Abstract
The delta opioid system is involved in the behavioral effects of various drugs of abuse. However, only few studies have focused on the possible interactions between the opioid system and the effects of MDMA. In order to examine the possible role of the delta opioid system in MDMA-induced behaviors in mice, locomotor activity and conditioned place preference were investigated in the presence of naltrindole, a selective delta opioid antagonist. Moreover, the consequences of acute and chronic MDMA administration on Penk (pro-enkephalin) and Pomc (pro-opioimelanocortin) gene expression were assessed by quantitative real-time PCR. The results showed that, after acute MDMA administration (9mg/kg; i.p.), naltrindole (5mg/kg, s.c.) was able to totally block MDMA-induced hyperlocomotion. Penk expression gene was not modulated by acute MDMA but a decrease of Pomc gene expression was observed that was not antagonized by naltrindole. Administration of the antagonist prevented the acquisition of MDMA-induced conditioned place preference, suggesting an implication of the delta opioid receptors in this behavior. Following chronic MDMA treatment only the level of Pomc was modulated. The observed increase was totally blocked by naltrindole pretreatment. All these results confirm the interactions between the delta opioid system (receptors and peptides) and the effects of MDMA.
Keywords: Amphetamine-Related Disorders; genetics; Animals; Behavior, Animal; drug effects; Brain; drug effects; Choice Behavior; drug effects; Conditioning, Classical; drug effects; Enkephalins; genetics; Gene Expression; drug effects; genetics; Hallucinogens; toxicity; Mice; Motor Activity; drug effects; N-Methyl-3,4-methylenedioxyamphetamine; toxicity; Naltrexone; analogs & derivatives; pharmacology; Narcotic Antagonists; pharmacology; Pro-Opiomelanocortin; genetics; Protein Precursors; genetics; RNA, Messenger; genetics; Receptors, Opioid, delta; antagonists & inhibitors; Social Environment; Transcription, Genetic; drug effects
Keywords: conditioned place preference, gene expression, locomotor activity MDMA, opioid system, striatum
Introduction
MDMA (3, 4-methylenedioxymethamphetamine, “ecstasy”) is a phenylethylamine derivative, with psychostimulant properties, widely used by young adults during night parties. MDMA has various acute effects in humans: hyperactivity, hyperthermia, cognitive disturbances, elevated anxiety, serotonin syndrome, etc. (Morgan, 2000, Morton, 2005, Parrott, 2002). Some of these effects can be found in animals (Callaway and Geyer, 1992, Gold and Koob, 1989, Green et al., 2003, O’Shea et al., 2006). Surprisingly, while the rewarding effects of MDMA have been well established in several species (monkeys, rats, mice) by the use of various paradigms, including intravenous self-administration or conditioned place preference (CPP) (Lile et al., 2005, Robledo et al., 2004, Salzmann et al., 2003, Schenk et al., 2003), the mechanisms leading to these effects are still unclear. MDMA is a complex drug that may act on several neurochemical systems. This substituted amphetamine increases the extracellular concentrations of monoamines, including dopamine (DA) and serotonin (5-HT), primarily by acting as a substrate for reuptake transporters (Bankson and Cunningham, 2001, Di Chiara, 1995, Gough et al., 1991, Simantov, 2004).
The endogenous opioid system is known to be involved in the processes of reward and reinforcement. The effects induced by various psychostimulants can be modulated by the opioid system (Bilsky et al., 1992, Kuzmin et al., 1997, Suzuki et al., 1994). Naltrindole (NTI) is a selective delta-opioid receptor antagonist. The delta opioid system is notably localized in the ventral and dorsal striatum, the hippocampus and the amygdala. The implication of the delta opioid system in the effects of various drugs has been highlighted by studies showing that NTI can block or attenuate cocaine (Menkens et al., 1992; Suzuki et al., 1994a), morphine (Suzuki et al., 1994b) and methamphetamine (Suzuki et al., 1994a)-induced conditioned place preference. NTI has also been shown to block cocaine-induced facilitation of pressing for rewarding brain stimulation (Reid et al., 1993). In the case of MDMA, previous studies have shown that the delta opioid system is involved in the effects of this drug. The delta opioid receptor antagonist NTI blocks MDMA’s enhancement of pressing for reinforcing brain stimulation (Reid et al., 1996). Moreover, the delta opioid receptor interacting peptides, enkephalins have been shown to mediate at least in part the MDMA-induced hyperlocomotion in mice (Compan et al., 2003).
In order to better understand the implication of the delta opioid system in both acute and chronic MDMA effects, we investigated the effects of the delta opioid antagonist naltrindole. First, we demonstrated the total selectivity of naltrindole on delta receptor using the locomotor activity and the hot plate test. Then, we investigated the effect of naltrindole on MDMA-induced hyperlocomotion and conditioned place preference (CPP). Finally, we evaluated the consequences of acute and chronic MDMA administration on the mRNA levels of two delta-opioid receptor interacting peptides: pro-enkephalin (Penk) and pro-opiomelanocortin (Pomc) using quantitative real-time PCR (Q-PCR).
Materials and methods
Animals
Male CD1 mice (20–22 g at the beginning of the experiments) (Charles River, L’arbresle, France) were housed in groups of 12 in a room with a 12 h light/dark cycle and temperature controlled environment (20–21°C). Food and water were provided ad libitum. Animal experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) as well as French law, with the standard ethical guidelines and under the control of the Ethical Committee of the Faculty. Every effort was made to minimize the number of animals used and their discomfort.
Chemicals and pharmacological treatments
MDMA hydrochloride (3,4-methylenedioxymethamphetamine) was synthesized in the laboratory of Pr H. Galons (Université Paris Descartes, Paris, France). SNC80 ((+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide) and U-50,488 (trans-(+)-3,4-dichloro-N-methyl-N-(2-(1 pyrrolidinyl) cyclohexyl) benzeneacetamide methanesulfonate) were purchased from TOCRIS (Missouri, USA). Methadone and naltrindole (NTI) were purchased from Sigma (Saint-Quentin Fallavier, France). MDMA, methadone, U-50,488, and naltrindole were dissolved in saline solution (0.9% NaCl). Naltrindole (5 mg/kg) was administered subcutaneously (s.c.) immediately before testing. This dose was chosen on the basis of the literature, with higher doses we would have lost the selectivity of the antagonist. It has been shown that there is no dose-effect in the CPP paradigm (Groblewski et al, 2008), the use of a higher dose of naltrindole would therefore alter the selectivity of this compound without changing its effect in that behavioural test. SNC80 (5 mg/kg, i.p.), methadone (3 mg/kg, i.p.) and U-50,488 (10 mg/kg, s.c.) were administered immediately before the test. These doses were chosen to ensure selectivity for their respective opioids receptors respectively. The dose of MDMA (9 mg/kg, i.p.) was chosen based on previous behavioral and transcriptional studies in the same mouse strain (Salzmann et al., 2006, Salzmann et al., 2003). In all cases the injection volume was 0.1 ml/10 g.
Locomotor activity
The locomotor activity of mice was measured just after the drug injections during 60 min in an actimeter (Immetronic, Bordeaux, France) composed of eight cages of transparent plastic of equal size (19 × 11 × 14 cm) under low illumination (< 5 lux). One mouse was placed in each box to record its movements. Displacements were measured by photocell beams located across the long axis and above the floor. Locomotor activity was recorded for 60 minutes. Horizontal locomotor activities were expressed in scores (mean ± s.e.m) as the number of interruptions of the photocell beams recorded on total activity (60 min).
Conditioned Place Preference (CPP) paradigm
As previously described an unbiased place preference conditioning procedure was used (Salzmann et al., 2003). The place preference apparatus consisted of two conditioning compartments (15×15×15 cm) separated by a neutral area. The conditioning compartments were differentiated by a distinctive sensory cue: the wall-coloring pattern (black or strips). Movements and location of mice were recorded by computerized monitoring software (Video track, Viewpoint, Lyon, France). The protocol consisted of three phases. Preconditioning phase: drug-naïve mice had free access to all compartments for 20 min, and the time spent in each compartment was recorded. Conditioning phase: this phase consisted of 6 days where each conditioning chamber was closed. On the first conditioning day, mice were treated with NTI (5 mg/kg s.c.) or saline just before MDMA (9 mg/kg, i.p) or saline injection according to their group and placed immediately after the last injection in one of the conditioning environments individually for 20 min. The following conditioning day, all mice were given saline in the opposite compartment, and this sequence was repeated during the next 4 days. The designation of drug-paired chamber was random and resulted in an approximately equal representation of the two conditioning chambers as the drug-paired chamber across groups for all experiments. Post-conditioning phase: this phase took place 24h after the final conditioning session (day 7). This phase was carried out exactly as the preconditioning phase. Results were expressed in scores (mean ± s.e.m.) calculated as the difference between the time spent in the drug-paired compartment during the post-conditioning phase minus the time spent in the same compartment in the preconditioning phase.
Hot plate test
The test was based on that described by Eddy and Leimbach (Eddy and Leimbach, 1953). A glass cylinder (16 cm high, 16 cm diameter) was used to keep the mouse on the heated surface (52 +/− 0.5 °C) of the plate. The latency period until the mouse jumped was registered by means of a stop-watch (cut-off time = 240 sec). Results were expressed as means of jump latency ± s.e.m.
Dissection, RNA extraction and reverse transcription for quantitative real-time PCR (Q-PCR)
For acute treatment of MDMA, mice were killed 2h after injection of the drugs, (a different group of mice had been used for behavioural study) whereas for CPP experience mice were killed the day of the test just after behavioural study, by cervical dislocation. The brain was quickly removed, frozen in isopentane at −50°C, and placed in an acrylic matrice (David Kopf Instruments, Phymep, France) allowing the reproducible slicing of 1mm coronal sections. A section of 2 mm was cut, corresponding approximately to bregma +0.26 mm to −0.46mm according to The Mouse Brain Paxinos and Franklin Atlas (Academic Press, 2nd edition, 2001). Dorsal striatum was then dissected free-hand on ice within the slice, frozen in isopentane and stored at −80 °C until processing.
Total RNA was extracted from bilateral striata with RNeasy Qiagen mini kit according to the lipid tissue protocol of the manufacturer (Qiagen, Courtaboeuf, France). Quantification of total RNA was assessed using a NanoDrop® ND-1000 spectrophotometer. Reverse transcription of RNA was performed in a final volume of 20μl containing 1X RT-PCR buffer (3mM MgCl2, 75mM KCl and 50mM tris-HCl), 500μM each deoxynucleotide triphosphate, 20U RNasin ribonuclease inhibitor (Promega, France), 10mM DTT, 100U of superscript II RNase H− reverse transcriptase (Invitrogen, France), 1.5μM random hexamers (GE Healthcare, France) and 500ng of total RNA. Samples were incubated at 25°C for 10 min, then 42°C for 30 min and at 99°C for 5 min. cDNAs were kept at −20°C.
Real-time quantitative PCR (Q-PCR)
The primer nucleotide sequences were chosen with the assistance of Oligo 6.42 software (MedProbe, Oslo, Norway). Sequences of the primers used for amplification were as followed: Pomc upper 5′-GCAGGGGTCTTCTCATTCC-3′ and lower 5′-AGAGCCGACTGTGAAATCTG-3′; Penk upper 5′-ACGCCCCGAGTGGTGGAT-3′ and lower 5′-GCCCCCGTATCTTTTCTC-3′. The primer nucleotide sequences used for Hprt (hypoxanthine guanine phosphoribosyl transferase) have been previously described (Salzmann et al, 2006). Gene expression was assessed by real-time quantitative RT–PCR. Amplification was detected by SYBR® Green fluorescence on 7900HT sequence detection system (Applied Biosystems, France). Thermocycling was carried out in a final volume of 20μl containing 0.5 μM of each upper and lower primer and 10μl of Power SYBR® Green PCR Master Mix 2X. 8μl of diluted cDNA were added to this mix. The thermal cycling conditions were 2 min at 50°C then 10 min at 95°C followed by 40 amplification cycles at 95°C for 15 sec, 65°C or 60°C for 45 sec and 95°C for 15 sec. Quantification was made on the basis of a calibration curve using cDNA from an untreated mouse striata. In addition to the genes of interest, the Hprt transcript was also quantified and each sample was normalized on the basis of its Hprt content. Results were expressed as gene of interest transcript/Hprt transcript.
Statistical analysis
All series of data were analyzed with Statview 5.0 software (SAS Institute Inc., NC, USA). For locomotor activity, conditioned place preference and real time quantitative RT-PCR results, data were analyzed using one-way ANOVA between subjects, followed by a Bonferroni test for post hoc comparisons. The level of significance was set at P <0.05.
Results
Selectivity of the delta opioid antagonist: naltrindole
In a first series of experiments, selectivity of the delta opioid antagonist, naltrindole (5mg/kg, s.c) on opioid system was investigated. We used opioid agonists and appropriate behavioral tests.
1. Delta response
The delta response was assessed with the selective delta opioid receptor agonist SNC80 using locomotor activity test. One-way ANOVA showed a significant treatment effect (F(3,36) = 5.567, p<0.0033). Post-hoc analysis revealed that NTI totally blocked the SNC80-induced locomotor activity. (Fig 1a)
Figure 1. Selectivity of naltrindole (5 mg/kg) on delta, kappa and mu opioid receptors-induced behaviors.
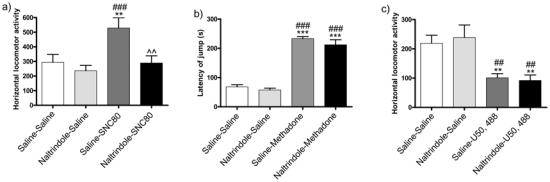
Mice were injected with the delta selective agonist, SNC80 (5 mg/kg) (a) or the kappa selective agonist, U50, 488 (10 mg/kg) (c) in presence or in absence of naltrindole and were immediately placed in the actimeter to record horizontal locomotor activity for 60 min. For the mu receptor (b) mice were injected with the mu agonist, methadone (3 mg/kg, i.p.) in presence or in absence of naltrindole and were immediately placed on the hot plate. The latency period until the mouse jumped was registered. **p<0.01, ***p<0.001 as compared to saline-saline treated group, ##p<0.01, ###p<0.001 as compared to naltrindole-saline treated group, ^^ p<0.01 as compared to saline-MDMA treated group. (n = 9–10 per group).
2. Mu response
The mu response was assessed with the mu opioid receptor agonist methadone using the hot plate test. One-way ANOVA showed a significant treatment effect (F(3,39) = 77.10, p<0.0001). Post-hoc analysis revealed that NTI had no effect on methadone-induced antinociception response. (Fig 1b).
3. Kappa response
The kappa response was assessed with the kappa opioid receptor agonist U-50,488 using the locomotor activity test. One-way ANOVA showed a significant treatment effect (F(3,36 = 7.06, p = 0.0009). Post-hoc analysis revealed that NTI had no effect on U50, 488-induced hypolocomotion (Fig 1c).
Effect of naltrindole on MDMA-induced hyperlocomotion
One-way ANOVA showed a significant treatment effect (F (3, 33) = 8.345, p = 0.0003). Administration of MDMA increased locomotor activity as compared to saline-treated animals (saline/MDMA vs saline/saline). Post-hoc analysis revealed that NTI (5 mg/kg s.c.) was able to block this hyperactivity (saline/MDMA vs naltrindole/MDMA). No significant difference was found between saline/saline and naltrindole/saline treated animals (Fig. 2).
Figure 2. Effects of naltrindole treatment on MDMA-induced horizontal locomotor activity.
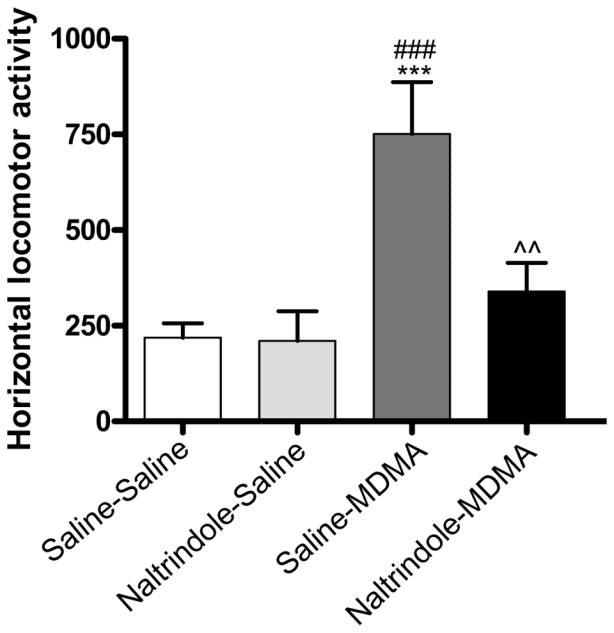
Mice were injected with MDMA (9 mg/kg) ± naltrindole (5 mg/kg) and were immediately placed in the actimeter to record horizontal locomotor activity for 60 min. ***p<0.001 as compared to saline-saline treated group, ###p<0.001 as compared to naltrindole-saline treated group, ^^ p<0.01 as compared to Saline-MDMA treated group. (n = 9–10 per group).
Effect of acute MDMA and NTI administrations on Pomc and Penk mRNA levels in the striatum
The effects of naltrindole pretreatment on MDMA-induced Pomc and Penk gene expression were analyzed by real-time quantitative RT–PCR (Fig. 3a, b). In the case of Pomc, one-way ANOVA revealed significant differences in transcript levels in the striatum (Fig. 3a) (F(3,41) = 4.745, p = 0.0062). Post hoc comparisons showed a significant decrease of Pomc transcription 2h after MDMA administration (9 mg/kg). The administration of NTI did not alter MDMA-induced decrease of Pomc (saline-saline vs NTI-MDMA p=0.05). Thus NTI had no effect on this MDMA-induced decrease of Pomc mRNA level. No significant effects were observed on Penk gene expression following MDMA and MDMA + naltrindole (F (3, 43) = 1.622, p = 0.1981 (Fig. 3b).
Figure 3. Real-time quantitative PCR of Pomc and Penk gene expression on MDMA-induced hyperlocomotion.
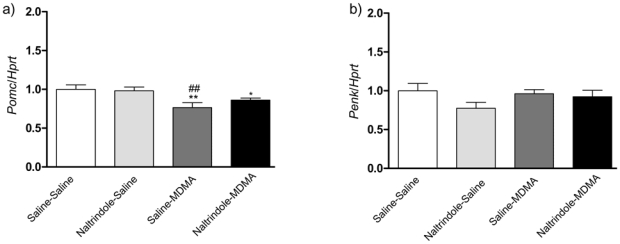
Mice were injected with MDMA (9mg/kg) ± naltrindole (5 mg/kg) and sacrificed 2h after treatments. Expression of Pomc (Fig. 5a) and Penk (Fig. 5b) were analyzed by real-time quantitative RT–PCR. Results are expressed as mean ± s.e.m of gene interest/Hprt ratio (n = 12 per group). *p<0.05 **p<0.01 as compared to saline-saline treated group, ##p<0.01 as compared to naltrindole-saline treated group.
Role of delta opioid receptors activation on the rewarding properties of MDMA
NTI was used in order to assess the role of delta opioid receptors in MDMA-induced conditioned place preference. One-way ANOVA (F (3, 84) = 5.682, p = 0.0014) revealed a significant treatment effect between the four groups of animals. Post hoc comparisons showed a significant effect of MDMA as compared to control group (MDMA/saline vs saline/saline: P = 0.0002) and a significant effect of naltrindole pretreatment on the acquisition of MDMA-induced conditioned place preference (saline/MDMA vs naltrindole/MDMA: P = 0.0068) (Fig. 4).
Figure 4. Effect of naltrindole on the acquisition of MDMA-induced CPP.
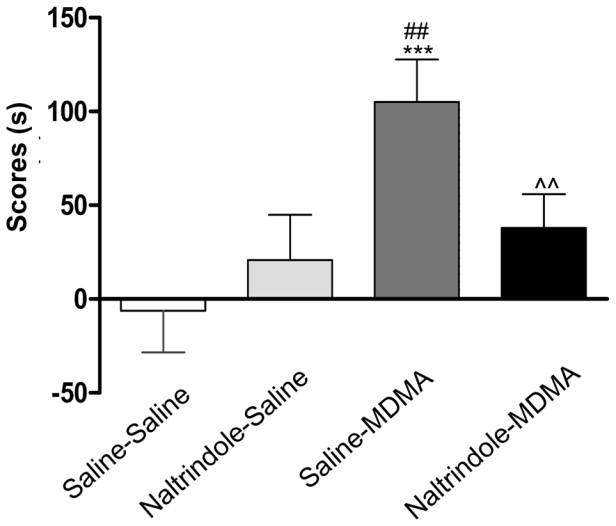
Mice (n=9–10 per group) received naltrindole (5 mg/kg) or saline immediately before MDMA (9 mg/kg) or saline during the conditioning phase. Data are expressed as mean ± s.e.m in score values calculated as difference between post-conditioning and preconditioning time spent in the compartment associated with the drug (n = 10–12 per group). ***p<0.001 as compared to saline-saline treated group, ##p<0.01 as compared to naltrindole-saline treated group, ^^ p<0.01 as compared to saline-MDMA treated group. (n = 9–10 per group).
Effect of chronic MDMA and NTI administrations on Pomc and Penk gene expression in the striatum
Mice were killed the day of the test just after behavioral study. One-way ANOVA showed no significant effect of MDMA chronic treatment on Penk transcript level (Fig 5b) (F(3,40) = 0.669, p = 0.5763), while a significant treatment effect on the Pomc transcript (Fig. 5a) (F(3,34) = 6.293, p = 0.0016) was found. Post hoc comparisons showed a significant increase of Pomc in MDMA-treated animals as compared to control mice (MDMA/saline vs saline/saline). This increase was blocked by co-administration of the delta opioid antagonist, naltrindole. No significant difference was found between saline/saline and naltrindole/saline treated animals
Figure 5. Real-time quantitative PCR of Pomc and Penk gene expression on MDMA-induced conditioned place preference.
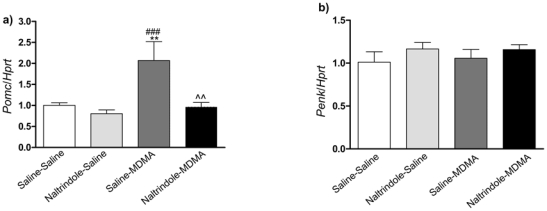
Mice were conditioned with MDMA (9 mg/kg) ± naltrindole (5 mg/kg.). Animals were sacrificed the day of the test just after behavioral study. Results are expressed as mean ± s.e.m of gene of interest/Hprt ratio (n=10–12 per group). **p<0.01 as compared to saline-saline treated group, ###p<0.001 as compared to naltrindole-saline treated group treated, ^^p<0.01 as compared to saline-MDMA treated group.
Discussion
Behavioral effects of MDMA have been studied in rodents for many years (Ball and Rebec, 2005, Daza-Losada et al., 2007, Gold and Koob, 1989, Green et al., 2003), but the mechanisms underlying these effects are still poorly understood. Few studies have focused on the possible interaction between the delta opioid system and the effects of this substituted amphetamine (Compan et al., 2003; Reid et al., 1996). Indeed this system is known to be involved in the behavioral effects of various drugs of abuse such as cocaine (Menkens et al., 1992; Reid et al. 1993), morphine (Suzuki et al., 1994b) and methamphetamine (Suzuki et al., 1994a). However, only partial approaches have been used until now, studying either the peptides or the opioid receptors. These observations led us to study the role of the delta opioid receptor and the regulation of its interacting peptides to have an overview of the role that endogenous delta opioid system may have in MDMA-induced behaviors in mice. Since Robledo and coworkers have already shown that the rewarding properties of MDMA are preserved in mice lacking μ-opioid receptor, we focused on the delta system because (Robledo et al., 2004).
We first verified that, in our experimental conditions, NTI selectively blocked a delta opioid behavioral response and did not interfere with mu or kappa responses. We then studied the effect of NTI on MDMA-induced behavioral responses. Acute administration of naltrindole blocked the MDMA-induced hyperlocomotion in mice suggesting a role of the delta receptors in this behavior. Two endogenous opioid peptides may bind to these receptors: enkephalins and beta-endorphin (β-endorphin). A contribution of enkephalins in acute locomotor effects of MDMA has already been proposed by Compan et al. (Compan et al., 2003). MDMA could facilitate a release of these peptides, and their interaction with delta opioid receptor activation might increase the dopamine release (Svingos et al., 1999) leading to D1 dopamine receptor activation known to be involved in the effect of MDMA on locomotion (Benturquia et al., 2008). Another possible mechanism would be that MDMA-induced increase of the extracellular levels of 5–HT and dopamine could increase enkephalins release (Compan et al., 2003, Gobaille et al., 1994).
In order to determine whether MDMA could modulate the genomic expression of delta opioid peptides, we studied the transcript levels of the precursors of the two peptides: Pomc and Penk. We chose to work on the dorsal striatum, this dopaminergic structure is implicated in the two studied behaviours: locomotion (Dankova et al., 1975, Hruska and Silbergeld, 1979) and reward (Hurd and Herkenham, 1993, Robbins et al., 1990). We observed that acute MDMA administration had no significant effects on the transcription level of the Penk gene suggesting that these peptides are not involved in the observed behaviour. However, we cannot exclude the hypothesis that the synthesis of new enkephalins might be needless, the pre-existing pool being sufficient to mediate the action of this peptide. In the case of Pomc gene expression, data showed that MDMA-induced a decrease that was not antagonised by naltrindole. These results suggest that MDMA exerts an inhibition of Pomc gene expression in the striatum that is independent of delta opioid receptors.
In order to determine whether the delta opioid system was implicated in MDMA rewarding properties we studied the effect of naltrindole on MDMA-induced CPP. In agreement with previous studies, MDMA (9mg/kg, i.p.) induced a conditioned place preference (Salzmann et al., 2003). Naltrindole significantly blocked the rewarding properties of the substituted amphetamine. These data suggest that activation of delta opioid receptors, subsequent to MDMA administration, plays an important role in the acquisition of MDMA-induced conditioned place preference consistent with an involvement of the delta opioid system in the reinforcing properties of MDMA as previously suggested by the study of Reid and coworkers (Reid et al., 1996).
In order to assess the expression levels of the opioid peptides during the post-conditioning CPP test, we studied their mRNA levels. We found that, as observed after the acute treatment, the mRNA level of Penk was not affected by chronic MDMA administration. In the case of Pomc, an increase of the mRNA level was found, that could be blocked by naltrindole pretreatment. The Pomc gene encode various peptides among which β-endorphin. It has been shown that β-endorphin peptide plays a key role in reinforcing properties of drugs of abuse as cocaine (Marquez et al., 2008, Roth-Deri et al., 2003), ethanol (Marinelli et al., 2003), amphetamine (Olive et al., 2001), heroin (Amalric et al., 1987) and THC (Solinas et al., 2004). It has been shown that these drugs of abuse produced elevated level of β-endorphin in mesolimbic structures. Our results suggest that the rewarding effects of MDMA may involve a strong release of β-endorphin during the CPP that would subsequently necessitate de novo synthesis of the peptide. Indeed, further studies are needed to assess if the observed increase in Pomc mRNA level, after MDMA-induced CPP, is associated or not with an increase of β-endorphin peptide level. Interestingly, an increase in 5-HT levels has been shown to induce an increase in Pomc expression and β-endorphin level in various brain structures (Winberg and Lepage, 1998, Zangen et al., 1999). It is therefore possible that chronic MDMA treatment increases Pomc expression through its action on 5-HT transporter.
Increase in Pomc mRNA could have been due to either the effects of repeated MDMA administration or to the effects of learning the CPP procedure. In our experimental conditions, we cannot differentiate the contributions of these two effects. The observed modulation is likely due to a combination of both effects since the learning procedure is important for the establishment of CPP.
The results of the present study demonstrate that the blockade of delta opioid receptors is able to antagonize MDMA-induced hyperlocomotion and CPP. Moreover, our molecular results suggest that chronic MDMA administration may increase the release of endogenous opioid peptides, which activate delta opioid receptors and participate in the MDMA-induced hyperlocomotion and reward. Although, the peptide precursor affected in acute and chronic MDMA treatment is the same, the mechanisms involved seem different. Further studies are needed to determine the implication of the β-endorphin peptides in the observed modulation of the behavioral effects of MDMA by the delta opioid system.
Acknowledgments
The authors thank Stefano Palminteri for preliminary studies and Didier Fauconnier for technical assistance.
List of references
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- Ball KT, Rebec GV. Role of 5-HT2A and 5-HT2C/B receptors in the acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on striatal single-unit activity and locomotion in freely moving rats. Psychopharmacology (Berl) 2005;181:676–687. doi: 10.1007/s00213-005-0038-z. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Benturquia N, Courtin C, Noble F, Marie-Claire C. Involvement of D1 dopamine receptor in MDMA-induced locomotor activity and striatal gene expression in mice. Brain Res. 2008;1211:1–5. doi: 10.1016/j.brainres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Montegut MJ, Delong CL, Reid LD. Opioidergic modulation of cocaine conditioned place preferences. Life Sci. 1992;50:PL85–90. doi: 10.1016/0024-3205(92)90105-x. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Stimulant effects of 3,4-methylenedioxymethamphetamine in the nucleus accumbens of rat. Eur J Pharmacol. 1992;214:45–51. doi: 10.1016/0014-2999(92)90094-k. [DOI] [PubMed] [Google Scholar]
- Compan V, Scearce-Levie K, Crosson C, Daszuta A, Hen R. Enkephalin contributes to the locomotor stimulating effects of 3,4-methylenedioxy-N-methylamphetamine. Eur J Neurosci. 2003;18:383–390. doi: 10.1046/j.1460-9568.2003.02767.x. [DOI] [PubMed] [Google Scholar]
- Dankova J, Boucher R, Poirier LJ. Role of the strio-pallidal system and motor cortex in induced circus movements in rats and cats. Exp Neurol. 1975;47:135–149. doi: 10.1016/0014-4886(75)90242-3. [DOI] [PubMed] [Google Scholar]
- Daza-Losada M, Ribeiro Do Couto B, Manzanedo C, Aguilar MA, Rodriguez-Arias M, Minarro J. Rewarding Effects and Reinstatement of MDMA-Induced CPP in Adolescent Mice. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301309. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Gobaille S, Schmidt C, Cupo A, Herbrecht F, Maitre M. Characterization of methionine-enkephalin release in the rat striatum by in vivo dialysis: effects of gamma-hydroxybutyrate on cellular and extracellular methionine-enkephalin levels. Neuroscience. 1994;60:637–648. doi: 10.1016/0306-4522(94)90492-8. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF. MDMA produces stimulant-like conditioned locomotor activity. Psychopharmacology (Berl) 1989;99:352–356. doi: 10.1007/BF00445556. [DOI] [PubMed] [Google Scholar]
- Gough B, Ali SF, Slikker W, Jr, Holson RR. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacol Biochem Behav. 1991;39:619–623. doi: 10.1016/0091-3057(91)90137-q. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008;201:97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Abnormal locomotion in rats after bilateral intrastriatal injection of kainic acid. Life Sci. 1979;25:181–193. doi: 10.1016/0024-3205(79)90390-4. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Kuzmin AV, Gerrits MA, van Ree JM, Zvartau EE. Naloxone inhibits the reinforcing and motivational aspects of cocaine addiction in mice. Life Sci. 1997;60:PL-257–264. doi: 10.1016/s0024-3205(97)00130-6. [DOI] [PubMed] [Google Scholar]
- Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78:135–140. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl) 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology (Berl) 2000;152:230–248. doi: 10.1007/s002130000545. [DOI] [PubMed] [Google Scholar]
- Morton J. Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- O’Shea E, Orio L, Escobedo I, Sanchez V, Camarero J, Green AR, Colado MI. MDMA-induced neurotoxicity: long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br J Pharmacol. 2006;148:778–785. doi: 10.1038/sj.bjp.0706783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome, and serotonergic neurotoxicity. Pharmacol Biochem Behav. 2002;71:837–844. doi: 10.1016/s0091-3057(01)00711-0. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hubbell CL, Glaccum MB, Bilsky EJ, Portoghese PS, Porreca F. Naltrindole, an opioid delta receptor antagonist, blocks cocaine-induced facilitation of responding for rewarding brain stimulation. Life Sci. 1993;52:PL67–71. doi: 10.1016/0024-3205(93)90084-g. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hubbell CL, Tsai J, Fishkin MD, Amendola CA. Naltrindole, a delta-opioid antagonist, blocks MDMA’s ability to enhance pressing for rewarding brain stimulation. Pharmacol Biochem Behav. 1996;53:477–480. doi: 10.1016/0091-3057(95)02020-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Giardini V, Jones GH, Reading P, Sahakian BJ. Effects of dopamine depletion from the caudate-putamen and nucleus accumbens septi on the acquisition and performance of a conditional discrimination task. Behav Brain Res. 1990;38:243–261. doi: 10.1016/0166-4328(90)90179-i. [DOI] [PubMed] [Google Scholar]
- Robledo P, Mendizabal V, Ortuno J, de la Torre R, Kieffer BL, Maldonado R. The rewarding properties of MDMA are preserved in mice lacking mu-opioid receptors. Eur J Neurosci. 2004;20:853–858. doi: 10.1111/j.1460-9568.2004.03532.x. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Mayan R, Yadid G. A hypothalamic endorphinic lesion attenuates acquisition of cocaine self-administration in the rat. Eur Neuropsychopharmacol. 2006;16:25–32. doi: 10.1016/j.euroneuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Salzmann J, Canestrelli C, Noble F, Marie-Claire C. Analysis of transcriptional responses in the mouse dorsal striatum following acute 3,4-methylenedioxymethamphetamine (ecstasy): identification of extracellular signal-regulated kinase-controlled genes. Neuroscience. 2006;137:473–482. doi: 10.1016/j.neuroscience.2005.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology (Berl) 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- Simantov R. Multiple molecular and neuropharmacological effects of MDMA (Ecstasy) Life Sci. 2004;74:803–814. doi: 10.1016/j.lfs.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9-tetrahydrocannabinol. Eur J Neurosci. 2004;19:3183–3192. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mori T, Tsuji M, Misawa M, Nagase H. The role of delta-opioid receptor subtypes in cocaine- and methamphetamine-induced place preferences. Life Sci. 1994;55:PL339–344. doi: 10.1016/0024-3205(94)00774-8. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM. Localization of the delta-opioid receptor and dopamine transporter in the nucleus accumbens shell: implications for opiate and psychostimulant cross-sensitization. Synapse. 1999;34:1–10. doi: 10.1002/(SICI)1098-2396(199910)34:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Winberg S, Lepage O. Elevation of brain 5-HT activity, POMC expression, and plasma cortisol in socially subordinate rainbow trout. Am J Physiol. 1998;274:R645–654. doi: 10.1152/ajpregu.1998.274.3.R645. [DOI] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Yadid G. Serotonin-mediated increases in the extracellular levels of beta-endorphin in the arcuate nucleus and nucleus accumbens: a microdialysis study. J Neurochem. 1999;73:2569–2574. doi: 10.1046/j.1471-4159.1999.0732569.x. [DOI] [PubMed] [Google Scholar]


