Activated Akt suppresses checkpoint activation by cells in late G2: although they are able to detect DNA damage, the repair pathway is put on hold until mitosis is complete.
Abstract
Using chemical genetics to reversibly inhibit Cdk1, we find that cells arrested in late G2 are unable to delay mitotic entry after irradiation. Late G2 cells detect DNA damage lesions and form γ-H2AX foci but fail to activate Chk1. This reflects a lack of DNA double-strand break processing because late G2 cells fail to recruit RPA (replication protein A), ATR (ataxia telangiectasia and Rad3 related), Rad51, or CtIP (C-terminal interacting protein) to sites of radiation-induced damage, events essential for both checkpoint activation and initiation of DNA repair by homologous recombination. Remarkably, inhibition of Akt/PKB (protein kinase B) restores DNA damage processing and Chk1 activation after irradiation in late G2. These data demonstrate a previously unrecognized role for Akt in cell cycle regulation of DNA repair and checkpoint activation. Because Akt/PKB is frequently activated in many tumor types, these findings have important implications for the evolution and therapy of such cancers.
Introduction
Because of its central role in initiating homologous recombination repair (HRR), DNA strand resection at DNA double-strand breaks (DSBs) is regulated during the cell cycle, thus restricting HRR to S and G2. In yeasts, Cdk activity plays a major role in controlling DNA strand resection (Wohlbold and Fisher, 2009). In part, this is controlled through Cdk-mediated phosphorylation of Sae2, a protein required to initiate the resection process (Huertas et al., 2008). Vertebrate cells express an orthologue of Sae2, CtIP (C-terminal interacting protein), which is also crucial for DSB resection (Sartori et al., 2007). Strand resection is also cell cycle regulated in vertebrate cells, and evidence suggests that Cdks may regulate this process at least in part via direct phosphorylation of CtIP in a manner analogous to yeast (Huertas and Jackson, 2009; Yun and Hiom, 2009). However, whether this is the only mechanism underlying cell cycle regulation of DSB resection in vertebrates is unclear.
In addition to forming a substrate for HRR, tracts of single-stranded DNA play a key role in triggering aspects of the DNA damage checkpoint response by recruiting and activating the PIKK (PI3-kinase–like kinase) ATR (ataxia telangiectasia and Rad3 related; Cimprich and Cortez, 2008). Unlike the related PIKK ATM (ataxia telangiectasia mutated), which can be activated simply through association with DSBs (Harrison and Haber, 2006), ATR is activated through recruitment to regions of single-stranded DNA in association with its partner protein, ATRIP (ATR-interacting protein; Cimprich and Cortez, 2008). Once activated, ATR and ATM selectively phosphorylate and activate two downstream checkpoint effector kinases, Chk1 and Chk2 (Harrison and Haber, 2006). Phosphorylation of Chk1 by ATR at serine 345 (S345) within the C-terminal regulatory domain in particular is essential for both the DNA damage and replication checkpoint responses in vertebrates (Walker et al., 2009).
Interestingly, recent data indicate that phosphorylation and activation of Chk1 by ATR in response to DSBs is also cell cycle regulated. Thus, in human T24 cultures released from density arrest, Chk1 was activated in response to irradiation only in cells that had reached S and G2 phase (Jazayeri et al., 2006). Consistent with this, Chk1 was activated most strongly in fractions enriched for S- and G2-phase cells when irradiated DT40 cell cultures were fractionated by elutriation (Walker et al., 2009). Cell cycle–dependent DSB processing to generate single-stranded DNA is likely to play a role in determining this pattern of ATR–Chk1 activation (Jazayeri et al., 2006); however, it remains possible that other cell cycle phase–specific processes could also contribute. Finally, it has been reported that Chk1 becomes refractory to activation by DNA damage in mitotic cells (Shiromizu et al., 2006); however, when this desensitization occurs and whether it is imposed via the same regulatory processes that operate during interphase are unknown.
Results and discussion
DNA damage fails to activate Chk1 or delay mitotic entry in late G2
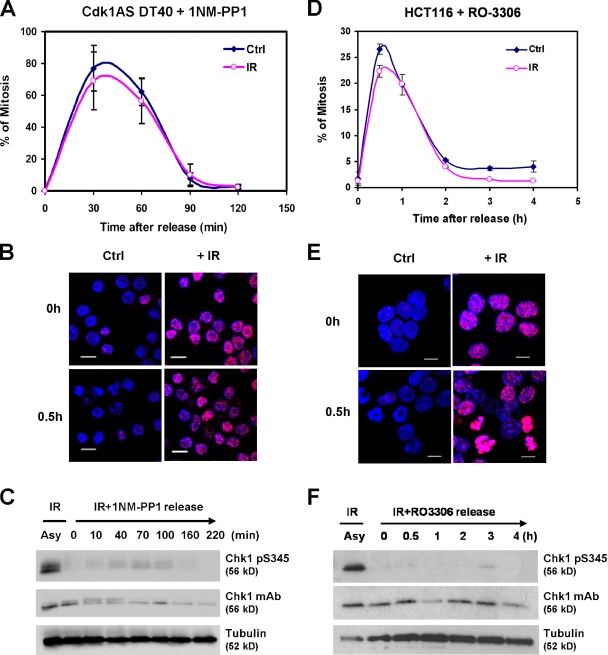
Chk1 is refractory to activation by DNA damage in mitotic cells (Shiromizu et al., 2006); however, when desensitization occurs is unclear. To assess checkpoint proficiency in late G2, we used a DT40 cell line, Cdk1AS, in which a mutant, analogue-sensitive (AS) form of Cdk1 replaces the endogenous kinase (Hochegger et al., 2007). When exposed to the ATP analogue 1NM-PP1, Cdk1AS cells accumulated homogenously in G2, and when the drug was washed away, the great majority rapidly entered mitosis and divided (Fig. 1 A; Hochegger et al., 2007). Importantly, the negative regulatory phosphorylation on tyrosine 15 (Y15), which restrains Cdk1 catalytic activity before mitosis and forms the principal target of the DNA damage checkpoint, is maintained in 1NM-PP1–arrested cells (Hochegger et al., 2007).
Figure 1.
DNA damage fails to activate Chk1 or mitotic delay in late G2–arrested Cdk1AS or HCT116 cells. (A) Mitotic entry in Cdk1AS cells released from 1NM-PP1 arrest with or without irradiation. (B) γ-H2AX staining of 1NM-PP1–arrested Cdk1AS cells before and after release with or without irradiation. (C) Western blot of total and pS345 Chk1 in asynchronous (Asy) and 1NM-PP1–arrested Cdk1AS cells 30 min after exposure to 10 Gy ionizing radiation (IR) and after release. (D–F) As for A–C using RO-3306–arrested HCT116 colon carcinoma cells. (A and D) Values represent the mean and standard deviation of three independent experiments. Ctrl, control. Bars, 10 µm.
Remarkably, when G2-arrested Cdk1AS cells were irradiated (10 Gy) before release, we observed a complete absence of G2 checkpoint proficiency (Fig. 1 A). This was unexpected because asynchronous cultures of Cdk1AS cells arrested in G2 efficiently after irradiation (unpublished data). Furthermore, DNA damage was sensed in the G2-arrested cells because irradiation induced prominent γ-H2AX foci (Fig. 1 B) and nuclear recruitment of Mre11, both of which were attenuated by treatment with the ATM inhibitor Ku55933 (Fig. S1 B, right). These observations indicate that ATM is activated by DNA damage in late G2 cells; however, irradiation-induced mitotic delay in DT40 cells has been shown to be dependent on ATR-mediated activation of Chk1 (Zachos et al., 2003; Walker et al., 2009). Strikingly, although Chk1 S345 phosphorylation could be induced by irradiation of asynchronous cultures, no corresponding activation occurred in G2-arrested cells (Fig. 1 C).
To rule out the possibility that loss of checkpoint proficiency might be an artifact of prolonged 1NM-PP1 exposure, elutriated fractions of Cdk1AS cells enriched in G1/early S or late S/G2/M cells (Fig. S1 A) were irradiated either immediately or after culture for a further 4 h in the presence of 1NM-PP1. Although irradiation activated Chk1 only weakly in the G1/early S population immediately after fractionation, activation was much stronger after 4 h, when most cells (95%) had entered S phase (Fig. S1 A). Conversely, Chk1 was activated strongly in the late S/G2/M population immediately after isolation but only weakly after 4-h culture in 1NM-PP1, when most cells had arrested in late G2, as indicated by high levels of cyclin B2 and Y15-phosphorylated Cdk1 (Fig. S1 A). Thus, loss of checkpoint proficiency is a consequence of cell cycle position and not of Cdk1 inhibition by itself.
We next used the reversible Cdk1-selective inhibitor, RO-3306 (Vassilev et al., 2006), to arrest HCT116 cells in late G2 phase and then released them with or without prior irradiation. As shown in Fig. 1 (D and E), irradiation had no effect on mitotic entry after release, despite the formation of prominent γ-H2AX foci in damaged cells. Neither did we observe Chk1 pS345 phosphorylation in RO-3306–arrested HCT116 cells, although activation was robust in asynchronous cultures (Fig. 1 F). Thus, we conclude that loss of DNA damage checkpoint proficiency is a general feature of cells arrested in late G2.
Checkpoint deficiency in late G2 stems from lack of DNA damage processing
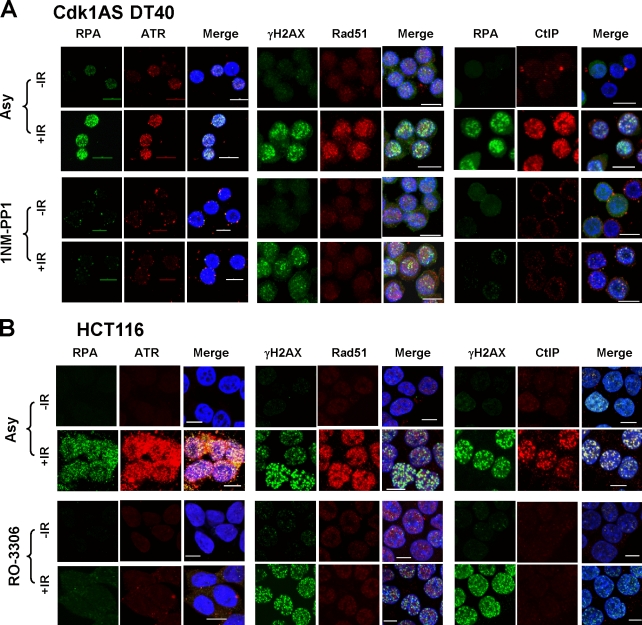
Chk1 S345 phosphorylation is mediated primarily by ATR, whose activity depends on concentration at stretches of single-stranded DNA coated with RPA (replication protein A) generated by DNA strand resection from DSBs. To gain insight into the processing of DSBs in late G2, we examined the subcellular localization of RPA and ATR.
As shown in Fig. 2 (A and B), irradiation induced a marked concentration of RPA and ATR in nuclear foci in a majority of asynchronous Cdk1AS and HCT116 cells; however, no chromatin-associated foci were observed in late G2–arrested cells. In cells proficient for HRR, RPA is displaced from single-stranded DNA by Rad51 to initiate homologous recombination. Consistent with this, irradiation induced the formation of numerous prominent Rad51 foci in many proliferating Cdk1AS and HCT116 cells, which often colocalized with γ-H2AX (Fig. 2, A and B). Strikingly, although γ-H2AX foci formed in late G2–arrested Cdk1AS and HCT116 cells, no recruitment of Rad51 was observed. Collectively, these data indicate that late G2 cells fail to resect DSBs to generate single-stranded DNA, thus leading to failure to recruit or activate ATR or initiate later events required for HRR.
Figure 2.
Lack of DNA damage processing in late G2–arrested Cdk1AS and HCT116 cells. (A) Subcellular localization of RPA, ATR, Rad51, γ-H2AX, and CtIP in asynchronous (Asy) and 1NM-PP1–arrested Cdk1AS cells with or without prior irradiation (10 Gy). DAPI staining is included in the merged images to visualize nuclei. (B) As for A, but using asynchronous or RO-3306–arrested HCT116 cells. IR, ionizing radiation. Bars, 10 µm.
To gain insight into possible explanations for this defect, we examined the effect of irradiation on the subcellular distribution of CtIP, a key protein involved in DSB strand resection. In proliferating Cdk1AS and HCT116 cells, CtIP was observed to concentrate in nuclear foci after irradiation, where it partially colocalized with RPA; however, this did not occur in irradiated late G2 cells (Fig. 2, A and B). Because CtIP is required for DNA strand resection at DSBs (Sartori et al., 2007), it seems likely that failure to recruit this component to sites of damage contributes to the resection defect.
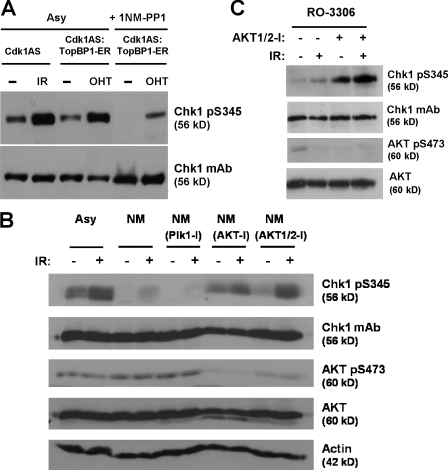
To confirm that the ATR–Chk1 pathway was potentially functional in late G2–arrested cells, we expressed a TopBP1-ER fusion protein that activates ATR in response to tamoxifen (Toledo et al., 2008). Tamoxifen activated Chk1 as potently as irradiation when added to proliferating Cdk1AS–TopBP1-ER cells (Fig. 3 A). Tamoxifen also stimulated Chk1 S345 phosphorylation in late G2–arrested cells, albeit more weakly, demonstrating that the ATR–Chk1 pathway remains competent for activation.
Figure 3.
DNA damage–induced Chk1 S345 phosphorylation can be restored in late G2–arrested cells by Akt inhibition. (A) Comparison of TopBP1-mediated activation of ATR and Chk1 pS345 phosphorylation in asynchronous (Asy) and 1NM-PP1–arrested Cdk1AS cells exposed to tamoxifen or irradiation. (B) Western blot of asynchronous or G2-arrested Cdk1AS cells pretreated with the indicated kinase inhibitors for 1.5 h plus or minus irradiation. (C) Western blot of G2-arrested HCT116 cells pretreated with Akt inhibitor for 1.5 h plus or minus irradiation. IR, ionizing radiation.
Inhibition of Akt/PKB restores Chk1 activation and DNA damage processing in late G2
1NM-PP1–arrested Cdk1AS cells express high levels of Cdk2 activity (Hochegger et al., 2007; Bourke et al., 2010), which is known to be sufficient to sustain both resection and checkpoint activation (Cerqueira et al., 2009). Therefore, we considered that some other pathway or kinase active in G2 might actively suppress DNA damage processing.
Plk1 kinase is active in G2 and is required for recovery from DNA damage–induced checkpoint arrest (Macůrek et al., 2008); however, pretreatment with Plk1 inhibitor did not restore Chk1 activation after irradiation in late G2–arrested cells (Fig. 3 B). Because Akt/PKB kinase activity has been reported to override DNA damage–induced G2 arrest (Henry et al., 2001; Shtivelman et al., 2002; Nimbalkar and Quelle, 2008), we examined the effect of two selective Akt inhibitors, Akt-I and Akt1/2-I. Remarkably, pretreatment with both inhibitors reactivated Chk1 S345 phosphorylation in G2-arrested Cdk1AS cells after irradiation (Fig. 3 B and Fig. S2 A). Akt inhibition alone did not result in DNA damage because cells treated with inhibitor alone showed no γ-H2AX foci (Fig. S2 A). Akt inhibition was confirmed using an antibody against Akt phosphorylated on serine 473 (S473), a modification specific to the active form (Fig. 3 B; Bozulic and Hemmings, 2009). Chemical inhibition of Akt also restored irradiation-induced Chk1 S345 phosphorylation in RO-3306–arrested HCT116 cells (Fig. 3 C and Fig. S2 A). As an alternative means of inhibiting Akt catalytic activity, we depleted Akt isoform expression using siRNA. Interestingly, depletion of Akt2 restored irradiation-induced Chk1 S345 phosphorylation in late G2–arrested HCT116 cells, whereas depletion of Akt1 had little effect (Fig. S2 B and not depicted).
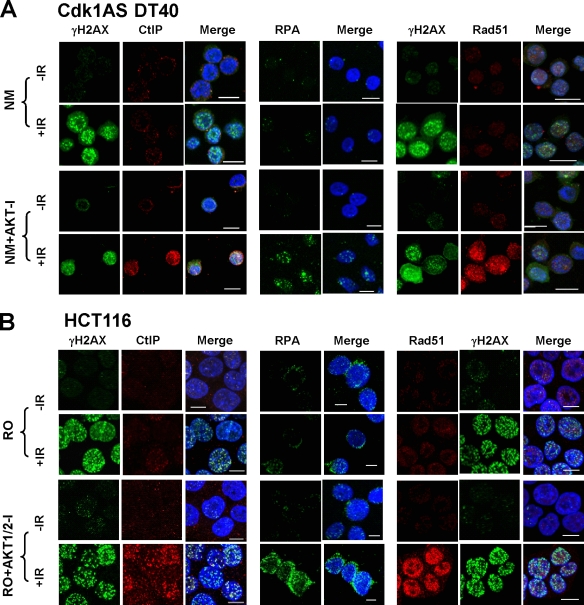
To determine whether inhibition of Akt could restore DNA damage processing in late G2, cells were irradiated with or without pretreatment with Akt inhibitor, and the subcellular distribution of CtIP, RPA, and Rad51 was visualized. As shown in Fig. 4 (A and B), inhibition of Akt restored focal accumulation of CtIP, RPA, and Rad51 in late G2–arrested Cdk1AS and HCT116 cells. Nuclear accumulation of CtIP after damage was also substantially restored in RO-3306–arrested HCT116 cells after simultaneous siRNA depletion of Akt1 and Akt2 (Fig. S2 C). Thus, reactivation of Chk1 by Akt inhibition in late G2–arrested cells after DNA damage is, at least in part, the result of restoration of DNA damage processing.
Figure 4.
DNA damage processing can be restored in late G2–arrested Cdk1AS and HCT116 cells by Akt inhibition. (A) Subcellular localization of CtIP, RPA, γ-H2AX, and Rad51 in 1NM-PP1–arrested Cdk1AS cells pretreated with Akt inhibitor for 1.5 h plus or minus irradiation (10 Gy). DAPI staining is included in the merged images to visualize nuclei. (B) As for A, but using RO-3306–arrested HCT116 cells. IR, ionizing radiation. Bars, 10 µm.
To determine whether augmenting Akt activity could suppress Chk1 activation in proliferating cells, we first used a constitutively active, oncogenic form of Akt (gag-Akt). As shown in Fig. S3 A, proliferating HCT116 cells transfected with gag-Akt failed to activate Chk1 efficiently; however, activation was restored by treatment with Akt inhibitor. Furthermore, whereas irradiation induced prominent γ-H2AX foci in gag-Akt–expressing cells, RPA, ATR, and CtIP failed to concentrate in nuclear foci, indicating that DNA damage processing was also suppressed (Fig. S3 A, right).
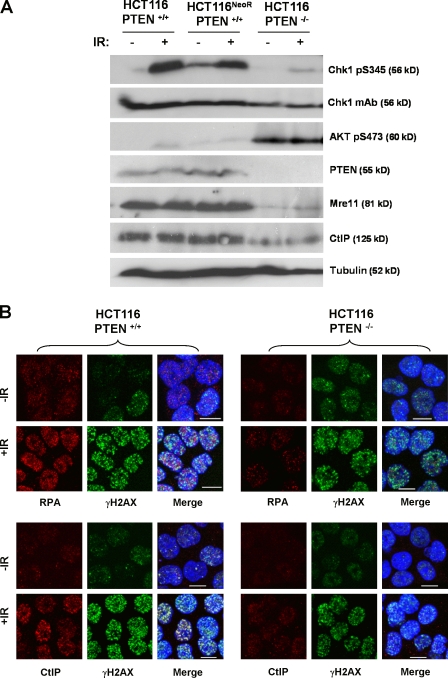
Oncogenic activation of Akt frequently results from loss of PTEN expression or function (Chalhoub and Baker, 2009). To determine how PTEN loss affects DNA damage signaling, we took advantage of a PTEN-deficient HCT116 derivative (Lee et al., 2004). PTEN−/− HCT116 cells exhibited much higher levels of active S473-phosphorylated Akt than either parental HCT116 cells or a drug-resistant control (HCT116NeoR; Fig. 5 A). Strikingly, activation of Chk1 S345 phosphorylation after irradiation was greatly attenuated in PTEN−/− HCT116 cells compared with controls, and this was associated with weaker nuclear recruitment of both RPA and CtIP (Fig. 5 B). Thus, physiological activation of Akt as a result of genetic inactivation of PTEN also leads to suppression of DNA damage signaling.
Figure 5.
PTEN inactivation suppresses DNA damage signaling. (A) Western blot analysis of total and pS345 Chk1, pS473 Akt, PTEN, Mre11, CtIP, and tubulin expression in parental HCT116 cells and neomycin-resistant and PTEN-deficient derivatives. (B) Subcellular localization of RPA, CtIP, and γ-H2AX in parental or PTEN-deficient HCT116 cells plus or minus irradiation (10 Gy). DAPI staining is included in the merged images to visualize nuclei. IR, ionizing radiation. Bars, 10 µm.
The finding that the ATR–Chk1 pathway, and thus DNA damage checkpoint proficiency, becomes refractory to activation late in G2 before the onset of mitosis because of lack of DSB resection is remarkable. In addition to loss of checkpoint proficiency, these data predict that proficiency for HRR will also be lost late in G2. Consistent with this idea, DNA repair is inefficient in late G2–arrested cells, as judged by the persistence of high levels of γ-H2AX 6–8 h after irradiation, when levels have substantially declined in asynchronous controls (Fig. S3 B). Interestingly, a recent study demonstrated a link between the dissolution of Rad51 foci and mitotic entry (Ayoub et al., 2009). Therefore, one possibility is that DNA strand resection may be switched off late in G2 simply to terminate recombination in preparation for mitotic entry and chromosome segregation. Because recombination and ATR activation are both initiated by single-stranded DNA, this could explain their coregulation. However, checkpoint deactivation might also be required for a normal G2/M transition because recent data have shown that Chk1 and Cdk1 interact in a feedback loop that contributes to timing mitotic entry (Enomoto et al., 2009).
One major question arising from these experiments concerns the molecular mechanism or mechanisms by which Akt antagonizes DNA damage processing and checkpoint signaling. We postulate that Akt inhibits the expression or activity of one or more factors required for DSB resection, perhaps opposing the positive effects of Cdk-mediated phosphorylation (Wohlbold and Fisher, 2009). Interestingly, Akt activity is strongly cell cycle regulated in Cdk1AS cells with very high levels in G1, low levels in S, and intermediate levels again in G2 (Fig. S1 A and not depicted). The basis of this regulation is not understood; however, similar fluctuations have been observed in other cell types in which Akt activity has also been implicated in regulation of the G2/M transition (Shtivelman et al., 2002). Interestingly, the level of Mre11, a factor required for strand resection, was markedly reduced in PTEN−/− HCT116 cells compared with controls (Fig. 5 A), whereas constitutive Akt activity has been reported to inhibit recombination by inducing cytoplasmic sequestration of Rad51 and Brca1 (Plo et al., 2008). Although suggestive of possible mechanisms, further work will be required to identify the target or targets through which Akt modulates DNA damage signaling.
A second important question is whether Akt activity also modulates DNA damage processing throughout the cell cycle. That ectopic expression of a constitutively active, oncogenic form of Akt markedly suppresses both DNA damage–induced Chk1 activation in proliferating cells and recruitment of RPA, ATR, and CtIP to sites of damage is consistent with this possibility. Also consistent is the finding that Akt inhibition markedly enhances DNA damage–induced Chk1 activation in proliferating cells. This is unlikely to be mediated via direct phosphorylation of Chk1 by Akt (Puc et al., 2005) because a Chk1 S280A mutant is similarly affected (Fig. S3 C); however, further work will be required to determine whether this amplification reflects more extensive DNA damage processing after Akt inhibition.
Finally, it is pertinent to consider the broader implications of these findings. Akt is deregulated in many human tumors (Yuan and Cantley, 2008). It has generally been considered that Akt deregulation either drives tumor cell proliferation directly or enhances cell survival (Manning and Cantley, 2007). Our findings suggest an alternative, complementary possibility, namely that Akt activation may allow cells to evade the deleterious consequences of DNA damage. The early stages of neoplasia are often associated with spontaneous genotoxic stress, and the resulting DNA damage signaling can elicit tumor-suppressive responses such as cell senescence or apoptosis (Bartkova et al., 2006; Di Micco et al., 2006; Toledo et al., 2008). Mutations that dampen the cellular response to DNA damage signals, such as loss of p53, or attenuate the initial generation of such signals, as we propose in this study for Akt, would be subject to powerful selection by allowing cells to proliferate successfully in the face of such damage. It is possible that tumors that evolve through such a path would exhibit both increased levels of genome instability and resistance to genotoxic anticancer therapies. Future work will seek to evaluate these possibilities.
Materials and methods
Cell culture, synchronization, siRNA, DNA transfection, and chemical inhibitors
Cdk1AS DT40 cells were grown in DME (Invitrogen) containing 10% fetal bovine serum, 1% chicken serum, 10−5 M β-mercaptoethanol, penicillin, and streptomycin at 39.5°C. To synchronize Cdk1AS cells in late G2, 10 µM 1NM-PP1 was added to the medium for 12–16 h. Wild-type and PTEN-deficient HCT116 cells (gift of T. Waldman, Lombardi Comprehensive Cancer Center, Georgetown University School of Medicine, Washington, DC; Lee et al., 2004) were grown in DME containing 10% fetal bovine serum at 37°C in 5% CO2. To synchronize HCT116 cells in late G2, cells were treated with 9 µM RO-3306 (EMD) for 20 h. Cdk1AS DT40 cells were separated in an elutriating rotor (JE-6B; Beckman Coulter) at room temperature at a flow rate of 40 ml/min, and fractions enriched for G1/early S (F1) and late S/G2/M (F2) cells were isolated as described previously (Walker et al., 2009). Cells were irradiated using an Alcyon II CGR MeV cobalt source as described previously (Rainey et al., 2008).
To generate the Cdk1AS–TopBP1-ER cell line, Cdk1AS cells were transfected with plasmid pMXPIE–TopBP1-ER (gift of O. Fernandez-Capetillo, Spanish National Cancer Research Centre, Madrid, Spain; Toledo et al., 2008) and selected for resistance to puromycin. Drug-resistant clones were screened, and a stable TopBP1-expressing clone was chosen for further study. TopBP1-ER function was activated by adding tamoxifen to the culture medium as described previously (Toledo et al., 2008). In general, cells were treated with tamoxifen for 2 h before harvesting. To augment Akt activity, HCT116 cells were transiently transfected with a plasmid encoding a constitutively active gag-Akt fusion protein (Burgering and Coffer, 1995). To inhibit Plk1, Akt, and ATM, cells were pretreated for 1 h with 0.1 µM Plk1 inhibitor (BI 2536), 25 µM Akt1/2-I inhibitor (Sigma-Aldrich), 5 µM Akt-I (EMD), or 10 µM ATM inhibitor Ku55933 (Tocris Bioscience) before experimental treatment as appropriate. Akt1 and Akt2 siRNAs (Thermo Fisher Scientific) were transfected into HCT116 cells using Nucleofector kit V (program D-032; Lonza). All siRNA transfection were performed with a 50-nM final concentration of smart pool. siRNA-treated cells were harvested 48 h after transfection.
Immunocytochemistry
For immunochemical detection of Chk1 pS345, γ-H2AX, and Rad51, cells were fixed in 4% paraformaldehyde in PBS for 15 min, followed by permeabilization with 0.5% Triton X-100 in PBS for 5 min, all at room temperature. For ATR and RPA immunocytochemistry, cells were pretreated with extraction buffer (25 mM Hepes, pH 7.4, 50 mM NaCl, 1 mM ETDA, 3 mM MgCl2, 300 mM sucrose, and 0.5% Triton X-100) as described previously (Huertas and Jackson, 2009) before fixation and permeabilization as for Chk1 pS345, γ-H2AX, and Rad51. For CtIP immunocytochemistry, cells were fixed with 4% paraformaldehyde for 15 min and then treated with 70% EtOH in PBS at −20°C. For Mre11 immunocytochemistry, cells were fixed in acetone/methanol (1:1) for 15 min at room temperature. For analysis of radiation-induced foci, cells were returned to culture for 1 h after irradiation before fixation. Fixed preparations were blocked with 3% BSA in PBS for 30 min at room temperature before incubation with primary and secondary antibodies. Primary antibodies used were pS345 Chk1 (1:100; Cell Signaling Technology), γ-H2AX (1:250; Millipore), RPA (1:200; EMD), ATR (1:100; Santa Cruz Biotechnology, Inc.), Rad51 (1:1,000; Merck Chemicals Ltd.), CtIP (1:100; Santa Cruz Biotechnology, Inc.), and Mre11 (1:100; Santa Cruz Biotechnology, Inc.). Secondary antibodies used were Alexa Fluor 488 or 555 conjugates (Invitrogen). Images were captured using a confocal microscope (A1R [Nikon]; or FV1000 [Olympus]) using either a Plan-Apochromat VC60× NA 1.40 oil or UPLSAPO 60× NA 1.35 oil objective together with NIS-Elements AR (Nikon) or Fluoview version 1.7c (Olympus) software, respectively. Scale bars on images correspond to 10 µm.
Flow cytometry
Cells were fixed in 70% ethanol in PBS overnight. For mitotic index determinations, fixed cells were incubated with polyclonal anti–phospho serine 10 histone H3 (pH3) antibodies, followed by FITC-conjugated secondary antibody. Cells were counterstained with propidium iodide and analyzed for pH3 fluorescence and DNA content by use of a FACScan flow cytometer (BD) as described previously (Zachos et al., 2003). For S-phase determinations, cells were labeled with 25 µM BrdU and then fixed and stained using anti-BrdU monoclonal antibody as described previously (Robinson et al., 2006).
Western blotting
Cells were lysed in ice-cold whole-cell extract buffer (20 mM Hepes, 5 mM EDTA, 10 mM EGTA, 0.4 M KCl, 0.4% Triton X-100, 10% glycerol, 5 mM NaF, 50 ng/ml Okadaic acid, 1 mM DTT, 5 mg/ml leupeptin, 50 mg/ml PMSF, 1 mM benzamidine, 5 mg/ml aprotinin, and 1 mM Na3VO4) for 30 min on ice. Lysates were cleared by centrifugation at 13,200 rpm for 15 min. Cell extracts were resolved by SDS-PAGE and analyzed by Western blotting. Antibodies used for Western blotting were as follows. Monoclonal antibodies against Chk1 (G-4) and actin (C-2) and polyclonal antibodies against Cdc2 phosphorylated at Y15 were purchased from Santa Cruz Biotechnology, Inc. Polyclonal antibodies against pS345 Chk1 and Mre11 were obtained from Cell Signaling Technology, whereas monoclonal antibody against α-tubulin (DM1A) was obtained from Sigma-Aldrich. A polyclonal antiserum against avian cyclin B2 was generated by Eurogentec. Polyclonal antibodies against pan Akt, pS473 Akt, Akt1, Akt2, and CtIP (T16) were purchased from Cell Signaling Technology. Monoclonal antibodies against γ-H2AX and PTEN were obtained from Millipore and Santa Cruz Biotechnology, Inc., respectively.
Online supplemental material
Fig. S1 shows that loss of Chk1 activation is a consequence of cell cycle position. Fig. S2 shows that inhibition of Akt restores irradiation-induced Chk1 activation in late G2. Fig. S3 shows that constitutively active Akt suppresses Chk1 activation and DNA damage processing in proliferating HCT116 cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201003004/DC1.
Acknowledgments
We thank Drs. O. Fernandez-Capetillo and T. Waldman for gifts of TopBP1-ER and PTEN−/− HCT116 cells and Dr. G. Inman for comments on the manuscript.
This work was supported by Cancer Research UK (CR-UK), the Medical Research Council of the UK, and the Wellcome Trust (grant ref: 082267/Z/07/Z). N. Xu is a CR-UK China Fellow.
Footnotes
Abbreviations used in this paper:
- ATM
- ataxia telangiectasia mutated
- ATR
- ataxia telangiectasia and Rad3 related
- CtIP
- C-terminal interacting protein
- DSB
- double-strand break
- HRR
- homologous recombination repair
- RPA
- replication protein A
References
- Ayoub N., Rajendra E., Su X., Jeyasekharan A.D., Mahen R., Venkitaraman A.R. 2009. The carboxyl terminus of Brca2 links the disassembly of Rad51 complexes to mitotic entry. Curr. Biol. 19:1075–1085 10.1016/j.cub.2009.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L.V., Kolettas E., Niforou K., Zoumpourlis V.C., et al. 2006. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 444:633–637 10.1038/nature05268 [DOI] [PubMed] [Google Scholar]
- Bourke E., Brown J.A., Takeda S., Hochegger H., Morrison C.G. 2010. DNA damage induces Chk1-dependent threonine-160 phosphorylation and activation of Cdk2. Oncogene. 29:616–624 10.1038/onc.2009.340 [DOI] [PubMed] [Google Scholar]
- Bozulic L., Hemmings B.A. 2009. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr. Opin. Cell Biol. 21:256–261 10.1016/j.ceb.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Burgering B.M., Coffer P.J. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 376:599–602 10.1038/376599a0 [DOI] [PubMed] [Google Scholar]
- Cerqueira A., Santamaría D., Martínez-Pastor B., Cuadrado M., Fernández-Capetillo O., Barbacid M. 2009. Overall Cdk activity modulates the DNA damage response in mammalian cells. J. Cell Biol. 187:773–780 10.1083/jcb.200903033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N., Baker S.J. 2009. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 4:127–150 10.1146/annurev.pathol.4.110807.092311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich K.A., Cortez D. 2008. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9:616–627 10.1038/nrm2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre’ M., Nuciforo P.G., Bensimon A., et al. 2006. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 444:638–642 10.1038/nature05327 [DOI] [PubMed] [Google Scholar]
- Enomoto M., Goto H., Tomono Y., Kasahara K., Tsujimura K., Kiyono T., Inagaki M. 2009. Novel positive feedback loop between Cdk1 and Chk1 in the nucleus during G2/M transition. J. Biol. Chem. 284:34223–34230 10.1074/jbc.C109.051540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.C., Haber J.E. 2006. Surviving the breakup: the DNA damage checkpoint. Annu. Rev. Genet. 40:209–235 10.1146/annurev.genet.40.051206.105231 [DOI] [PubMed] [Google Scholar]
- Henry M.K., Lynch J.T., Eapen A.K., Quelle F.W. 2001. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 98:834–841 10.1182/blood.V98.3.834 [DOI] [PubMed] [Google Scholar]
- Hochegger H., Dejsuphong D., Sonoda E., Saberi A., Rajendra E., Kirk J., Hunt T., Takeda S. 2007. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J. Cell Biol. 178:257–268 10.1083/jcb.200702034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas P., Jackson S.P. 2009. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J. Biol. Chem. 284:9558–9565 10.1074/jbc.M808906200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas P., Cortés-Ledesma F., Sartori A.A., Aguilera A., Jackson S.P. 2008. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature. 455:689–692 10.1038/nature07215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A., Falck J., Lukas C., Bartek J., Smith G.C., Lukas J., Jackson S.P. 2006. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8:37–45 10.1038/ncb1337 [DOI] [PubMed] [Google Scholar]
- Lee C., Kim J.S., Waldman T. 2004. PTEN gene targeting reveals a radiation-induced size checkpoint in human cancer cells. Cancer Res. 64:6906–6914 10.1158/0008-5472.CAN-04-1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macůrek L., Lindqvist A., Lim D., Lampson M.A., Klompmaker R., Freire R., Clouin C., Taylor S.S., Yaffe M.B., Medema R.H. 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 455:119–123 10.1038/nature07185 [DOI] [PubMed] [Google Scholar]
- Manning B.D., Cantley L.C. 2007. AKT/PKB signaling: navigating downstream. Cell. 129:1261–1274 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimbalkar D., Quelle F.W. 2008. Phosphoinositide 3-kinase signaling overrides a G2 phase arrest checkpoint and promotes aberrant cell cycling and death of hematopoietic cells after DNA damage. Cell Cycle. 7:2877–2885 [DOI] [PubMed] [Google Scholar]
- Plo I., Laulier C., Gauthier L., Lebrun F., Calvo F., Lopez B.S. 2008. AKT1 inhibits homologous recombination by inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res. 68:9404–9412 10.1158/0008-5472.CAN-08-0861 [DOI] [PubMed] [Google Scholar]
- Puc J., Keniry M., Li H.S., Pandita T.K., Choudhury A.D., Memeo L., Mansukhani M., Murty V.V., Gaciong Z., Meek S.E., et al. 2005. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 7:193–204 10.1016/j.ccr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Rainey M.D., Black E.J., Zachos G., Gillespie D.A. 2008. Chk2 is required for optimal mitotic delay in response to irradiation-induced DNA damage incurred in G2 phase. Oncogene. 27:896–906 10.1038/sj.onc.1210702 [DOI] [PubMed] [Google Scholar]
- Robinson H.M., Jones R., Walker M., Zachos G., Brown R., Cassidy J., Gillespie D.A. 2006. Chk1-dependent slowing of S-phase progression protects DT40 B-lymphoma cells against killing by the nucleoside analogue 5-fluorouracil. Oncogene. 25:5359–5369 10.1038/sj.onc.1209532 [DOI] [PubMed] [Google Scholar]
- Sartori A.A., Lukas C., Coates J., Mistrik M., Fu S., Bartek J., Baer R., Lukas J., Jackson S.P. 2007. Human CtIP promotes DNA end resection. Nature. 450:509–514 10.1038/nature06337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromizu T., Goto H., Tomono Y., Bartek J., Totsukawa G., Inoko A., Nakanishi M., Matsumura F., Inagaki M. 2006. Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1). Genes Cells. 11:477–485 10.1111/j.1365-2443.2006.00955.x [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Sussman J., Stokoe D. 2002. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr. Biol. 12:919–924 10.1016/S0960-9822(02)00843-6 [DOI] [PubMed] [Google Scholar]
- Toledo L.I., Murga M., Gutierrez-Martinez P., Soria R., Fernandez-Capetillo O. 2008. ATR signaling can drive cells into senescence in the absence of DNA breaks. Genes Dev. 22:297–302 10.1101/gad.452308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L.T., Tovar C., Chen S., Knezevic D., Zhao X., Sun H., Heimbrook D.C., Chen L. 2006. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA. 103:10660–10665 10.1073/pnas.0600447103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Black E.J., Oehler V., Gillespie D.A., Scott M.T. 2009. Chk1 C-terminal regulatory phosphorylation mediates checkpoint activation by de-repression of Chk1 catalytic activity. Oncogene. 28:2314–2323 10.1038/onc.2009.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold L., Fisher R.P. 2009. Behind the wheel and under the hood: functions of cyclin-dependent kinases in response to DNA damage. DNA Repair (Amst.). 8:1018–1024 10.1016/j.dnarep.2009.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T.L., Cantley L.C. 2008. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 27:5497–5510 10.1038/onc.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M.H., Hiom K. 2009. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 459:460–463 10.1038/nature07955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos G., Rainey M.D., Gillespie D.A. 2003. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 22:713–723 10.1093/emboj/cdg060 [DOI] [PMC free article] [PubMed] [Google Scholar]