
- 28.Waterlow JC, Garlick PJ, Millward DJ. Protein Turnover in Mammalian Tissues and in the Whole Body. New York: North-Holland Publishing; 1978. [Google Scholar]
- 29.Taylor KB. Enzyme Kinetics and Mechanisms. Boston: Kluwer Academic; 2002. p. 227. [Google Scholar]
- 30.Gene Ontology Consortium The Gene Ontology project in 2008. Nucleic Acids Res. 2008;36(Database issue):D440–D444. doi: 10.1093/nar/gkm883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J Roy Statist. Soc. 1922;85:87–94. [Google Scholar]
- 32.Mao LM, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz JH. Ubiquitination, protein turnover, and long-term synaptic plasticity. Sci STKE. 2003;2003:pe26. doi: 10.1126/stke.2003.190.pe26. [DOI] [PubMed] [Google Scholar]
- 34.Krüger M, et al. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134:353–364. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Ong S-E, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC) Nat Protoc. 2006;1:2650–2660. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 36.Doherty MK, Whitehead C, McCormack H, Gaskell SJ, Beynon RJ. Proteome dynamics in complex organisms: Using stable isotopes to monitor individual protein turnover rates. Proteomics. 2005;5:522–533. doi: 10.1002/pmic.200400959. [DOI] [PubMed] [Google Scholar]
- 37.Ishihama Y, et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, O'Connor TP, Heshka S, Heymsfield SB. The reconstruction of Kleiber's law at the organ-tissue level. J Nutr. 2001;131:2967–2970. doi: 10.1093/jn/131.11.2967. [DOI] [PubMed] [Google Scholar]
- 39.Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem. 2007;282:5617–5624. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 40.Journo D, Mor A, Abeliovich H. Aup1-mediated regulation of Rtg3 during mitophagy. J Biol Chem. 2009;284:35885–35895. doi: 10.1074/jbc.M109.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drews O, et al. Mammalian proteasome subpopulations with distinct molecular compositions and proteolytic activities. Mol Cell Proteomics. 2007;6:2021–2031. doi: 10.1074/mcp.M700187-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Wei N, Deng XW. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–286. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 43.Tomoda K, et al. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- 44.Prusiner SB. Prions. In: Knipe DM, Howley PM, editors. Fields Virology. 4th Ed. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 3063–3087. [Google Scholar]
- 45.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.