Abstract
Autism is one of the five disorders that falls under the umbrella of Pervasive Developmental Disorders (PDD) or Autism Spectrum Disorder (ASD), a category of neurological disorders characterized by “severe and pervasive impairment in several areas of development.” ASD is characterized by varying degrees of impairment in communication skills, social interaction and restricted, repetitive stereotyped patterns of behavior. The five disorders under PDD are autistic disorder, Asperger's disorder, childhood disintegrative disorder, Rett's disorder and PDD-not otherwise specified. ASD can often be reliably detected by the age of 3 years and, in some cases, as early as 18 months. The appearance of any warning signs of ASD is reason to have the child evaluated by a professional specializing in these disorders.
Keywords: Autism, genetics, mental handicap
Introduction
Autism is one of the five disorders that falls under the umbrella of Pervasive Developmental Disorders (PDD) or Autism Spectrum Disorder (ASD), a category of neurological disorders characterized by “severe and pervasive impairment in several areas of development.” ASD is characterized by varying degrees of impairment in communication skills, social interaction and restricted, repetitive stereotyped patterns of behavior.[1,2] The five disorders under PDD are autistic disorder, Asperger's disorder, childhood disintegrative disorder, Rett's disorder and PDD-not otherwise specified (PDD-NOS). ASD can often be reliably detected by the age of 3 years and, in some cases, as early as 18 months. The appearance of any warning signs of ASD is reason to have the child evaluated by a professional specializing in these disorders.
The likely causative factors for the condition are genetic, viral, toxins, pollution and hypersensitivity to thiomersol in vaccines etc. The exact cause of autism has not been yet established, although current research links autism to biological or neurological differences in TQthe brain.
This study has been undertaken to detect the prevalence of genetic conditions in children with ASD and intellectual disabilities.
Studies on associated medical conditions in autism assessed genetic and non-genetic risk factors. It is generally agreed that about 10-15% of individuals with ASD have a known medical condition that causes the disorder.[3] Most of these are the cytogenetic or single-gene disorders and non-genetic medical conditions are not common; however, they are especially relevant with regard to the prevention of ASD. In our study, we observed an association of ASD with intellectual disability in children with fragile X syndrome (50.6%), other chromosomal anomalies(11.6%) and with some of the single-gene disorders, like tuberous sclerosis and phenylketonuria. Our study also has a large number of children with autism and intellectual disabilities, where the causative factor is not traceable (33.3%).
Genetic factors play a very important role in the causation of autistic traits in children with intellectual disability. Among these, children falling into the category of classical autism do not have associated chromosomal abnormalities, whereas those with autistic traits and PDD have chances of associated chromosomal abnormalities present. It is mandatory that a detailed cytogenetic evaluation has to be recommended in all subjects with ASD, more so if the subject additionally shows intellectual disability, abnormal EEG patterns or seizures, muscular hypotonia, severe motor and gait problems or dysmorphic features.
Autism is a behavioral syndrome of varying intensity and probably encompasses a variety of etiological factors. It is associated with repetitive and stereotypic behaviors. About 5-10% of individuals with autism have “secondary” autism, in which an environmental agent, chromosomal abnormality or single-gene disorder can be identified. Ninety percent have idiopathic autism and a major gene has not yet been identified.[4] It is one of the most heritable complex genetic disorders. Despite strong heritability, there seems to be a likelihood of involvement of multiple genes and chromosomal regions that needs to be resolved.
Early screening studies of autistic individuals suggested that up to one-quarter of the cases were associated with fragile X anomaly.[5] On the basis of family and twin studies, there appears to be a genetic basis for a wide “autistic syndrome.” About a quarter of such cases with autism are associated with genetic disorders such as fragile X syndrome or with infectious diseases such as congenital rubella.[6] Despite the high heritability estimates for ASD, only a few genes increasing the risk for idiopathic ASD have been elucidated. As the disorder shows a high phenotypic variability and additional genetic heterogeneity, it is of crucial importance to, first, clearly define the phenotype, especially with regard to the broader spectrum of ASD and to the differential diagnosis of other PDDs.
There have been few studies that have examined the occurrence of chromosomal abnormalities in patients with autism and related PDDs. The prevailing view is that chromosomal abnormalities are uncommon in traditional autism and may be relatively more common in people with PDD-NOS.[7] Fragile X is not a common cause of autism, although the number of individuals with fragile X who meet the diagnostic criteria for autism is higher than can be accounted by chance.[8] At a cytogenetic level, fragile X is the most important marker for a genetic alteration that underlies autism.[9] There have been reports of evidence that the autism genes may be located on chromosomes 2, 3, 7, 15 and X.[10] Various studies have shown that autism tends to occur more frequently than expected among individuals who have certain medical conditions and genetic disorders, such as fragile X syndrome, tuberous sclerosis and congenital Rubella syndrome and untreated phenylketonuria.
Objectives
The heterogeneity and complex nature of autism necessitates further studies to determine the causative factors of this disorder in order to provide proper genetic counseling, to estimate the recurrence risk of the disorder and help the affected child and the parents. Genetic counseling for ASD is challenging because phenotype and genetic mechanisms are complex. There is a strong need to carefully assess the children and the family, and to exclude all known medical causes of the disorder. This paper describes the results of a pilot study undertaken by us that includes clinical, cytogenetic and biochemical investigations on children with autistic spectrum of disorders with intellectual disability, to determine the causative factors of autistic disorders.
Materials and Methods
Two groups of children have been taken up for our study. Group A consisted of 120 children with intellectual disability and autistic disorders identified through investigations by a group of professionals comprising of pediatrician, social worker, child psychologists, occupational therapists and speech therapists. The diagnosis is made systematically, employing diagnostic criteria from the Diagnostic and Statistical Manual of The American Psychiatric Association (DSM-IV, 1994) and The International Classification of Disease (ICD-10, 1993). Group B comprised of 120 non-autistic chilren with intellectual disability who had been assessed by us during the same period, selected as controls for comparison to ascertain the differentiating factors. The groups were appropriately age and sex matched. Clinical examination and family history, including pedigree charting, was carried out and wherever necessary, the cases were referred to other hospitals for specialized investigations like computed tomography scan, magnetic resonance imaging, etc.
Biochemical investigations were carried out in all the cases to detect inborn errors of metabolism (IEM). Cytogenetic investigations were carried out to detect chromosomal anomalies wherever indicated.
Biochemical investigations include:
Standardized screening tests for detection of IEM
1-D paper chromatography of plasma and urine samples to detect IEM of amino acid metabolism
Cellulose acetate electrophoresis for detection of mucopolysaccharidoses
Thin layer chromatography for amino acids and oligosaccharides
Cytogenetic Investigations
This comprised of study of the chromosomes obtained from cultured lymphocytes.
Peripheral leukocyte cultures were put in TC-199 medium with fetal calf serum and phytohemeagglutinin. Cultures were terminated after 69 h by the addition of colchicine and processed further for chromosome preparation by the standard procedure. The chromosomes were stained with Giemsa. Both plainstained and banded chromosomes were analyzed. A minimum of 20-50 metaphases were analyzed for the detection of chromosomal abnormality, including fragile X detection.
Observations and Discussion
In ASD, the sex ratio averages 4.3:1 and is greatly modified by cognitive impairment.[11] In our study, we have found the sex ratio in the same range of 4.45:1 in group A, comprising of children with intellectual disability and ASD [Table 1]. Children with intellectual disability alone (group B) show a sex ratio of 2.4:1.
Table 1.
Distribution of children by gender
| Group | Males (%) | Females (%) | Total (%) |
|---|---|---|---|
| A-ASD | 98 (81.7) | 22 (18.3) | 120 (100) |
| B-Control | 85 (70.8) | 35 (29.2) | 120 (100) |
| Total | 183 (76.3) | 57 (23.7) | 240 (100) |
Χ2 = 3.888, d.f. = 1, P < 0.05 (significant)
As observed from [Table 2], children with autism and intellectual disability have come to our center at a comparatively earlier age (between 3-6 and 6-12 years) than those with intellectual disability alone (between 6-12 and 12-16 years). This could be due to the reason that children with intellectual disability and autism have more complex and variable behavioral problems that parents could notice at an earlier age than children with intellectual disability alone.
Table 2.
Distribution of children by age
| Group | Age (in years) |
Total (%) | Mean (SD) | ||||
|---|---|---|---|---|---|---|---|
| 0-3 (%) | 3-6 (%) | 6-12 (%) | 12-16 (%) | > 16 (%) | |||
| A-ASD | 16 (55.8) | 66 (34.2) | 31 (7.5) | 5 (1.7) | 2 (0.8) | 120 (100) | 5.88 (10.56) |
| B-Control | 12 (19.2) | 20 (40.8) | 46 (29.2) | 30 (7.5) | 12 (3.3) | 120 (100) | 9.65 (9.37) |
t = 2.925, d.f. = 238, P < 0.01 (significant)
The division of cases depending on the IQ [Table 3] shows almost similar results with both the groups, having the highest number of children with severe intellectual disability followed by moderate and then profound levels. The genetic components have always been associated with severe to profound grade of intellectual disability and the same observation has been proven here, because the study shows a larger number of cases having genetic factor as a cause of intellectual disability and autism.
Table 3.
Distribution of children by IQ
| Category | (IQ) Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Profound | Severe | Moderate | Mild | Borderline intelligence | Dull normal intelligence | IQ not assessed* | Total (%) | |
| A-ASD | 9 (7.5) | 51 (42.5) | 37 (30.8) | 12 (10.0) | 1 (0.8) | 2 (1.7) | 8 (6.7) | 120 (100) |
| B-Control | 25 (20.8) | 41 (34.2) | 24 (20.0) | 14 (11.7) | 4 (3.3) | 12 (10.0) | 0 | 120 (100) |
The IQ could not be assessed in these children as they were non-cooperative during the entire test session
Many theories have been put forth to suggest genetic and environmental causes of autism, but the definite cause is yet to be established. About 10-15% of autism cases have an identifiable Mendelian (single gene) condition, chromosomal abnormality or other genetic syndromes.[3] In our study [Table 4], group A, which comprises of children with intellectual disability and autism, have 50% genetic causes and 15.8% environmental causes, resulting in this condition. Group B, comprising of children with intellectual disability alone, have 58% causes due to genetic factors and 23.3% due to environmental factors. As has been observed, the idiopathic category for group A has 33.35% cases, which is much higher than 14% cases in group B. This further suggests that a considerable number of cases with autism and intellectual disability remain unidentifiable with reference to causative factors.
Table 4.
Etiological factors
| Group |
Z | P | ||
|---|---|---|---|---|
| A-ASD | B-Control | |||
| Genetic (%) | 61 (50.8) | 70 (58.3) | 1.17 | > 0.05 NS |
| Environmental (%) | 19 (15.8) | 28 (23.3) | 1.47 | > 0.05 NS |
| Idiopathic (%) | 40 (33.3) | 17 (14.1) | 3.59 | < 0.01 Sig |
| Probable genetic (%) | 0 | 5 (4.2) | 2.29 | < 0.01 Sig |
| Total (%) | 120 (100) | 120 (100) | ||
Sig - Significant; NS - Not significant
Recent studies estimated a rate of 3-5% of cytogenetic anomalies in autistic disorders.[12,13] Our observation suggests 50.6% cases with fragile X syndrome and 11.6% cases with other chromosomal anomalies in group A and 23.3% cases with Fragile X syndrome and 30.5% cases with other chromosomal anomalies in group B [Table 5]. This observation further supports the higher number of cases with fragile X in the autistic population. This is in accordance with the recent report in the autism news of 2005,[9] where the fragile X condition has been reported as the most common anomaly in children with autistic traits [Figures 1‐2].
Table 5.
Chromosomal abnormalities found in the two groups of children
| Group |
Z | P | ||
|---|---|---|---|---|
| A-ASD | B-Control | |||
| Chromosomal abnormality | ||||
| Normal karyotype (%) | 38 (31.7) | 54 (42.5) | 2.138 | < 0.01 S |
| Fragile X (%) | 68 (50.6) | 30 (23.3) | 5.277 | < 0.001 S |
| Down(%) | 1 (0.83) | 23 (19.2) | 4.999 | < 0.001 S |
| Prader Willi (%) | 0 | 4 (3.3) | 2.023 | < 0.01 S |
| Other chromosomal anomalies (%) | 13 (10.8) | 9 (7.5) | 0.887 | > 0.05 NS |
Sig - Significant; NS - Not significant
Figure 1.
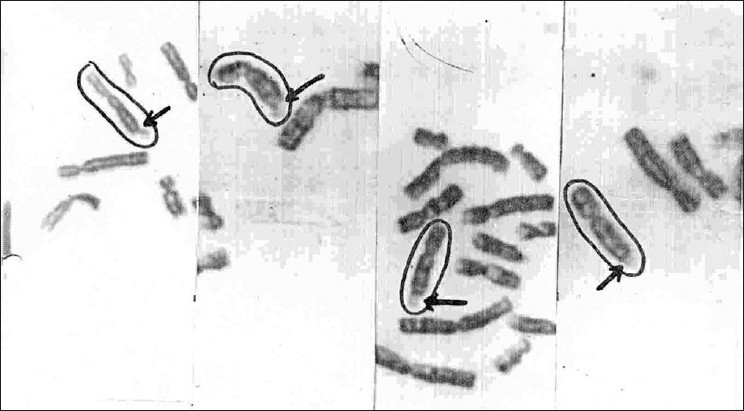
Parts of metaphases showing fragile sites on the X chromosome
Figure 2.
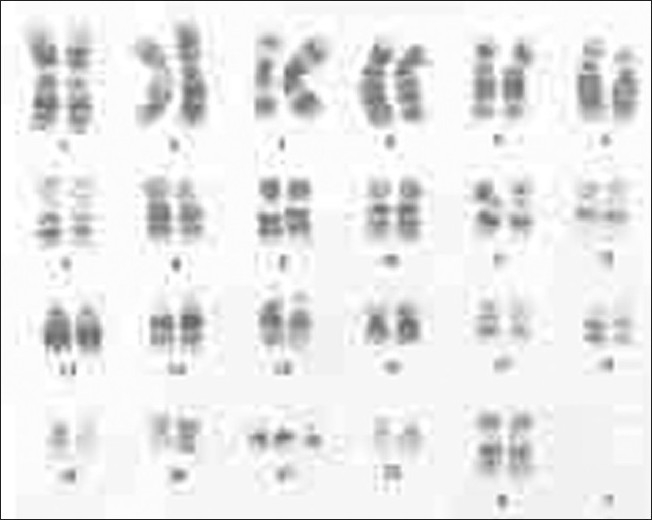
Karyotype of a down syndrome
Autism is one of the five PDDs or autistic spectrum disorders, which include autism, Asperger syndrome, PDD-NOS, Rett syndrome and childhood disintegrative disorder. We have divided occurrence of chromosomal anomalies in various ASDs, and it is observed that autism has a minimum number of cases with chromosomal anomalies, whereas ASD, ADHD (attention deficit hyperactive disorder) and PDD have a higher percentage of cases with chromosomal anomalies, with fragile X being the most common of all [Table 6]. A study showed chromosomal anomalies to be found rarely in children with classical autism. The same study showed chromosomal anomalies to be common in children diagnosed to have PDD and autistic traits.[7] Our results from this study also confirm these findings.
Table 6.
Various chromosomal abnormalities found in the subgroups of autism
| Subgroup/abnormality | Autism (n = 21) (%) | Autistic spectrum (n = 65) (%) | ADHD (n = 16) (%) | PDD (n = 16) (%) | Psychosis (n = 2) (%) | Autism classical (21-100) | Other autistic disorders (99-100) | Z | P |
|---|---|---|---|---|---|---|---|---|---|
| Normal karyotype | 16 (76.2) | 5 (7.7) | 7 (43.75) | 8 (50) | 2 (100) | 76.2 | 22.2 | 9.94 | < 0.001 S |
| Fragile X | 3 (14.3) | 54 (83.1) | 5 (31.25) | 6 (37.5) | 0 | 14.3 | 65.7 | 9.55 | < 0.001 S |
| Down | 1 (4.8) | 0 | 0 | 0 | 0 | 4.8 | 0 | 2.46 | < 0.01 S |
| Other chromosomal anomalies | 1 (4.8) | 6 (9.2) | 4 (25) | 2 (12.5) | 0 | 4.8 | 12.1 | 2.65 | < 0.01 S |
| Total | 21 (100) | 65 (100) | 16 (100) | 16 (100) | 2 (100) |
IEM are very rare and a few are associated with autism. We have been able to detect some of the metabolic and single-gene disorders in children with autism. A female child with profound intellectual disability came to us at the age of 3 years and 6 months, and she was diagnosed as having phenylketonuria at our center [Figure 3].
Figure 3.
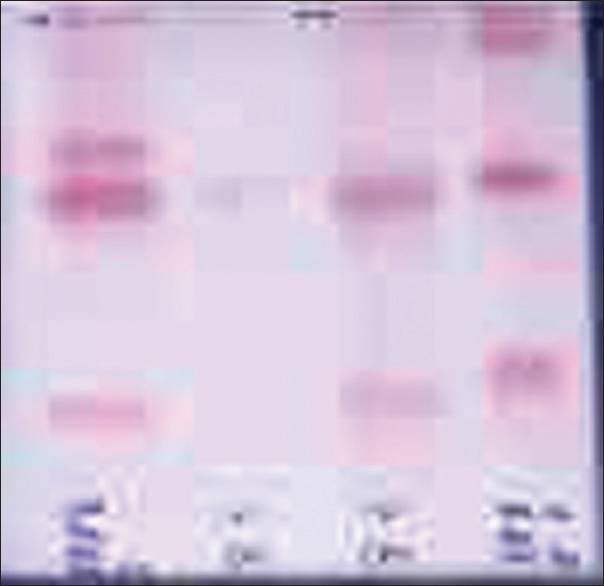
Thin layer chromatography of plasma and urinary amino acids showing phenylketonuria (from left to right) in lanes 2 and 3 and standard amino acids in lanes 1 and 4
Conclusions
From this study, it is evident that although autism is highly heritable, it is likely that several overlapping sets of predisposing genes result in the overall susceptibility in a complex and as-yet not understood mechanism. Genetic factors play a very important role in the causation of autistic traits in children with intellectual disability. Among these, children falling into the category of classical autism do not have associated chromosomal abnormalities whereas those with autistic traits and PDD have chances of associated chromosomal abnormalities present. It is mandatory that a detailed cytogenetic evaluation has to be recommended in all subjects with ASD, more so if the subject additionally shows intellectual disability, abnormal EEG patterns or seizures, muscular hypotonia, severe motor and gait problems or dysmorphic features.
Acknowledgments
The authors are grateful to the Director and the members of the board for having given us the necessary permission to publish the data. They express their sincere thanks to Prof. Sharma for his statistical analysis and valuable suggestions. The authors would also like to acknowledge the help and cooperation rendered by the other team members.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. [Google Scholar]
- 2.Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organisation; 1992. World Health Organisation. The ICD-10 Classification of Mental and Behavioural Disorders. [Google Scholar]
- 3.Folstein SE, Rosen-Sheidley B. Genetics of autism: Complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KS. Cytogenetic abnormalities and fragile X syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey A, Botton P, Butter L, Le Couteur A, Murphy M, Scott S, et al. Prevalence of the fragile X anomaly amongst autistic twins and singletons. J Child Psychol Psychiatry. 1993;34:673–88. doi: 10.1111/j.1469-7610.1993.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 6.Trotter G, Srivastava L, Walker CD. Etiology of infantile autism: A review of recent advances in genetic and neurobiological research. J Psychiatry Neurosci. 1999;24:103–15. [PMC free article] [PubMed] [Google Scholar]
- 7.Weidmer-Mikhail E, Sheldon S, Ghaziuddin M. Chromosomes in autism and related pervasive developmental disorders: A Cytogenetic study. Intellect Disabil Res. 1998;42:8–12. doi: 10.1046/j.1365-2788.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein C, Reiss AL. Autism: The point of view from fragile X studies. J Autism Dev Disord. 1998;28:393–405. doi: 10.1023/a:1026000404855. [DOI] [PubMed] [Google Scholar]
- 9.Autism News, in Medical News Today. 2005. Autism and X-fragile Syndrome. date 5th Feb. [Google Scholar]
- 10.Applied Genetics News. 1999. Possible Autism Genes on chromosome 13“ (Brief article) Dec 19. [Google Scholar]
- 11.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in autism spectrum disorder. BMC Med Genet. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatr Genet. 2001;11:57. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]


